Summary
SARS‐CoV‐2 infection can cause severe pneumonia (COVID‐19). There is evidence that patients with comorbidities are at higher risk of a severe disease course. The role of immunosuppression in the disease course is not clear. In the present report, we first describe two cases of persisting SARS‐CoV‐2 viraemia with fatal outcome in patients after rituximab therapy.
Keywords: SARS‐CoV‐2, COVID‐19, rituximab, viraemia, pneumonia
Coronavirus disease 2019 (COVID‐19) is an emerging novel infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). The virus was first described in Wuhan, China and meanwhile is spreading worldwide. 1 Many studies all over the world suggest that older persons and patients with chronic disease (especially with chronic lung disease) are at higher risk of developing SARS‐CoV‐2‐related pneumonia (COVID‐19) and respiratory failure. 2 If immunosuppressive treatment is a further risk factor or even has protective capabilities has not been elucidated to date. Historical data has demonstrated higher risk for immunocompromised patients after different viral infections, e.g. influenza infection. According to mortality data on Middle East respiratory syndrome (MERS)‐CoV, SARS‐CoV and SARS‐CoV‐2 infections, there is evidence that an immunosuppressive therapy alone may not determine a worse prognosis. 3 Immunocompromised patients after organ transplantation or with chronic inflammatory disease seem to be not extraordinarily affected by a severe disease course after infection with coronaviruses (SARS, MERS and SARS‐CoV‐2). 4 Standard medications after organ transplantation, such as calcineurin inhibitors, interact primarily selectively on T lymphocytes and suppress their function, while B‐cell activity largely remains. Mainly patients with haematological malignancies are treated with rituximab, an anti‐CD20 antibody. Rituximab leads to complete B‐cell depletion and therefore to severe combined immunosuppression and is possibly associated with severe disease course, contrary to existing data of other immunosuppressive drugs. Here we present two cases of SARS‐CoV‐2‐infected patients after rituximab therapy with fatal outcome.
Patients and methods
Two patients with SARS‐CoV‐2 infection and history of rituximab therapy were identified. The patients' charts were screened for patient history and actual clinical data and laboratory and radiological findings. SARS‐CoV‐2 viraemia was detected by semi‐quantitative real‐time polymerase chain reaction (PCR) in peripheral blood in both the plasma and cellular fraction. Ethylenediamine tetra‐acetic acid (EDTA) plasma samples were processed using an integrated automated pipetting system for nucleic acid extraction and reverse transcriptase (RT)‐PCR assay setup (Qiagen Symphony, DSP Virus/Pathogen Midi Kit; Qiagen AG, Hilden, Germany). Single‐step RT‐PCR primers and probes specific for SARS‐CoV‐2 E‐gene, RNA‐dependent RNA polymerase (RdRP)‐gene, RNA positive controls and RNA extraction control, respectively, were purchased from TIB molbiol GmbH, Berlin, Germany (LightMix Modular SARS and Wuhan CoV E‐gene, Wuhan CoV RdRP gene, equine arteritis virus [EAV] RNA extraction control). 5 RT, taq polymerase and nucleotides were obtained from ThermoFisher (Superscript III Platinum One‐Step qRT‐PCR Kit); ThermoFisher, Schwerte, Germany). Thermal cycling and fluorescence detection was performed on a LightCycler 480 II instrument (Roche AG, Mannheim, Germany). To calibrate the outcome of E‐gene specific RT‐PCR, the E‐gene target sequence was amplified from overlapping oligonucleotides (E‐gene forward 5′‐ACA GGT ACG TTA ATA GTT AAT AGC GTC ATT CTT TTT CTT GCT TTC GTG GTA TTC TTG CTA GTT ACA‐3′, E‐gene backward 5′‐ATA TTG CAG CAG TAC GCA CAC AAT CGA AGC GCA GTA AGG ATG GCT AGT GTA ACT AGC AAG AAT ACC A‐3′) and the 113 bp PCR product was inserted into the vector pCR‐Topo‐2.1 (ThermoFisher). Linearised plasmid DNA was added to SARS‐CoV‐2 negative human plasma pool at a final concentration of 106 to 102 copies/ml, extracted as described above and a standard curve was calculated.
