Coagulopathy and elevated D‐dimer levels were recognized as prognostic factors early in Wuhan, China, as accompanying more severe COVID‐19 patient cases. We sought to determine the accuracy of normal versus elevated D‐dimer blood levels at presentation, on day 1, and on day 3 for predicting 28‐day survival in a large cohort of consecutive PCR‐proven COVID‐19 patients to help with patient triage, reassurance, and follow‐up management.
This is an observational study of a cohort of consecutive patients presenting to Affiliated Hospital of Jianghan University, Wuhan, from January 10 through February 28, 2020. Before data collection, we obtained patients' consent and ethical approval from the Medical Ethics Committee of Jianghan University Affiliated Hospital and China‐Japan Friendship Hospital (WHSHIRB‐K‐2020015). D‐dimer levels (Nanopia immunoturbidimetric assay, Sekisui Medical; abnormal > 1.0 mg/L) were measured at day 1 presentation in nearly all patients and again on day 3 and afterward in many. Patients without day 1 D‐dimer levels were excluded from the analysis. Clinicians delivered care appropriate to the level of illness, including intensive care, assisted ventilation, and circulatory and other support such as hemodialysis. The primary outcome was 28‐day survival.
Of 761 PCR‐confirmed COVID‐19 patients admitted, 749 had presenting day 1 D‐dimer levels available. Twenty‐eight‐day mortality was 78 in the 749‐patient cohort, 10.4% (95% CI = 8.3% to 12.8%). D‐dimer levels on day 1 were normal in 586 of 671 survivors but elevated in 36 of 78 nonsurvivors, for a survival sensitivity of 87% (95% CI = 86% to 89%), positive predictive value of 93% (95% CI = 92% to 95%), specificity of 46% (95% CI = 36% to 57%), and negative predictive value of 30% (95% CI = 23 to 36%). Figure 1 shows 28‐day survival for normal versus elevated D‐dimer values in this population.
Figure 1.
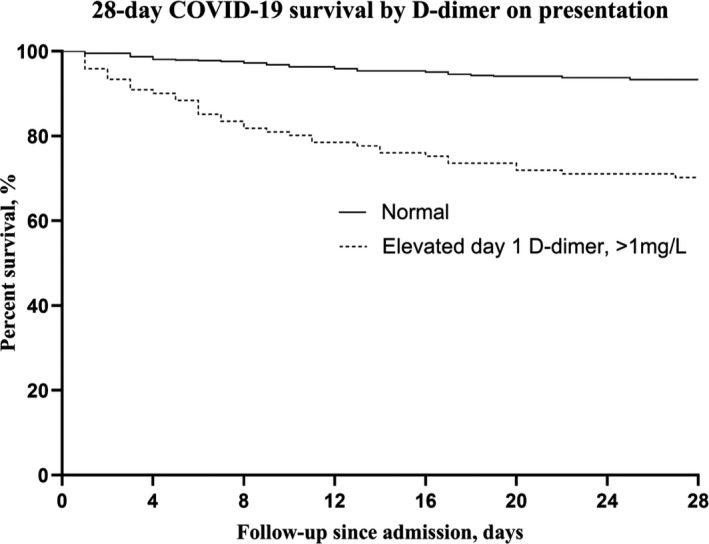
28‐day survival of PCR‐positive COVID‐19 patients by presenting D‐dimer result.
Day 3 D‐dimer values, available for 598 cohort patients (80%), were normal in 408 of the 28‐day survivors and 10 who died. They were elevated in 130 of the 28‐day survivors and 50 who died. Thus, a normal value was strongly associated with survival: sensitivity 76% (95% CI = 75% to 77%), positive predictive value 98% (95% CI = 96% to 99%), specificity 83% (95% CI = 72% to 91%), and negative predictive value 28% (95% CI = 24% to 30%). Survival odds with a normal day 1 D‐dimer were 5.9 (95% CI = 3.6% to 9.7%); for a normal day 3 D‐dimer, survival odds were 15.6 (95% CI = 7.7% to 31.8%).
