In December 2019, a novel coronavirus pneumonia, coronavirus disease 2019 (COVID‐19), caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) was reported in Wuhan, China. A hyperinflammatory immune response, or cytokine release syndrome (CRS) is globally observed in critically unwell patients with SARS‐CoV‐2. Severe lymphopenia, hyperactivated pro‐inflammatory T cells 1 and decreased regulatory T cells 2 are seen in these critically ill patients, suggesting dysregulated immune responses. The first report of pathological characteristics demonstrates an increased concentration of pro‐inflammatory cytokines 1 including tumour necrosis factor alpha (TNF‐α) and interleukin‐6 (IL‐6). Patients with CRS can rapidly progress to acute respiratory distress syndrome (ARDS), multi‐organ dysfunction and death. CRS is not unique to COVID‐19 and is a well‐recognised complication of chimaeric antigen receptor T cell (CAR‐T cell) therapy used for the management of haematological malignancies. CRS is an exaggerated response to an inflammatory stimulus with hyperactivation of immune effector cells and significant upregulation of interleukin (IL)‐6 without comparable production of other inflammatory cytokines. 3 IL‐6 is implicated as an important mediator of COVID‐19‐associated CRS. The onset of COVID‐19‐induced CRS is typically a clinical deterioration 7–14 days from symptom onset. Features include fever, hypotension, capillary leak, hypoxia, end organ dysfunction and evidence of hyperinflammation including hyperferritinaemia and an elevated C‐reactive protein (CRP). Early identification and management of COVID‐19‐induced CRS has the potential to minimise morbidity and mortality.
Tocilizumab, a humanised monoclonal antibody against soluble and membrane IL‐6 receptors (IL‐6R), blocks IL‐6 signalling and reduces inflammation. It is licenced in the management of rheumatoid arthritis and CAR‐T cell‐associated CRS. There have been published case series and reports of the successful treatment of COVID‐19‐induced CRS with tocilizumab. 4 , 5 , 6 There are limited high‐quality data on the outcomes of tocilizumab in this setting and this is being addressed in the RECOVERY trial. 7 We describe our experience of the successful treatment of a patient with chronic myeloid leukaemia (CML) and COVID‐19‐associated CRS with tocilizumab in a district general hospital setting.
A 52‐year‐old Indian man with poorly controlled CML, despite compliance with the tyrosine kinase inhibitor (TKI) imatinib, attended for outpatient review. In view of his poor haematological response, imatinib was discontinued and he was asked to start hydroxycarbamide cytoreduction pending results of tyrosine kinase domain mutation analysis. He reported fevers, which had started three days earlier. An infection screen was performed including testing for COVID‐19. He was prescribed oral antibiotics and advised to self‐isolate but presented to the emergency department 48 h later with ongoing fevers and breathlessness. The COVID‐19 antigen test confirmed infection with SARS‐CoV‐2. His admission chest radiograph demonstrated patchy consolidation bilaterally consistent with moderate radiological COVID‐19 disease. He was randomised to the liponavir and ritonavir anti‐viral therapy arm of the RECOVERY trial and commenced on broad‐spectrum, intravenous antibiotics. On day 10 of his illness he underwent a computed tomography pulmonary angiogram (CTPA) due to increasing oxygen requirements and an elevated D‐dimer. There was no pulmonary embolism but severe COVID pneumonia with ground‐glass shadows throughout the lungs, favouring peripheral and lower zones (Fig 1). He continued to deteriorate with persistent fevers and tachypnoea and on day 11 of symptoms required non‐invasive ventilation support with continuous positive airway pressure (CPAP). Biochemical evidence of hyperinflammation was present with an elevated ferritin and CRP. A local clinical guideline and pathway for the application of tocilizumab on compassionate grounds having been set up, an application for our patient was made and granted. On day 12 of symptom onset, he received 600 mg tocilizumab intravenously, in line with the RECOVERY trial dosing protocol. There was rapid cessation of his fevers and a significant reduction in his inflammatory markers within 24 h of tocilizumab therapy (Fig 2). A consistent improvement in his oxygen requirements was observed and he was weaned off CPAP on day 16 with discontinuation of supplementary oxygen therapy on day 19 of his illness.
