To the Editor:
Since the end of February 2020, Italy is facing an outbreak of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and the clinical picture associated with the infection has been named COVID‐19.
The COVID‐19 patients may develop multiple organ dysfunction with signs of endothelial derangement. Vascular damage has been proposed as a relevant mechanism that can perpetuate the activation of complement system, and might therefore enhance the inflammatory stimulus.
Recently post‐mortem analysis in a series of patients with COVID‐19 by Varga et al. 1 demonstrated endothelial cell involvement in different organs (kidney, lung, small bowel) with an accumulation of inflammatory cells, and evidence of endothelial and inflammatory cell death. In addition, viral‐like particles have been reported within endothelial cells, but direct viral infection of endothelial cells is a matter of debate 2 .
Another report 3 on pulmonary autopsy specimens compared COVID‐19 patients’ findings with specimens of patients who died of influenza H1N1. Besides common features of diffuse alveolar damage, COVID‐19 infected patients showed distinctive histologic features of severe endothelial injury, intracellular endothelial SARS‐CoV‐2 virus, microangiopathy with intravascular thrombi and intussusceptive angiogenesis.
Circulating endothelial cells (CECs) are mature cells considered reliable markers of endothelial derangement. Healthy subjects carry low CEC counts, whereas it has been demonstrated that levels increase significantly in conditions associated with vascular damage. 4 Note, CEC enumeration in peripheral blood using flow cytometry has been successfully used to correlate CEC titre and organ damage in ARDS, 5 and hematopoietic stem cell transplantation‐related sinusoidal obstruction syndrome. 6
We evaluated CECs in 30 COVID‐19 patients admitted to our institution and hospitalized in the intensive care unit or in the infectious disease unit; six healthy controls have been analyzed. Freshly collected peripheral blood EDTA samples from non‐traumatic venipuncture were analyzed according to standardized methods by trained operators, unaware of the patients’ clinical conditions. 7 The number of CECs was expressed as cells per milliliter of blood and counts were considered normal when inferior to 30/mL (Figure S1). At the same time peripheral blood smears were evaluated by hematologists to assess schistocyte count; the presence of ≥1% schistocytes on peripheral blood smears was considered abnormal.
Clinical features of patients, comorbidities and timing of analysis are summarized in Table S1. At the time of CEC evaluation, LDH and D‐dimer were elevated in 29/30 (97%) and 22/25 (88%) patients, respectively; schistocytes were detected in five patients (range 0%‐4.5%). Hemoglobin was less than 13 g/dL in 27 patients (90%), and platelets less than 150 × 109/L in eight patients (27%). Haptoglobin was normal in all tested patients.
In 23 out of 30 patients (76.7%) CECs were > 30/mL, while this was true in one out of six (16.7%) healthy subjects (P = .010). Circulating endothelial cells were higher in patients with respect to healthy subjects (46/mL; IQR: 32‐89/mL vs 14.5/mL; IQR: 9‐20/mL, P = .002) (Figure 1). Table S2 reports the comparison of laboratory features according to CECs values. Higher numbers of CECs were found in patients at earlier phase of disease (median time between hospitalization and assessment for elevated vs normal CECs: 5 vs 18 days). No differences were found between patients with elevated CECs and those with normal values of hemoglobin, leukocytes, lymphocytes, platelets, LDH, D‐dimer (Table S2).
FIGURE 1.
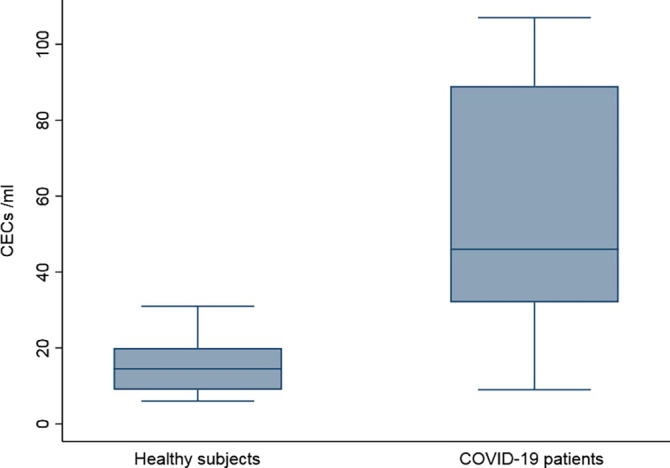
Circulating endothelial cells in 30 COVID‐19 patients and in six healthy subjects (Two COVID‐19 patients with CECs higher than 200 were omitted from figure)
Overall these data add a supplemental element to support the role of endothelial damage in COVID‐19. The presence of concomitant comorbidities known to be related to CECs elevation, such as cardiovascular and rheumatic diseases, diabetes and malignancies might exert a confounding effect on CEC assessment.