Results
Both patients were male, aged 65 and 66 years and of normal weight. Patient 1 had cerebral relapse of diffuse large B‐cell lymphoma and arterial hypertension, and was treated with R‐DeVIC (rituximab, dexamethason, etoposide, ifosfamide, carboplatin) for several months. The last therapy cycle was administered 2 weeks before infection with SARS‐CoV‐2. Patient 2 had mantle cell lymphoma and chronic renal insufficiency. He was in complete remission and received maintenance therapy with rituximab (last cycle was administered 2 weeks before infection) and ibrutinib daily. B cells were completely depleted and immunoglobulin G was decreased in both patients. Admission to our hospital was at 1 and 4 days after development of fever respectively. SARS‐CoV‐2 RNA was detected in pharyngeal swabs. Neither of them had hypoxia on the day of admission. High fever persisted in both patients for the next 14–21 days. Besides SARS‐CoV‐2 infection there were no alternative foci present. Patient 1 developed hypoxia on day 4, patient 2 on day 9. Both patients had chest X‐rays and thoracic computed tomography (CT) with detection of bilateral pulmonary peripheral ground glass infiltrates (Fig 1). Inflammatory markers (C‐reactive protein, interleukin 6 [IL‐6] and ferritin) were elevated on the day of admission and further increased significantly over time (Fig 2). Because of increasing procalcitonin levels both patients received anti‐infective therapy with piperacillin/tazobactam. Despite all therapy, the condition of both cases deteriorated. Chest X‐rays showed progredient bilateral pulmonary infiltration. Because of progressive respiratory failure both patients needed invasive ventilation after intubation on day 14. Because of persistent high temperature we analysed the plasma and cellular fraction of peripheral blood for SARS‐CoV‐2 viraemia. We detected significant viraemia with relatively low cycle threshold (Ct)‐values in the plasma and cellular fraction by PCR in both cases. To further quantify viraemia, we established a standardised PCR assay by using a plasmid standard as mentioned above. This assay provided evidence of a massive viraemia in both patients at different time points. The virus levels tended to further increase during the disease course and there was no indication of virus clearance at all. Patient 1 died at 22 days, patient 2 at 26 days after admission due to respiratory failure. Viraemia peaked shortly before death in both patients; furthermore there was no sign of viral clearance at 22 and 30 days after development of first symptoms or rather 22/26 days after first detection of SARS‐CoV‐2 RNA in pharyngeal swabs (Fig 2).
Fig 1.
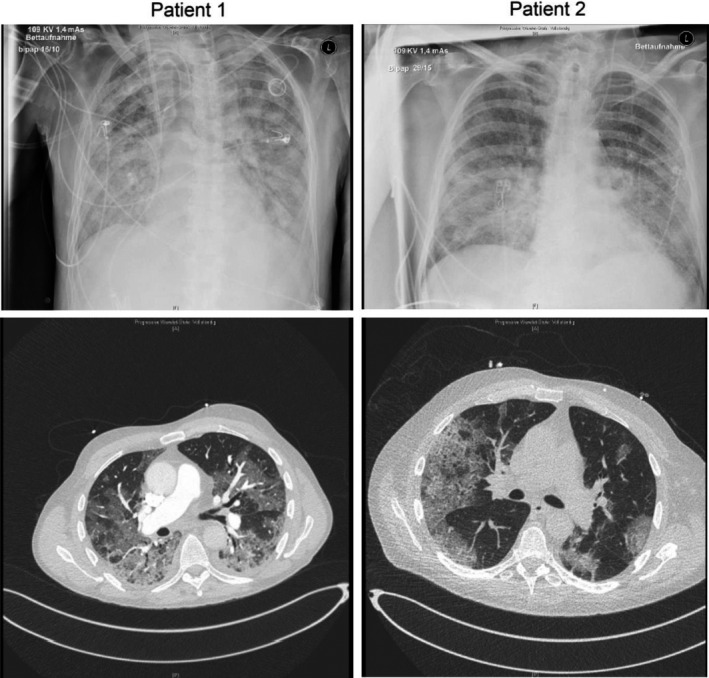
Chest X‐ray and thoracic CT imaging. Radiographic imaging showed bipulmonal, peripheral ground glass infiltration in both patients, a frequent finding in COVID‐19 pneumonia.