Fig 1.
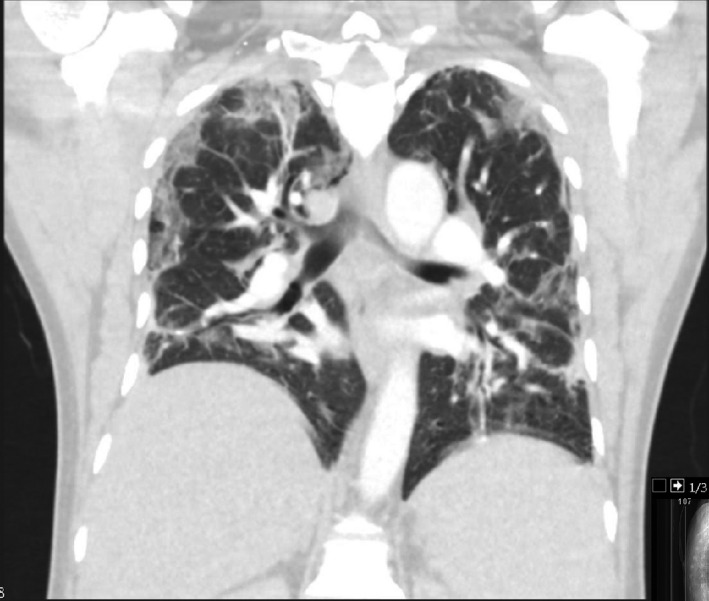
Coronal plane computed tomography pulmonary angiogram (CTPA) image taken on day 10 of COVID‐19 illness demonstrating bilateral patchy ground‐glass shadows throughout the lungs, more marked in the peripheral and lower zones, and minor bilateral basal consolidation.
Fig 2.
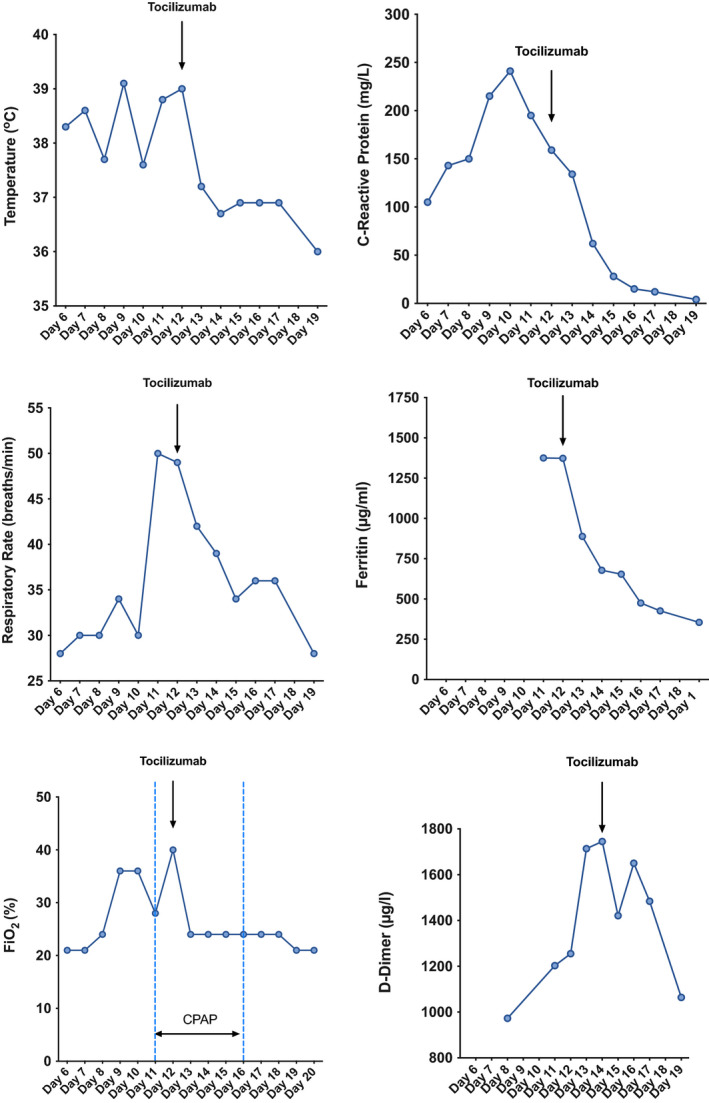
Clinical and biochemical observations in our patient with COVID‐19‐associated cytokine release syndrome treated with intravenous tocilizumab 600 mg on day 12 of symptom onset. Following tocilizumab therapy there was a rapid cessation of his fevers and improvement in his ferritin and C‐reactive protein (CRP), followed by a consistent improvement in his respiratory parameters. The ongoing elevation in D‐dimer is likely to represent the complex COVID‐19‐associated hypercoaguable state.
