The coronavirus disease 2019 (COVID‐19) pandemic has led to an abrupt transition to virtual healthcare in pregnancy in order to reduce dependence on hospital‐based care and minimize the risk of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, which appears to carry a similar risk in pregnancy compared with that in non‐pregnant adults 1 . This is true for all women, including the approximately 10% who have pregnancy hypertension and receive specialist hypertension care 2 .
Specific guidance for hypertensive pregnant women during the COVID‐19 pandemic has been provided in some jurisdictions 3 and has focused on provision of self‐monitoring at home and virtual consultation whenever possible. This is most likely for women with chronic or gestational hypertension, who can self‐monitor blood pressure (BP) at home, undertake proteinuria testing, and receive only remote review by the maternity‐care team unless otherwise attending hospital (such as for maternal blood tests or fetal ultrasound). While women with pre‐eclampsia may be cared for as outpatients, they are still advised to attend face‐to‐face visits frequently 3 . Regardless, key aspects of pregnancy‐hypertension care must be provided for all hypertensive pregnant women and within the constraints of the current healthcare system.
Measure BP with device validated for use in pregnancy
While home BP monitoring (HBPM) has been undertaken informally in maternity care, the COVID‐19 pandemic has facilitated rapid implementation of this practice. HBPM is a key part of a remote monitoring strategy in pregnancy, and is recommended based on acceptability to women, widespread informal use and lack of safety concerns 4 . Women with chronic hypertension are ideally suited for HBPM and may have practiced this before pregnancy. Women with gestational hypertension are also capable of undertaking HBPM 5 .
As a national example, HBPM is being facilitated for use in the UK. First, the Royal College of Obstetricians and Gynaecologists (RCOG) provides guidance on BP monitoring devices that are appropriate for home use and validated for use in pregnancy and pre‐eclampsia specifically (https://STRIDEBP.org/BP‐monitors), along with clear patient instructions for BP device loans and details of monitoring 4 . Second, UK government agencies have procured and validated BP monitors for purchase by hospitals, for domiciliary use by hypertensive pregnant women. Third, use of BP apps is being encouraged to facilitate recording of BP and transmission of BP values to care providers; K2 Hampton (https://www.k2ms.com) is the only pregnancy BP app certified as a Class‐I medical device in the UK and extensively evaluated within the NHS 5 , 6 , 7 .
It is unclear whether HBPM targets should be the same as those used in the clinical setting for either screening (among previously normotensive women, whether they are at low or increased risk of pre‐eclampsia) or management among hypertensive women. While BP measured at home (vs the clinic) may be lower, at least among hypertensive women (by up to 16 mmHg systolic and 7 mmHg diastolic), there is wide variation between women 8 . As such, it is difficult to justify routine use of lower target BP values at home.
The implications on pregnancy outcomes and costs of a reliance on HBPM to replace many clinic measurements are unknown. Preliminary evidence in hypertensive women attending for specialist care suggests that use of HBPM and a BP app may reduce outpatient healthcare utilization (even among women with recently diagnosed gestational hypertension 5 ) and costs 7 .
Assess risk of pre‐eclampsia at antenatal care booking and prescribe aspirin for women at increased risk
Low‐dose aspirin decreases the risk of pre‐eclampsia, particularly preterm pre‐eclampsia, when 150 mg/day of aspirin is administered to women identified as being at high risk based on first‐trimester multivariable screening 9 . While concerns have been raised about use of non‐steroidal anti‐inflammatory drugs (NSAIDs) and an associated risk of disease progression, this remains unproven, and the World Health Organization considers use of NSAIDs acceptable for relief of COVID‐19 symptoms 10 . The dose of aspirin for pre‐eclampsia prevention is lower than that used to achieve anti‐inflammatory effects, and there are no reports of accelerated COVID‐19 disease progression in patients so‐treated. Furthermore, it is even more important to decrease the risk of pre‐eclampsia in this era of virtual care.
Treat hypertension (BP ≥ 140/90 mmHg) with antihypertensive therapy
Oral antihypertensive therapy halves the risk of severe hypertension (systematic review, 31 trials, 3485 women) 11 , which is an outcome that warrants face‐to‐face assessment in all jurisdictions, even during the COVID‐19 pandemic. As avoidance of unnecessary face‐to‐face visits is an objective goal during this pandemic, avoidance of severe hypertension is a particularly worthy goal.