Fig 2.

Development of pro‐inflammatory markers during disease course. C‐reactive protein (standard value <0·5 mg/l), IL‐6 (standard value <6 pg/ml) and ferritin (standard value 30–400 µg/l) increased over time. Vertical lines indicate the date of intubation. Detection of SARS‐CoV‐2 viraemia by real‐time PCR. Viral load increased over time in both patients until death. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
We present two immunocompromised patients with severe COVID‐19 pneumonia with persisting viraemia until death without any sign of viral clearance. Both patients developed a massive cytokine storm during their disease course and died of progressive respiratory failure. Viraemia is not typical in viral respiratory diseases. Usually diagnosis of pulmonary viral infection results from specimens from the oropharyngeal and respiratory tract. Therefore, the role of viraemia in this strong immune reaction in these two cases remains speculative. There is evidence that viraemia itself and the duration of the viraemic period is correlated with increased morbidity. 6 Many patients with COVID‐19 develop a massive cytokine storm during severe disease course. 7 This immune reaction is possibly related to fatal outcome, as the host's uncontrolled immune reaction after infection with zoonotic viruses seems to be a main driver of tissue damage. 8 Therefore, immunosuppressive treatment is possibly effective by suppressing pro‐inflammatory host reaction to SARS‐CoV‐2. Some studies showed therapeutic effects of immunosuppressive treatment in these patients, for example anti‐IL‐6 antibody treatment possibly improves disease course in COVID‐19 by preventing lung tissue damage. 9 Available data of pandemic SARS, MERS and SARS‐CoV‐2 infections indicate that immunocompromised patients are not inevitably at higher risk of a severe disease course. 10 Reported cases often show successful recovery of COVID‐19 pneumonia in renal and liver transplant recipients. Moreover some immunosuppressive drugs such as FK506 show anti‐viral effects on coronaviruses. 11
On the other hand some studies indicate disadvantages of immunosuppressive therapy and indicate a delayed viral clearance in immunosuppressed conditions and therefore a severe disease course in renal transplant recipients among others. 12
Rituximab, an anti‐CD20 antibody, is an effective treatment option in many haematological malignancies, especially different B‐cell lymphomas. It leads to complete eradication of peripheral B lymphocytes and therefore suppresses B‐cell function such as immunoglobulin production. After rituximab application patients develop a severe combined cellular and humoral immunodeficiency and therefore many viral infections cause severe diseases in these patients (e.g. John Cunningham virus, hepatitis B virus and cytomegalovirus). Besides rituximab, ibrutinib therapy too can increase the risk of viral opportunistic infections. 13 Whether or not rituximab therapy has an impact on disease course and outcome in SARS‐CoV‐2 infected patients is not clear. To our knowledge there is no report available about SARS‐CoV‐2 infection in patients after rituximab therapy. There are only two published cases of patients with X‐linked agammaglobulinaemia, aged 34 and 26 years, without peripheral B‐cell function who survived SARS‐CoV‐2 infection after development of COVID‐19 pneumonia. Soresina et al. 14 concluded that B‐cell function might be dispensable in the immune response against SARS‐CoV‐2 and that remaining T‐cell function might be sufficient to overcome infection. Here we describe two fatal cases with persistent and over time increasing viraemia without any sign of viral clearance. Both patients were older and had haematological malignancies. Besides rituximab Patient 2 was treated with ibrutinib, a Bruton tyrosine kinase inhibitor, which targets B lymphocytes and inhibits the cell cycle. Patient 1 received ifosfamid, carboplatin and etoposide, each cell cycle inhibitors and besides B cell also effecting T‐cell immunity.
