Abstract
Coronavirus disease‐2019 (COVID‐19) is a global pandemic with high infectivity and pathogenicity, accounting for tens of thousands of deaths worldwide. Recent studies have found that the pathogen of COVID‐19, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), shares the same cell receptor angiotensin converting enzyme II (ACE2) as SARS‐CoV. The pathological investigation of COVID‐19 deaths showed that the lungs had characteristics of pulmonary fibrosis. However, how SARS‐CoV‐2 spreads from the lungs to other organs has not yet been determined. Here, we performed an unbiased evaluation of cell‐type‐specific expression of ACE2 in healthy and fibrotic lungs, as well as in normal and failed adult human hearts, using published single‐cell RNA‐seq data. We found that ACE2 expression in fibrotic lungs mainly locates in arterial vascular cells, which might provide a route for bloodstream spreading of SARS‐CoV‐2. Failed human hearts have a higher percentage of ACE2‐expressing cardiomyocytes, and SARS‐CoV‐2 might attack cardiomyocytes through the bloodstream in patients with heart failure. Moreover, ACE2 was highly expressed in cells infected by respiratory syncytial virus or Middle East respiratory syndrome coronavirus and in mice treated by lipopolysaccharide. Our findings indicate that patients with pulmonary fibrosis, heart failure, and virus infection have a higher risk and are more susceptible to SARS‐CoV‐2 infection. The SARS‐CoV‐2 might attack other organs by getting into the bloodstream. This study provides new insights into SARS‐CoV‐2 blood entry and heart injury and might propose a therapeutic strategy to prevent patients from developing severe complications.
Keywords: ACE2, COVID‐19, heart failure, pulmonary fibrosis, SARS‐CoV2
We found that angiotensin converting enzyme II (ACE2) expression in fibrotic lungs mainly locates in arterial vascular cells, and might provide a route for bloodstream spreading of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Failed human hearts have a higher percentage of ACE2‐expressing cardiomyocytes, and SARS‐CoV‐2 might attack cardiomyocytes through the bloodstream in patients with heart failure. Moreover, ACE2 was highly expressed in cells infected by RSV or Middle East respiratory syndrome coronavirus (MERS‐CoV) and in mice treated by lipopolysaccharide (LPS).
1. INTRODUCTION
Coronavirus disease‐2019 (COVID‐19) is a global pandemic with high infectivity and pathogenicity, accounting for tens of thousands of deaths worldwide. The pathogen named severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) showed 79.5% genome similarity to SARS‐CoV and 96% to a bat coronavirus RaTG13 (P. Zhou, Yang, et al., 2020). Angiotensin converting enzyme II (ACE2) can specifically bind to the S1 domain of SARS‐CoV S protein as a functional receptor (Li et al., 2003) and is also the cellular receptor of SARS‐CoV‐2 (Lan et al., 2020). ACE2 has been widely recognized as a negative regulatory enzyme of the renin‐angiotensin system (RAS) that converts Ang II into Ang1‐7/Ang1‐9 and has a protective role in cardiovascular function (Donoghue et al., 2000). Upon the spike protein of coronavirus binding to the cellular receptor ACE2, the type II transmembrane serine protease (TTSP) TMPRSS2 cleaves the SARS‐CoV‐2 spike protein to promote fusion of viral and cellular membranes (Hoffmann et al., 2020). FURIN also cleaves the SARS‐CoV‐2 spike protein (Walls et al., 2020). Both TMPRSS2 and FURIN play an essential role in viral entry (Hoffmann et al., 2020; Walls et al., 2020).
ACE2 was shown to be present in human lung alveolar epithelial cells (Hamming et al., 2004). Analysis of single‐cell RNA‐Seq (scRNA‐seq) data in normal human lung tissues demonstrated that ACE2 is mainly expressed in type II alveolar cells (AT2; Zhao et al., 2020). The pathological investigation of COVID‐19 fatal cases showed exudation and hemorrhage, epithelium injuries, infiltration of macrophages, and fibrosis in the lungs (C. Wang et al., 2020). Pulmonary fibrosis is not only due to SARS‐CoV‐2 directly attacking the lung as its target organ, the activation of the cytokine storm in COVID‐19 patients also contributes (Huang et al., 2020; Wan et al., 2020). A retrospective cohort study indicates that patients with chronic obstructive pulmonary disease (COPD) and bilateral pulmonary infiltration are associated with higher mortality (F. Zhou, Yu, et al., 2020). This may be due to the change of ACE2 gene expression profile in lung tissues in patients with lung diseases, and makes these patients more susceptible to SARS‐CoV‐2 infection, but the cell‐type specific expression change of ACE2 in healthy populations and patients with underlying lung diseases has not been reported.