The international Control of Hypertension In Pregnancy Study (CHIPS) trial showed that ‘tight’ control (aiming for a target diastolic BP of 85 mmHg) was better than ‘less‐tight’ control (aiming for a target diastolic BP of 100 mmHg to minimize use of antihypertensive therapy), not only to reduce the incidence of severe hypertension, but also that of a platelet count < 100 × 10 9 /L and elevated liver enzymes with symptoms 12 . Importantly, there was no impact (positive or negative) of ‘tight’ control on perinatal mortality or morbidity, birth weight < 10th centile or preterm birth 13 . BP control was achieved by a simple algorithm of up or down titration of antihypertensive medication (Figure 1), using single or multiple medications; in Figure 2, we provide practical advice and a protocol for dosing escalation from starting to maximum dosage and medication combinations. Initial antihypertensive therapy should be monotherapy using an accepted first‐line drug; while no antihypertensive agent has been proven superior to others, oral labetalol (as used by the majority of women in CHIPS), nifedipine and methyldopa are used most commonly in pregnancy 11 , 14 . As is the case outside of pregnancy, pregnant women of African or Caribbean ethnic origin would be expected to respond best to a calcium‐channel blocker based on the high prevalence of low‐renin hypertension in this population, but the majority still respond to oral labetalol 15 . Additional antihypertensive drugs should be used if target BP levels are not achieved with standard‐dose monotherapy 16 , at least to a mid‐range dose; add‐on drugs should be from a different drug class chosen from first‐ or second‐line options 16 .
Figure 1.
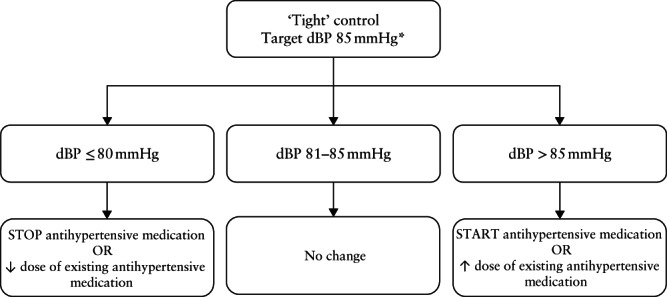
Algorithm for ‘tight’ blood‐pressure (BP) control in CHIPS trial. *If systolic BP is ≥ 160 mmHg, increase dose of existing medication or start new antihypertensive medication to get systolic BP < 160 mmHg, regardless of diastolic BP (dBP). Figure adapted from Magee et al. 13 .
Figure 2.
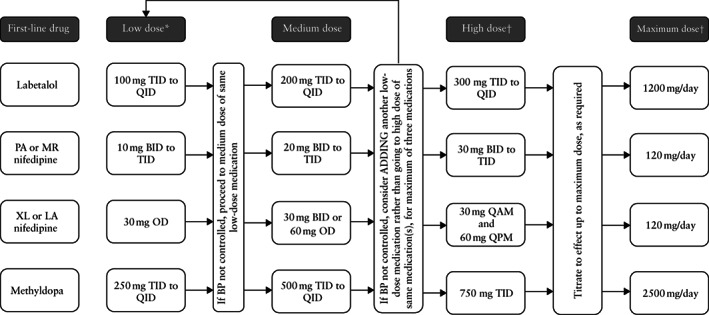
Suggested dose titration of first‐line antihypertensive therapy in pregnancy. *Starting doses are higher than those generally recommended for non‐pregnant adults, given more rapid clearance in pregnancy. †When medication is at high (or maximum) dosage, consider using different medication to treat any severe hypertension that may develop. BID, twice/day; BP, blood pressure; LA, long‐acting; MR, modified release; OD, once/day; PA, prolonged action; QAM, every morning; QID, four times/day; QPM, every evening; TID, three times/day; XL, extended release.
