Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is spreading worldwide and is a now a pandemic virus that has infected almost 5 million individuals, causing 300 000 deaths as of mid‐May 2020. Because SARS‐CoV‐2 is a new virus in humans there are currently no vaccines, monoclonal antibodies or even effective drugs available. Human convalescent plasma transfusion is an option for either prophylactic or therapeutic treatment of patients with coronavirus disease 2019 (COVID‐19), but its administration to patients who are affected by severe pulmonary disease is associated with increased risk of transfusion‐related acute lung injury. Antibody‐dependent enhancement of infection is an additional risk that has already been reported to occur in several viral diseases and involves an enhancement of disease in the presence of certain antibodies. Finally, to date there are relatively few people who have recovered from COVID‐19 and who can donate immunoglobulin‐containing plasma. 1
Cluster of differentiation 8 (CD8) cytotoxic T lymphocytes are essential for immune protection against viruses and several studies have shown that high levels of virus‐specific CD8 T cells are associated with decrease in virus load and favourable outcome. Interestingly, using human leucocyte antigen (HLA) class I predicted peptide ‘megapools’, SARS‐CoV‐2‐specific CD8 T cells were identified in the circulation of approximately 70% of COVID‐19 convalescent patients. 2
In a correspondence letter to the British Journal of Haematology published on‐line on 5 May 2020, Hanley et al.3 propose to utilise SARS‐CoV‐2‐specific and HLA‐matched cytotoxic T cells prepared from convalescent COVID‐19 patients for ‘the need of the hour’ therapy of patients with COVID‐19.
Adoptive transfer of donor‐derived and virus‐specific CD8 T cells has shown efficacy for the treatment of several different viral infections in immunocompromised individuals. 4
However, there are problems with adoptive T‐cell therapy. First, due to genetic (HLA class I) restriction, it is not possible to utilise allogeneic T cells from unrelated individuals and only donor‐derived T cells can be used. Second, donor T cells are expanded in vitro by prolonged stimulation with specific virus antigen to achieve sufficient numbers of effector T cells to be infused. This is a major obstacle in situations where CD8 T cells are exhausted and fail to proliferate and expand, as occurs in patients with COVID‐19, where peripheral blood CD8 T cells counts are profoundly reduced and CD8 T cells are functionally exhausted. 5 Third, an important side‐effect of T‐cell therapy is the so‐called ‘cytokine storm’, a massive inflammatory response that contributes to acute respiratory distress and multiple organ failure in patients with COVID‐19. 6
The aim of this letter is to discuss the potential for adoptive therapy of patients with severe COVID‐19 with HLA‐E‐restricted unconventional CD8 T cells.
HLA‐E is a non‐classical HLA class Ib molecule expressed on all nucleated cells. It is considered as non‐classical because it is basically monomorphic. There are in fact only two alleles, HLA‐E*01:01 and *01:03, which differ by a single amino acid substitution located outside the peptide binding groove, thus suggesting that the two alleles have an identical epitope‐binding repertoire. 7 HLA‐E typically binds peptides derived from the signal sequence of other HLA class Ia alleles, and these complexes in turn bind to natural killer (NK) group 2 member A (NKG2A)/CD94 and inhibit NK cell functions. However, most recent studies have demonstrated that HLA‐E binds peptides derived from different microbes including cytomegalovirus (CMV), human immunodeficiency virus (HIV), M. tuberculosis, etc., and presents them to CD8 T cells. 7 The crystal structure of HLA‐E bound to CMV, HIV and M. tuberculosis‐derived peptides has been very recently resolved 8 and may be then exploited to design peptides capable of activating HLA‐E‐restricted CD8 T cells.
Human HLA‐E‐restricted CD8 T cells possess cytolytic and microbicidal activities. 9 , 10 However, these CD8 T cells in addition to the typical Th1 cytokine interferon γ, also produce interleukin 4 (IL‐4), IL‐5, IL‐13, and to a variable extent IL‐10 and transforming growth factor β (TGF‐β). 9
Recent studies in non‐human primates have shown that vaccination with simian immunodeficiency virus‐derived gag protein expressed in rhesus CMV elicits a potent HLA‐E‐restricted CD8 T cell response, which causes eradication of a subsequent simian immunodeficiency virus challenge. 11
Most interestingly, in an experimental mouse model of tuberculosis, Qa‐1 (the murine orthologue of HLA‐E)‐restricted CD8 T cells mediate a protective immune response against M. tuberculosis by both killing infected cells and the intracellular bacilli, and also limiting the extent of tissue damage. 12
More than 1000 clinical trials for the treatment of patients with COVID‐19 are registered as of mid‐May 2020, with only one study from Singapore investigating the efficacy of adoptive cell therapy with SARS‐CoV‐2‐specific T cells in patients with severe COVID‐19 (https://clinicaltrials.gov/ct2/show/NCT04351659).
Here, we would like to propose that the utilisation of HLA‐E‐restricted CD8 T cells may offer several advantages to improve T‐cell immunotherapy in patients with COVID‐19, such as the simultaneous capacity to kill infected cells and inhibit intracellular infections, and to reduce the extent of the inflammatory response and limit collateral tissue damage, which is an important component in the pathogenesis of COVID‐19 (Fig 1). Another important aspect is represented by the monomorphic model of antigen recognition of HLA‐E‐restricted CD8 T cells, which permits their utilisation for the global heterogenic population. Moreover, HLA‐E‐restricted CD8 T cells are unlikely to mount alloreactive responses and cause graft‐versus‐host disease phenomena, making these cells more amenable to ‘off‐the‐shelf’ conventional T‐cell therapies. In principle, HLA‐E‐restricted and SARS‐CoV‐2‐specific CD8 T cells could be rapidly and cost‐effectively prepared in large numbers from COVID‐19 convalescent allogeneic donors, banked and used immediately upon request for patients with severe COVID‐19.