Epidemiology analysis of COVID‐19 has identified cardiac injury as among the most severe organ function damages, with symptoms of arrhythmia and cardiac arrest (Huang et al., 2020). Elevated high sensitivity Troponin I (hs‐cTnI) or new ECG/echocardiographic abnormalities suggested that cardiac injury was present in 7.2% of patients and 22% of those who required ICU care (D. Wang, Hu, et al., 2020). Moreover, advanced age and pre‐existing cardiovascular diseases were high‐risk factors of infection and lethality (Wu & McGoogan, 2020). In a study involving 138 COVID‐19 patients, many had cardiovascular‐related comorbidities and/or complications, including severe hypertension (31.2%), cardiovascular disease (14.5%), and arrhythmia (16.7%; D. Wang, Hu, et al., 2020). However, how SARS‐CoV‐2 spreads from the lungs to other organs and the cause of the high mortality rate in COVID‐19 patients with pre‐existing cardiovascular diseases are still not fully understood.
To investigate the possible route of SARS‐CoV‐2 spreading from the lungs to other organs, we performed an unbiased evaluation of cell‐type specific expression of ACE2 in healthy and fibrotic lungs, as well as in normal and failed adult human hearts, using published single‐cell RNA‐seq data. We found that ACE2 is mainly expressed in arterial vascular cells in fibrotic lungs, and might be essential for SARS‐CoV‐2 to enter the bloodstream and to start its circulatory spreading. We observed higher enrichment of ACE2 expression in cardiomyocytes of failed adult human hearts, and higher gene expression of ACE2 in cells infected by the respiratory syncytial virus (RSV) or Middle East respiratory syndrome coronavirus (MERS‐CoV). Our findings indicate that patients with pulmonary fibrosis, heart failure, and virus infection have a higher risk and are more susceptible to SARS‐CoV‐2 infection.
2. MATERIALS AND METHODS
2.1. Cell filtering and cell‐type clustering analysis
The R package Seurat was used for cell filtration, clustering analysis, and uniform manifold approximation and projection (UMAP) as described in the vignettes (https://satijalab.org/seurat/vignettes.html). Cells expressing <200 or >3000 genes in normal lung samples, <200 or >4000 genes in lung fibrosis samples, <200 or >7000 genes in normal heart samples, and <200 or >5000 genes in heart failure samples were filtered out for exclusion of noncell or cell aggregates. Next, the data were log‐normalized and we identified highly variable genes using the FindVariableFeatures function. The ScaleData function were used for linear regression and principle component analysis was performed on the scaled data for linear dimensional reduction. Then, a graph‐based clustering approach (FindClusters function) was performed to cluster cells and UMAP was used to visualize clusters. Visualization of gene expression was shown with violin plot, feature plot was implemented by the VlnPlot, FeaturePlot function. The FindAllMarkers function (min.pct = 0.25, logfc. threshold = 0.25) was used to identify marker genes. Type II alveolar cells, endothelial cells, smooth muscle cells, immunocytes, ciliated cells, fibroblasts, and cardiomyocytes clusters were removed based on their expression of known type II alveolar cell markers such as SFTPC, endothelial cell markers such as PECAM1, VWF, and GJA4, smooth muscle cell markers such as ACTA2 and MYH11, immunocyte markers such as CD163, HLA‐DRA, GNLY, CD68, and PTPRC, ciliated cell markers such as FCN3 and TPPP3, fibroblast markers such as DCN and COL1A2, and cardiomyocyte markers such as MYH6, MYH7 and TNNT2. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were performed with marker genes on DAVID website (https://david.ncifcrf.gov/).
2.2. Immunofluorescence
Normal heart and heart failure samples were permeabilized with 0.3% Triton X‐100 for 10 min and blocked with 5% donkey serum in phosphate buffered saline (PBS) for 2 hr, incubated with primary antibodies ACE2 (1:50; affinity #AF5165) overnight at 4°C, washed with PBS, incubated with secondary antibodies for 1 hr at room temperature, washed with PBS and then stained with 4',6‐diamidino‐2‐phenylindole.
2.3. Clinical specimens
We collected four cases of normal heart and four cases of heart failure where death occurred suddenly and unexpectedly. Reviewing patients' charts was exempted from informed consent by the Ethics Committee Board at the School of Basic Medical Sciences, Fudan University.