Define pre‐eclampsia broadly and assess risk of adverse maternal outcomes
Chronic (≈ 25%) or gestational (up to ≈ 35%) hypertension frequently evolves into pre‐eclampsia. Detection of this progression is why professional societies and advocacy groups emphasize evaluation of maternal symptoms 14 , and many societies have adopted a broad definition of pre‐eclampsia that includes maternal/fetoplacental end‐organ involvement (including symptoms) 17 .
In a systematic review of maternal risk stratification in pregnancy hypertension (32 studies), miniPIERS (Pre‐eclampsia Integrated Estimate of Risk Score) was the only model for all pregnancy hypertension types 18 . Importantly, during the COVID‐19 pandemic, miniPIERS can also be used for outpatients. miniPIERS has been externally validated 19 and quantifies the risk of adverse maternal outcome by BP, symptoms, urinalysis (if performed), gestational age and parity (of particular importance for nulliparous women who have no history of ongoing pregnancy) 19 . According to the model, women are classified as being at high risk if their predicted probability of adverse outcome is ≥ 25%, which as a ‘rule‐in’ test has a good likelihood ratio (5.1) and classifies correctly 86% of women.
Any woman with suspected pre‐eclampsia requires a face‐to‐face evaluation by her healthcare team. Angiogenic markers have been recommended as part of this evaluation in the UK 20 , based on their good‐to‐excellent performance at ruling out a diagnosis of pre‐eclampsia (defined as new‐onset proteinuria) within 7 days or pre‐eclampsia requiring delivery within 14 days 21 , 22 , 23 , 24 . However, angiogenic markers may be useful even if women meet diagnostic criteria for pre‐eclampsia; many women in ‘suspected’ pre‐eclampsia studies likely had pre‐eclampsia at baseline 22 , and preliminary evidence suggests that angiogenic markers may further improve prediction of the need for delivery 25 and guide place of care.
Time delivery from 37 weeks for women with pre‐eclampsia
By global consensus, women with preterm pre‐eclampsia who reach 37 + 0 weeks, and those who develop pre‐eclampsia at term gestational age, should be induced within 24 h in order to decrease the risk of maternal disease progression and complications 26 . While guidelines are inconsistent regarding timed delivery for women with chronic or gestational hypertension, local standard of care should be maintained. When considering induction of labor, if a woman is also symptomatic with COVID‐19, it may be advisable to delay induction if possible 3 ; under those circumstances, strict attention to BP control would be prudent as severe hypertension is the most common complication avoided by labor induction.
Use antenatal corticosteroids for fetal lung maturation
Dexamethasone is being evaluated as a therapeutic intervention for SARS‐CoV‐2 infection requiring hospitalization outside of pregnancy (https://www.recoverytrial.net/). As such, there is no maternal harm anticipated from use of antenatal corticosteroids for acceleration of fetal pulmonary maturity, and many women with pre‐eclampsia will require iatrogenic preterm birth. However, for outpatient hypertensive women prior to elective Cesarean delivery, clinicians should weigh the diminishing benefits of antenatal corticosteroids with advancing gestational age up to 38 + 6 weeks against the risks of SARS‐CoV‐2 infection, as women need to attend hospital twice to receive the injections 3 .
Use magnesium sulfate to prevent or treat eclampsia
There are no published reports of magnesium sulfate altering the natural history of SARS‐CoV‐2 infection. As magnesium sulfate halves the risk of eclampsia incidence and recurrence, it should be used during the COVID‐19 pandemic as normally indicated.
Measure BP postpartum on days 3–6 after hypertensive pregnancy
Despite its importance, there is limited evidence to support how to use antihypertensive therapy postpartum 27 . One trial found that HBPM and postnatal down‐titration of antihypertensives improved BP control 28 . The most commonly used antihypertensives, and most others, are acceptable for use when breastfeeding 29 . Given that BP rises postpartum and peaks on days 3–6 after birth, by which time women have usually left hospital, and as hypertension increases the risk of postnatal stroke 30 , it would be reasonable to continue ‘tight’ BP control for the first 6 weeks postpartum.
While drugs that block the renin‐angiotensin system may be used for postpartum hypertension, based on low drug levels in breast milk, the effect of angiotensin‐converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) on the natural history of COVID‐19 has been questioned. Mechanisms have been postulated for both harmful and beneficial effects mediated through upregulation of membrane‐bound ACE‐2 by ACE inhibitors or ARBs 31 . While reassuring information is emerging 32 , given the greater difficulty in monitoring maternal serum electrolytes and creatinine during the COVID‐19 pandemic, it may be prudent to avoid use of these medications postpartum until after the pandemic.