Conflict of interest
The authors declare no conflict of interest.
Fig 1.
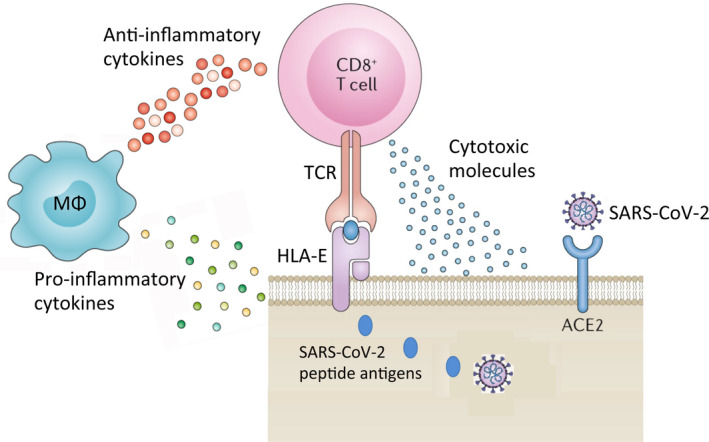
HLA‐E molecule in immunity to SARS‐CoV‐2 infection. HLA‐E molecule, expressed on the surface of all nucleated cells, can present SARS‐CoV‐2‐derived peptide antigens to CD8 T cells, which recognise these HLA‐E/peptide complexes on the surface of virus‐infected cells through their T‐cell receptor (TCR). Upon specific recognition, activated CD8 T cells de‐granulate and release cytotoxic molecules such as perforin, granzyme and granulysin, which cause killing of infected target cells and of the intracellular virus. In addition, HLA‐E‐restricted CD8 T cells also produce anti‐inflammatory cytokines (IL‐4, IL‐10, TGF‐β), which down‐modulate the production of pro‐inflammatory cytokines by macrophages and other inflammatory cells, and thus inhibit ‘cytokine storm’ and reduce the extent of tissue damage.
References
- 1. Casadevall A, Pirofski LA. The convalescent sera option for containing COVID‐19. 2020. J Clin Invest. 2020;130:1545–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, et al. Targets of T cell responses to SARS‐CoV‐2 coronavirus in humans with COVID‐19 disease and unexposed individuals. Cell. 2020. [Epub ahead of print]. 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanley B, Roufosse CA, Osborn M, Naresh KN. Convalescent donor SARS‐COV‐2‐specific cytotoxic T lymphocyte infusion as a possible treatment option for COVID‐19 patients with severe disease has not received enough attention till date. Br J Haematol. 2020. [Epub ahead of print]. DOI: 10.1111/bjh.16780. [DOI] [PubMed] [Google Scholar]
- 4. Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, et al. Monoculture‐derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–6. [DOI] [PubMed] [Google Scholar]
- 5. Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cell Mol Immunol. 2020;17:533–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGonagle D, Sharif K, O'Regan A, Bridgewood C. The role of cytokines including interleukin‐6 in COVID‐19 induced pneumonia and macrophage activation syndrome‐like disease. Autoimmun Rev. 2020;19:102537. 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petrie EJ, Clements CS, Lin J, Sullivan LC, Johnson D, Huyton T, et al. CD94‐NKG2A recognition of human leukocyte antigen (HLA)‐E bound to an HLA class I leader sequence. J Exp Med. 2008;205:725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walters LC, Harlos K, Brackenridge S, Rozbesky D, Barrett JR, Jain V, et al. Pathogen‐derived HLA‐E bound epitopes reveal broad primary anchor pocket tolerability and conformationally malleable peptide binding. Nat Commun. 2018;9:3137. 10.1038/s41467‐018‐05459‐z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prezzemolo T, van Meijgaarden KE, Franken KL, Caccamo N, Dieli F, Ottenhoff TH, et al. Detailed characterization of human Mycobacterium tuberculosis specific HLA‐E restricted CD8+ T cells. Eur J Immunol. 2018;48:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. La Manna MP, Orlando V, Prezzemolo T, Di Carlo P, Cascio A, Delogu G, et al. HLA‐E‐restricted CD8+ T lymphocytes efficiently control Mycobacterium tuberculosis and HIV‐1 coinfection. Am J Respir Cell Mol Biol. 2020;62:430–9. [DOI] [PubMed] [Google Scholar]
- 11. Hansen SG, Wu HL, Burwitz BJ, Hughes CM, Hammond KB, Ventura AB, et al. Broadly targeted CD81 T cell responses restricted by major histocompatibility complex E. Science. 2016;351:714–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bian Y, Shang S, Siddiqui S, Zhao J, Joosten SA, Ottenhoff TH, et al. MHC Ib molecule Qa‐1 presents Mycobacterium tuberculosis peptide antigens to CD8+ T cells and contributes to protection against infection. PLoS Pathog. 2017;13:e1006384. 10.1371/journal.ppat.1006384. [DOI] [PMC free article] [PubMed] [Google Scholar]