2.4. Statistical analysis
Data are presented as mean ± SEM. Statistical analyses were conducted with GraphPad prism 7. Differences among groups were determined with one‐way analysis of variance followed by Tukey's test for multiple comparisons. Differences between two groups were determined with unpaired Student's t test.
2.5. Data availability
Public data for normal and fibrotic lungs scRNA‐seq is available in GSE132771. Public data for healthy hearts scRNA‐seq was available in GSE109816, failed hearts scRNA‐seq in GSE121893. Public data for respiratory syncytial virus infection RNA‐seq is available in GSE140226, MERS‐CoV in GSE139516, and lipopolysaccharide (LPS) injection in GSE136848.
3. RESULTS
3.1. ACE2 is mainly expressed in arterial vascular cells in fibrotic lungs
To explore whether there is a cell‐type‐specific expression difference of the ACE2 gene in healthy populations and patients with pulmonary fibrosis, we analyzed a published data set containing scRNA‐seq data of the normal whole‐lung tissues from three healthy donors and fibrotic lung samples from three patients (GSE132771). 11,594 cells in normal lung tissues and 13,069 cells in fibrotic lung tissues were analyzed. We classified the entire population of normal lung tissue into five major cell types, including type I and type II alveolar cells (AT1, AT2), ciliated bronchial epithelial cells, immune cells, fibroblasts, and vascular cells based on their respective molecular features (Figure S1A‐S1B). Similar cell types were also observed in fibrotic lung samples (Figure S2A‐S1B). We found that the majority of ACE2‐expressing cells in normal human lung tissues were AT2 cells (SFTPC positive; Figure 1a,c), which is consistent with a previous study (Zhao et al., 2020). Unexpectedly, ACE2 was mainly expressed in arterial vascular cells, but rarely in AT2 in fibrotic lung tissues (Figure 1b,d), indicating that the distribution of ACE2 in fibrotic lung tissues has shifted compared to the normal.
Figure 1.
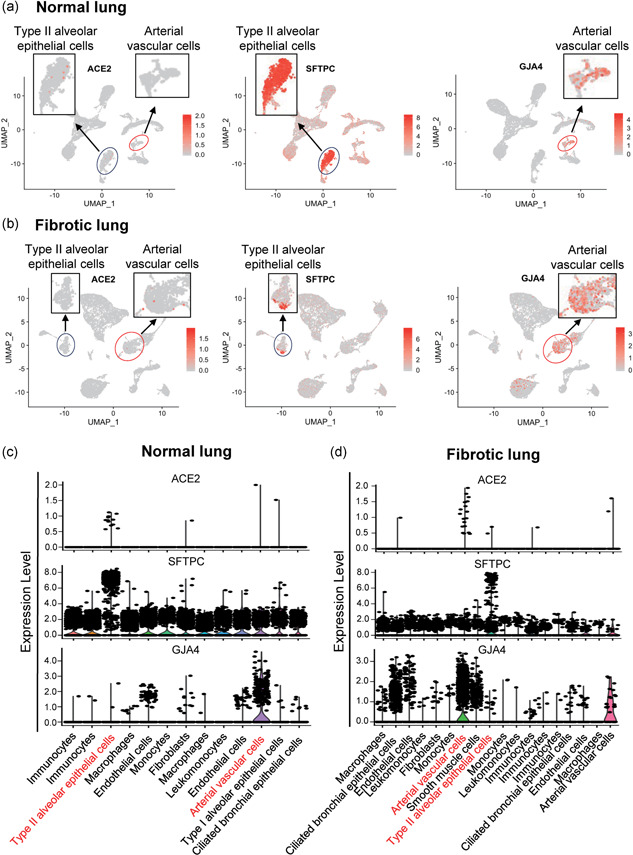
Different distribution pattern of angiotensin converting enzyme II (ACE2)‐expressing cells in normal and fibrotic lungs. (a and b) Feature plot showing the distribution of ACE2, SFTPC, and GJA4 expression levels in normal lungs (a) and in fibrotic lungs (b). (c and d) Violin plot showing the distribution of ACE2, SFTPC, and GJA4 expression levels in normal lungs (c) and in fibrotic lungs (d)
Further analysis showed that chemokines (CCL2, CXCL12) and the complement component (C1R) were highly expressed in ACE2‐positive arterial vascular cells of fibrotic lungs, but showed a lower expression in ACE2‐positive AT2 cells of normal lungs (Figure 2a,b), which suggests that ACE2‐expressing arterial vascular cells in fibrotic lungs may be associated with inflammation. SARS‐CoV‐2 depends on TMPRSS2 and FURIN to infect target cells (Hoffmann et al., 2020; Walls et al., 2020). Both TMPRSS2 and FURIN were expressed in AT2 cells of normal lungs, whereas only FURIN was expressed in arterial vascular cells of fibrotic lungs (Figure 2a,b), indicating that the entryway of SARS‐CoV‐2 might depend on FURIN in fibrotic lungs, which is different from that in normal lungs. These observations show that ACE2 is mainly expressed in arterial vascular cells in fibrotic lungs, and might help SARS‐CoV‐2 to enter blood vessels and cause bloodstream spreading of SARS‐CoV‐2 after infection.