Conclusions
Hypertension complicates approximately 10% of pregnancies and is a leading cause of maternal and perinatal morbidity and mortality worldwide. The COVID‐19 crisis has rapidly broadened a shared model of care with women in order to diagnose and remotely manage pregnancy hypertension. This health‐system transition is superimposed on significant shifts in thought about pre‐eclampsia definitions, maternal risk stratification and ‘tight’ BP control. As Winston Churchill said, ‘Never let a good crisis go to waste.’
REFERENCES
- 1. Khalil A, Kalafat E, Benlioglu C, O'Brien P, Morris E, Draycott T, Thangaratinam S, Le Doare K, Heath P, Ladhani S, von Dadelszen P, Magee L. SARS‐CoV‐2 infection in pregnancy: a systematic review and meta‐analysis of clinical features and pregnancy outcomes. EClinicalMedicine 2020; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Magee LA, Sharma S, Nathan HL, Adetoro OO, Bellad MB, Goudar S, Macuacua SE, Mallapur A, Qureshi R, Sevene E, Sotunsa J, Valá A, Lee T, Payne BA, Vidler M, Shennan AH, Bhutta ZA, von Dadelszen P; CLIP Study Group . The incidence of pregnancy hypertension in India, Pakistan, Mozambique, and Nigeria: A prospective population‐level analysis. PLoS Med 2019; 16: e1002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Royal College of Obstetricians and Gynaecologists (RCOG). Guidance for maternal medicine in the evolving coronavirus (COVID‐19) pandemic ‐ Information for healthcare professionals: Version 8. 2020. rcog.org.uk/globalassets/documents/guidelines/2020‐04‐17‐coronavirus‐covid‐19‐infection‐in‐pregnancy.pdf
- 4.Royal College of Obstetricians and Gynaecologists (RCOG). Self‐monitoring of blood pressure in pregnancy. Information for healthcare professionals. 2020. rcog.org.uk/globalassets/documents/guidelines/2020‐03‐30‐self‐monitoring‐of‐blood‐pressure‐in‐pregnancy.pdf
- 5. Kalafat E, Leslie K, Bhide A, Thilaganathan B, Khalil A. Pregnancy outcomes following home blood pressure monitoring in gestational hypertension. Pregnancy Hypertens 2019; 18: 14–20. [DOI] [PubMed] [Google Scholar]
- 6. Perry H, Sheehan E, Thilaganathan B, Khalil A. Home blood‐pressure monitoring in a hypertensive pregnant population. Ultrasound Obstet Gynecol 2018; 51: 524–530. [DOI] [PubMed] [Google Scholar]
- 7. Xydopoulos G, Perry H, Sheehan E, Thilaganathan B, Fordham R, Khalil A. Home blood‐pressure monitoring in a hypertensive pregnant population: cost‐minimization study. Ultrasound Obstet Gynecol 2019; 53: 496–502. [DOI] [PubMed] [Google Scholar]
- 8. Tucker KL, Bankhead C, Hodgkinson J, Roberts N, Stevens R, Heneghan C, Rey É, Lo C, Chandiramani M, Taylor RS, North RA, Khalil A, Marko K, Waugh J, Brown M, Crawford C, Taylor KS, Mackillop L, McManus RJ. How Do Home and Clinic Blood Pressure Readings Compare in Pregnancy? Hypertension 2018; 72: 686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rolnik DL, Wright D, Poon LC, O'Gorman N, Syngelaki A, de Paco Matallana C, Akolekar R, Cicero S, Janga D, M1 Singh, Molina FS, Persico N, Jani JC, Plasencia W, Papaioannou G, Tenenbaum‐Gavish K, Meiri H, Gizurarson S, Maclagan K, Nicolaides KH. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med 2017; 377: 613–622. [DOI] [PubMed] [Google Scholar]