Critical care beds are a limited resource and patients with haematological disorders are considered to have poor outcomes following admission to intensive care units (ICU). Despite overall improved outcomes in haematology patients, 8 the presence of a cancer diagnosis frequently precludes admission to ICU. In a global pandemic, this disadvantage is only magnified. Comorbidity is associated with poorer outcomes in COVID‐19 infection 9 and the impact of ethnicity, particularly those of black and minority ethnic subgroups, on the prognosis of COVID‐19 is under review.
The national RECOVERY trial aims to identify therapies that may be beneficial for people hospitalised with confirmed COVID‐19. Tocilizumab randomisation is included in the trial design for patients with progressive COVID‐19 with features consistent with CRS. This arm of the trial was not available at our hospital but Roche (Basel, Switzerland), the manufacturer of tocilizumab, has a scheme to provide patients who are not eligible to clinical trials access to investigational medicines through an expanded access programme or compassionate use. Compassionate access to drugs such as tocilizumab can alter the course, prognosis and need for ICU admission in COVID‐19.
Tocilizumab is an immunosuppressive therapy and serious and sometimes fatal infections have been reported following its use. CRS is indistinguishable from sepsis and the presence of superadded bacterial infections in patients with COVID‐19‐associated CRS should be aggressively screened for and treated. CRP is typically used to confirm the presence of significant bacterial infection but its specificity for sepsis is low. Procalcitonin (PCT), a prohormone of calcitonin, is a more specific biomarker for bacterial infection and sepsis. The low serum PCT (0·98 μg/l) in our patient, made a diagnosis of sepsis unlikely. 10
We describe the successful treatment of COVID‐19 associated CRS with tocilizumab in a patient with an uncontrolled haematological malignancy. Equality in access to investigational therapies should be actively pursued, especially in disadvantaged patient cohorts, and can be achieved outside of tertiary referral centres and the clinical trials process. Therapies such as this have the potential to change the outcome of severe COVID‐19 infection and prevent ICU admission, particularly in patients believed to be unsuitable for critical care or where there is a great demand on critical care resources.
References
- 1. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tanaka T, Narazaki M, Kishimoto T. IL‐6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang X, Song K, Tong F, et al. First case of COVID‐19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4:1307–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID‐19: A single center experience. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaidos A, Katsarou A, Mustafa C, Milojkovic D, Karadimitris A. Interleukin 6‐blockade treatment for severe COVID‐19 in two patients with multiple myeloma. Br J Haematol. 2020. https://doi.org/10.111/bjh.16787 [DOI] [PubMed] [Google Scholar]
- 7. https://www.recoverytrial.net V4.0 2020‐01‐14 I, EudraCT 2020‐001113‐21.
- 8. Pene F, Soares M. Can we still refuse ICU admission of patients with hematological malignancies? Intensive Care Med. 2008;34:790–2. [DOI] [PubMed] [Google Scholar]
- 9. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoeboer SH, van der Geest PJ, Nieboer D, Groeneveld AB. The diagnostic accuracy of procalcitonin for bacteraemia: a systematic review and meta‐analysis. Clin Microbiol Infect. 2015;21:474–81. [DOI] [PubMed] [Google Scholar]