Association of coagulopathy with COVID‐19 is now widely reported. 1 , 2 In the United States and elsewhere, rapid results of PCR coronavirus testing are not widely available and a positive swab result does not inform prognosis. In this cohort of 100% COVID‐19 patients, a day 1 and particularly a day 3 normal D‐dimer had high precision for predicting 28‐day survival. Similar to how D‐dimer is used to assist diagnosis of deep vein thrombosis and pulmonary embolism, a normal result supports a decision to triage a patient to watchful waiting as opposed to hospital admission. For symptomatic COVID‐19 suspects awaiting swab results but ill enough to require hospitalization, elevated D‐dimer levels could be presumptively diagnosed as COVID‐19 and triaged as higher risk. Those with normal D‐dimer level and without another reason for hospitalization could be managed expectantly as outpatients with reassurance and appointment for follow‐up day 3 D‐dimer level, while other etiologies were also considered. Evaluation of subjective outpatient deterioration could be assisted by an on‐site, real‐time, commercially available point‐of‐care D‐dimer determination. While qualitative bedside tests may be somewhat less accurate than quantitative ones, 3 real‐time D‐dimer testing can even be performed in the field and has been reported to be helpful in expediting emergency department evaluation of pulmonary embolism. 4 , 5 We speculate that field D‐dimer testing may be similarly useful to make prehospital decisions about transport of patients with suspected COVID‐19.
Our results differ from previous reports. 6 , 7 Because D‐dimer assays have different upper limits of normal and a multiple of some level from one assay is not necessarily proportional to that of another, 6 we used the upper limit of normal as the cutoff to allow generalization as universally as possible. We assess D‐dimer in the largest number of COVID‐19 PCR‐confirmed consecutive cohort patients yet prospectively reported rather than selecting patients. We include day 3 data, available for 80% of our cohort. These advantages allow tighter precision of the survival positive predictive value and other accuracy values, reduce possible selection bias, and allow insight for day 3 follow‐up with interpretation of those D‐dimer level results.
A normal D‐dimer on presentation is highly predictive of survival, and a day 3 normal value even more so. This readily available information can help guide physicians with triage and follow‐up, reassure patients and help to bring confidence to identifying those patients warrant closest surveillance.
Academic Emergency Medicine 2020;27:612–613.
†Drs. Li, Hu, Zhang, and Qin contributed equally.
Drs Zhai and Davidson take responsibility for the content and accuracy of the publication.
Funded by the National Key Research and Development Program of China (2016FYC0901104), the CAMS Innovation Fund for Medical Sciences (CIFMS, 2018‐12M‐1‐003), and the National Natural Science Foundation of China (81570049 and 81970058).
The authors have no potential conflicts of interest to disclose.
Author contributions: CL, ZGZ, BD, and CW had the idea for and designed the study and had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; CL, ZZ, WQ, BD, and ZGZ drafted the paper; CL, ZZ, BH, ZGZ, CW, and BD performed the analysis; all authors critically reviewed for important intellectual content and gave final approval for the version to be published; and CL, BH, WQ, and ZYZ collected the data.
Contributor Information
Zhenguo Zhai, Email: zhaizhenguo2011@126.com.
Bruce L. Davidson, Email: brucedavidson@pobox.com.
REFERENCES
- 1. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med 2020;382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID‐19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow‐up. J Am Coll Cardiol 2020;75:2950–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Geersing GJ, Janssen KJ, Oudega R, et al. Excluding venous thromboembolism using point of care D‐dimer tests in outpatients: a diagnostic meta‐analysis. BMJ 2009;339:b2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee‐Lewandrowski E, Nichols J, Van Cott E, et al. Implementation of a rapid whole blood D‐dimer test in the emergency department of an urban academic medical center: impact on ED length of stay and ancillary test utilization. Am J Clin Pathol 2009;132:326–31. [DOI] [PubMed] [Google Scholar]
- 5. Lucassen WA, Erkens PM, Geersing GJ, et al. Qualitative point‐of‐care D‐dimer testing compared with quantitative D‐dimer testing in excluding pulmonary embolism in primary care. J Thromb Haemost 2015;13:1004–9. [DOI] [PubMed] [Google Scholar]
- 6. Zhang L, Yan X, Fan Q, et al. D‐dimer levels on admission to predict in‐hospital mortality in patients with COVID‐19. J Thromb Haemost 2020;18:1324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou F, Yu T, Ronghui D, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]