- 10. Kwiatkowski S, Borowski D, Kajdy A, Poon LC, Rokita W, Wielgo SM. Why we should not stop giving aspirin to pregnant women during the COVID‐19 pandemic. Ultrasound Obstet Gynecol 2020; 55: 841–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abalos E, Duley L, Steyn DW, Gialdini C. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev 2018; 10: CD002252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Magee LA, von Dadelszen P, Rey E, Ross S, Asztalos E, Murphy KE, Menzies J, Sanchez J, Singer J, Gafni A, Gruslin A, Helewa M, Hutton E, Lee SK, Lee T, Logan AG, Ganzevoort W, Welch R, Thornton JG, Moutquin JM. Less‐tight versus tight control of hypertension in pregnancy. N Engl J Med 2015; 372: 407–417. [DOI] [PubMed] [Google Scholar]
- 13. Magee LA, Rey E, Asztalos E, Hutton E, Singer J, Helewa M, Lee T, Logan AG, Ganzevoort W, Welch R, Thornton JG, von Dadelszen P. Management of non‐severe pregnancy hypertension ‐ A summary of the CHIPS Trial (Control of Hypertension in Pregnancy Study) research publications. Pregnancy Hypertens 2019; 18: 156–162. [DOI] [PubMed] [Google Scholar]
- 14. Webster K, Fishburn S, Maresh M, Findlay SC, Chappell LC, Guideline C. Diagnosis and management of hypertension in pregnancy: summary of updated NICE guidance. BMJ 2019; 366: l5119. [DOI] [PubMed] [Google Scholar]
- 15. Stott D, Bolten M, Salman M, Paraschiv D, Douiri A, Kametas NA. A prediction model for the response to oral labetalol for the treatment of antenatal hypertension. J Hum Hypertens 2017; 31: 126–131. [DOI] [PubMed] [Google Scholar]
- 16. Butalia S, Audibert F, Cote AM, Firoz T, Logan AG, Magee LA, Mundle W, Rey E, Rabi DM, Daskalopoulou SS, Nerenberg KA; Hypertension Canada. Hypertension Canada's 2018 Guidelines for the Management of Hypertension in Pregnancy. Can J Cardiol 2018; 34: 526–531. [DOI] [PubMed] [Google Scholar]
- 17. Gillon TE, Pels A, von Dadelszen P, MacDonell K, Magee LA. Hypertensive disorders of pregnancy: a systematic review of international clinical practice guidelines. PLoS One 2014; 9: e113715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ukah UV, De Silva DA, Payne B, Magee LA, Hutcheon JA, Brown H, Ansermino JM, Lee T, von Dadelszen P. Prediction of adverse maternal outcomes from pre‐eclampsia and other hypertensive disorders of pregnancy: A systematic review. Pregnancy Hypertens 2018; 11: 115–123. [DOI] [PubMed] [Google Scholar]
- 19. Payne BA, Hutcheon JA, Ansermino JM, Hall DR, Bhutta ZA, Bhutta SZ, Biryabarema C, Grobman WA, Groen H, Haniff F, Li J, Magee LA, Merialdi M, Nakimuli A, Qu Z, Sikandar R, Sass N, Sawchuck D, Steyn DW, Widmer M, Zhou J, von Dadelszen P; miniPIERS Study Working Group . A risk prediction model for the assessment and triage of women with hypertensive disorders of pregnancy in low‐resourced settings: the miniPIERS (Pre‐eclampsia Integrated Estimate of RiSk) multi‐country prospective cohort study. PLoS Med 2014; 11: e1001589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institute for Health and Care Excellence (NICE). Diagnostic Guidance DG23. PlGF‐based testing to help diagnose suspected pre‐eclampsia (Triage PlGF test, Elecsys immunoassay sFlt‐1/PlGF ratio, DELFIA Xpress PlGF 1‐2‐3 test, and BRAHMS sFlt‐1 Kryptor/BRAHMS PlGF plus Kryptor PE ratio). 2016. https://www.nice.org.uk/guidance/dg23