Conclusion
Taken together, both patients had rituximab therapy in common and were additionally immunocompromised because of further therapies. These two cases provide evidence that B‐cell function might be one important mechanism in resolving SARS‐CoV‐2 viraemia. Those patients with depleted B‐cell function and further risk factors such as additional oncological therapies or older age might be at high risk of a fatal disease course after SARS‐CoV‐2 infection.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors.
Competing interests
The authors have no competing interests.
Acknowledgements
Radiological images were provided by courtesy of the Institute of Clinical Radiology, University Hospital Muenster. Phil‐Robin Tepasse, Richard Vollenberg and Hartmut Schmidt designed and wrote the paper. Mathias Lutz and Rainer Wiewrodt revised the haematological part of the paper. Jan Sackarnd, Martin Keller and Christian Wilms revised the clinical part of the paper. Wali Hafezi and Joachim Kuehn conducted the laboratory work.
References
- 1. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses . The species severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5:536–44. DOI: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in Covid‐19. N Engl J Med. 2020. [Online ahead of print]. DOI: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3. Hui DS, Azhar EI, Kim YJ, Memish ZA, Oh MD, Zumla A. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. 2018;18:e217–7. DOI: 10.1016/S1473-3099(18)30127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Minotti C, Tirelli F, Barbieri E, Giaquinto C, Dona D. How is immunosuppressive status affecting children and adults in SARS‐CoV‐2 infection? A systematic review. J Infect. 2020. [Epub ahead of print]. DOI: 10.1016/j.jinf.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25:2000045. DOI: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, et al. Viral load dynamics and disease severity in patients infected with SARS‐CoV‐2 in Zhejiang province, China, January‐March 2020: retrospective cohort study. BMJ. 2020;369:m1443. DOI: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jose RJ, Manuel A. COVID‐19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020. [Online ahead of print]. DOI: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peiris JS, Hui KP, Yen HL. Host response to influenza virus: protection versus immunopathology. Curr Opin Immunol. 2010;22:475–81. DOI: 10.1016/j.coi.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alattar R, Ibrahim TB, Shaar SH, Abdalla, S , Shukri, K , Daghfal, JN , et al. Tocilizumab for the Treatment of Severe COVID‐19. J Med Virol. 2020. [Online ahead of print]. DOI: 10.1002/jmv.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. DOI: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carbajo‐Lozoya J, Muller MA, Kallies S, Thiel V, Drosten C, von Brunn A. Replication of human coronaviruses SARS‐CoV, HCoV‐NL63 and HCoV‐229E is inhibited by the drug FK506. Virus Res. 2012;165:112–7. DOI: 10.1016/j.virusres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fishman JA, Grossi PA. Novel Coronavirus‐19 (COVID‐19) in the immunocompromised transplant recipient: #Flatteningthecurve. Am J Transplant. 2020. [online ahead of print]. DOI: 10.1111/ajt.15890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lutz M, Schulze AB, Rebber E, Wiebe S, Zoubi T, Grauer OM, et al. Progressive multifocal leukoencephalopathy after ibrutinib therapy for chronic lymphocytic leukemia. Cancer Res Treat. 2017;49:548–52. DOI: 10.4143/crt.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Soresina A, Moratto D, Chiarini M, Paolillo C, Baresi G, Focà E, et al. Two X‐linked agammaglobulinemia patients develop pneumonia as COVID‐19 manifestation but recover. Pediatr Allergy Immunol. 2020. [Online ahead of print]. DOI: 10.1111/pai.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]