Figure 2.
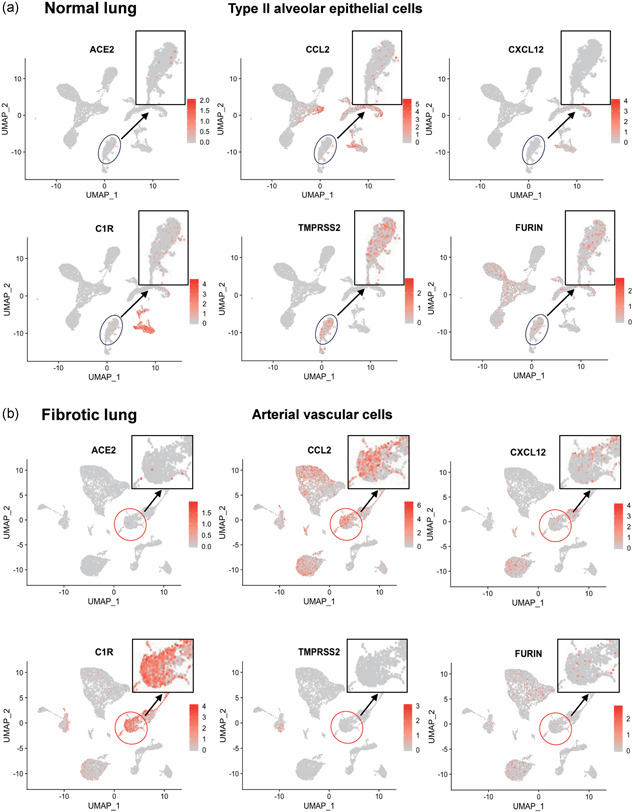
Distribution of chemokine and proteases in normal and fibrotic lungs. (a,b) Feature plot showing the distribution of ACE2, CCL2, CXCL12, C1R, TMPRSS2, and FURIN expression levels in normal lungs (a) and in fibrotic lungs (b)
3.2. Failed human hearts have a higher percentage of ACE2‐expressing cardiomyocytes
To assess the cell‐type specific expression of ACE2 gene in the heart, we analyzed the published scRNA‐seq data of adult human heart tissues from 12 healthy donors (GSE109816) and six patients with heart failure (GSE121893; L. Wang, Yu, et al., 2020). After quality filtering for detected genes, we analyzed 9,994 cells in normal heart tissues and 4,221 cells in heart failure tissues. Four major cell types were identified, including cardiomyocytes, fibroblasts, vascular cells, and immune cells (Figures S3A‐S3B and S4A‐S4B). Cells in failed hearts showed a higher ACE2‐positive rate than in normal hearts (7.60% of all cells vs. 5.88% of all cells). We observed higher enrichment of ACE2 expression in cardiomyocytes of failed hearts than that of normal hearts (9.87% of cardiomyocytes vs. 6.75% of cardiomyocytes). In contrast, there was a lower expression of ACE2 in arterial vascular cells of failed hearts compared to that of normal (7.93% of arterial vascular cells vs. 19.4% of arterial vascular cells; Figure 3a,b). Immunofluorescence staining revealed a significantly higher percentage of ACE2‐positive cells in human heart sections of heart failure compared with healthy donors (Figure 3c).
Figure 3.