- 21. Bian X, Biswas A, Huang X, Lee KJ, Li TK, Masuyama H, Ohkuchi A, Park JS, Saito S, Tan KH, Yamamoto T, Dietl A, Grill S, Verhagen‐Kamerbeek WDJ, Shim JY, Hund M. Short‐Term Prediction of Adverse Outcomes Using the sFlt‐1 (Soluble fms‐Like Tyrosine Kinase 1)/PlGF (Placental Growth Factor) Ratio in Asian Women With Suspected Preeclampsia. Hypertension 2019; 74: 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chappell LC, Duckworth S, Seed PT, Griffin M, Myers J, Mackillop L, Simpson N, Waugh J, Anumba D, Kenny LC, Redman CW, Shennan AH. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation 2013; 128: 2121–2131. [DOI] [PubMed] [Google Scholar]
- 23. Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC , Sennstrom M, Olovsson M, Brennecke SP, Stepan H, Allegranza D, Schoedl M, Grill S, Hund M, Verlohren S. Predictive Value of the sFlt‐1:PlGF Ratio in Women with Suspected Preeclampsia. N Engl J Med 2016; 374: 13–22. [DOI] [PubMed] [Google Scholar]
- 24. Zeisler H, Llurba E, Chantraine FJ, Vatish M, Staff AC , Sennstrom M, Olovsson M, Brennecke SP, Stepan H, Allegranza D, Schoedl M, Grill S, Hund M, Verlohren S. Soluble fms‐like tyrosine kinase‐1 to placental growth factor ratio: ruling out pre‐eclampsia for up to 4 weeks and value of retesting. Ultrasound Obstet Gynecol 2019; 53: 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perry H, Binder J, Kalafat E, Jones S, Thilaganathan B, Khalil A. Angiogenic Marker Prognostic Models in Pregnant Women With Hypertension. Hypertension 2020; 75: 755–761. [DOI] [PubMed] [Google Scholar]
- 26. Koopmans CM, Bijlenga D, Groen H, Vijgen SM, Aarnoudse JG, Bekedam DJ, van den Berg PP, de Boer K, Burggraaff JM, Bloemenkamp KW, Drogtrop AP, Franx A, de Groot CJ, Huisjes AJ, Kwee A, van Loon AJ, Lub A, Papatsonis DN, van der Post JA, Roumen FJ, Scheepers HC, Willekes C, Mol BW, van Pampus MG; HYPITAT study group . Induction of labour versus expectant monitoring for gestational hypertension or mild pre‐eclampsia after 36 weeks' gestation (HYPITAT): a multicentre, open‐label randomised controlled trial. Lancet 2009; 374: 979–988. [DOI] [PubMed] [Google Scholar]
- 27. Cairns AE, Pealing L, Duffy JMN, Roberts N, Tucker KL, Leeson P, MacKillop LH, McManus RJ. Postpartum management of hypertensive disorders of pregnancy: a systematic review. BMJ Open 2017; 7: e018696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cairns AE, Tucker KL, Leeson P, Mackillop LH, Santos M, Velardo C, Salvi D, Mort S, Mollison J, Tarassenko L, McManus RJ; Investigators SNAP‐HT. Self‐Management of Postnatal Hypertension: The SNAP‐HT Trial. Hypertension 2018; 72: 425–432. [DOI] [PubMed] [Google Scholar]
- 29. Drugs and Lactation Database (LactMed) [Internet]. National Library of Medicine : Bethesda, MD, USA, 2006. https://www.ncbi.nlm.nih.gov/books/NBK501922/?term=lactmed [Google Scholar]
- 30. Lappin JM, Darke S, Duflou J, Kaye S, Farrell M. Fatal Stroke in Pregnancy and the Puerperium. Stroke 2018; 49: 3050–3053. [DOI] [PubMed] [Google Scholar]
- 31. Brett AS, Rind DM. ACE inhibitors and ARBs during the COVID‐19 pandemic. 2020. https://www.jwatch.org/na51345/2020/04/09/ace‐inhibitors‐and‐arbs‐during‐covid‐19‐pandemic [DOI] [PMC free article] [PubMed]
- 32. Jarcho JA, Ingelfinger JR, Hamel MB, D'Agostino RB, Sr. , Harrington DP. Inhibitors of the Renin‐Angiotensin‐Aldosterone System and Covid‐19. N Engl J Med 2020. DOI: 10.1056/NEJMe2012924 [DOI] [PMC free article] [PubMed] [Google Scholar]