On the other hand it has been demonstrated that COVID‐19 patients affected by such comorbidities have worse outcomes; 8 it can be hypothesized that viral endothelial damage might be enhanced in presence of additional factors that contribute to endothelial impairment.
Among possible limitations of the study, the heterogeneous timing of CEC assessment and the lack of sequential analysis have to be acknowledged.
Moreover, our assay is not able to identify non vital endothelial cells that already underwent apoptosis after viral injury and, therefore, our analysis might not fully depict the entirety of endothelial damage.
It would also be of interest to extend our evaluation to the circulating endothelial progenitor cells (CEPs) compartment in order to identify possible correlation between CEC and CEP counts and to investigate angiogenic activity.
Finally, from a therapeutic perspective, it will be useful to assess the value of CEC monitoring during treatment with low molecular weight heparin and other drugs under investigation for COVID‐19. Complement inhibitors such as eculizumab and IFX‐1, and the endothelial protecting drug defibrotide, could limit the extension of endothelial damage and the progression of respiratory failure in COVID‐19.
The results of clinical trials (NCT04335201; NCT04288713, NCT04333420) investigating these agents are eagerly awaited.
CONFLICT OF INTEREST
L.A. received advisory honoraria from Celgene, Gilead, Roche, Janssen‐Cilag, Verastem and research support from Gilead, all unrelated to this correspondence.
AUTHOR CONTRIBUTIONS
M.E.N. and G.M. contributed equally as first authors, and L.A., R.B. and M.B. contributed equally as last authors. L.A., G.A.I., R.B. and M.B. are designed the study: M.E.N., G.M., L.P., M.S. and A.D.S. collected data: A.T., C.P., and E.C. performed flow cytometry, V.V.F. performed statistical analysis, M.E.N., G.M., and L.A. wrote paper: all authors approved the final version.
Supporting information
Figure S1 ‐ Flow cytometry identification of circulating endothelial cells
Panel A: lympho‐monocytes were gated on a FSC‐A/SSC‐A dot plot; Panel B: nucleated events (Syto‐16+) were selected; Panel C: dead cells were excluded on the basis of their positivity to 7‐AAD; Panel D: two subpopulations were identified and gated separately: CD34+/CD45 dim (hematopoietic stem cells, yellow dots) and CD34 bright/CD45‐ (green dots); Panel E: both subpopulations were analyzed for CD146 expression. Circulating Endothelial Cells (CD34 bright/CD45‐/CD146+) were identified.
REFERENCES
- 1. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395:1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goldsmith CS, Miller SE, Martines RB, Bullock HA, Zaki SR. Electron microscopy of SARS‐CoV‐2: a challenging task. The Lancet. 2020;395(10238):e99. 10.1016/s0140-6736(20)31188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid‐19. New England Journal of Medicine. 2020. 10.1056/nejmoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farinacci M, Krahn T, Dinh W, et al. Circulating endothelial cells as biomarker for cardiovascular diseases. Res Pract Thromb Haemost. 2019;3:49‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moussa MD, Santonocito C, Fagnoul D, et al. Evaluation of endothelial damage in sepsis‐related ARDS using circulating endothelial cells. Intensive Care Med. 2015;41:231‐238. [DOI] [PubMed] [Google Scholar]
- 6. Moiseev IS, Babenko EV, Sipol AA, Vavilov VN, Afanasyev BV. Measurement of circulating endothelial cells to support the diagnosis of veno‐occlusive disease after hematopoietic stem cell transplantation. International Journal of Laboratory Hematology. 2014;36(4):e27–e29. 10.1111/ijlh.12137. [DOI] [PubMed] [Google Scholar]
- 7. Lanuti P, Simeone P, Rotta G, et al. A standardized flow cytometry network study for the assessment of circulating endothelial cell physiological ranges. Sci Rep. 2018;8:5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid‐19 in China: a Nationwide analysis. Eur Respir J. 2020;55:2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 ‐ Flow cytometry identification of circulating endothelial cells
Panel A: lympho‐monocytes were gated on a FSC‐A/SSC‐A dot plot; Panel B: nucleated events (Syto‐16+) were selected; Panel C: dead cells were excluded on the basis of their positivity to 7‐AAD; Panel D: two subpopulations were identified and gated separately: CD34+/CD45 dim (hematopoietic stem cells, yellow dots) and CD34 bright/CD45‐ (green dots); Panel E: both subpopulations were analyzed for CD146 expression. Circulating Endothelial Cells (CD34 bright/CD45‐/CD146+) were identified.