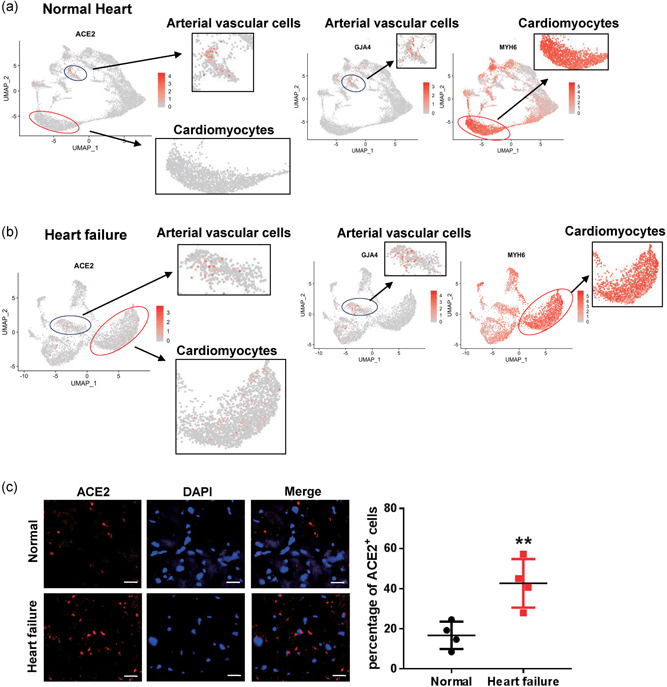
Different distribution of angiotensin converting enzyme II (ACE2)‐expressing cells in normal and failed hearts. (a,b) Feature plot showing the distribution of ACE2, GJA4, and MYH6 expression levels in normal hearts (a) and in failed hearts (b). (c) Immunofluorescence staining of ACE2 (red) expression and DAPI, 4',6‐diamidino‐2‐phenylindole (DAPI) (blue) in normal hearts, and in failed hearts. Scale bar = 20 μm (n = 4). Significance is determined with a two‐tailed unpaired Student's t test, **p < .01
Given that the ACE2 gene is highly expressed in arterial vascular cells, we next employed GO enrichment analysis in ACE2‐expressing arterial vascular cells (cluster 7, GJA4‐ and HES1‐positive cells) in the normal hearts. The highly expressed genes in these vascular cells were associated with virus infection response, including viral processes, response to the virus, and response to interferon‐gamma (Figure 4a). KEGG pathway analysis revealed that these highly expressed genes might be involved in viral myocarditis and pathogenic Escherichia coli infection, indicating that these arterial vascular cells may be the afferent pathway of infectious heart diseases (Figure 4b). Moreover, these cells highly expressed chemokine (CXCL12, CCL2) and integrin subunit (ITGA1, ITGA7; Figure 4c), which may participate in viral infection, postinfection immune response, migration, and adhesion of immune cells. GO and KEGG analysis of ACE2‐expressing arterial vascular cells in the failed heart also suggested that these cells were highly correlated with virus response and infectious diseases (Figure 5a,b). Unlike the normal heart, these cells in the failed heart expressed some regulators of monocyte and B cell differentiation, such as SOX4 and MEF2C (Figure 5c), which might be related to immune response and inflammation.
Figure 4.
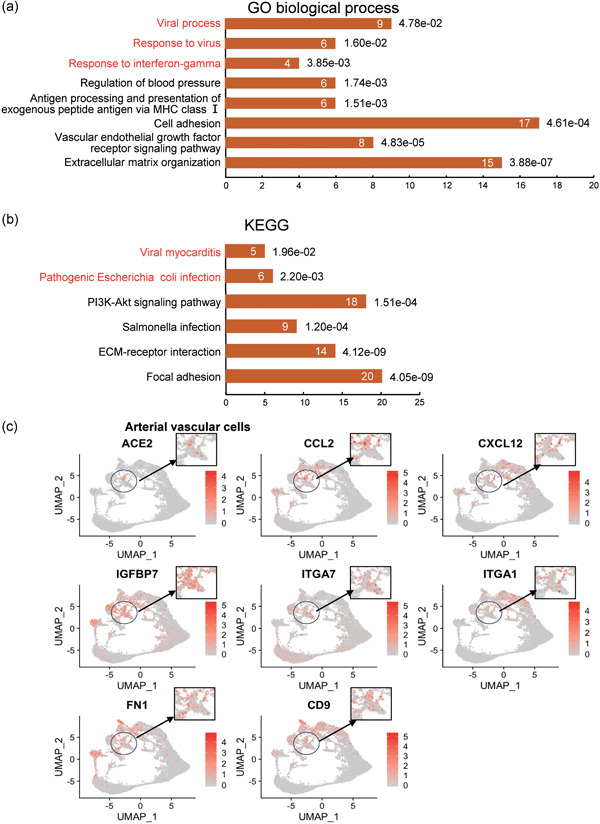
Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) analysis of artery vascular cells in normal hearts. (a) GO term (biological process) analysis of enriched Marker genes of cluster 7. (b) KEGG pathway analysis of enriched Marker genes of cluster 7. (c) Feature plot showing the distribution of expression levels of ACE2, CCL2, CXCL12, IGFBP7, ITGA7, ITGA1, FN1, and CD9 in normal hearts
Figure 5.
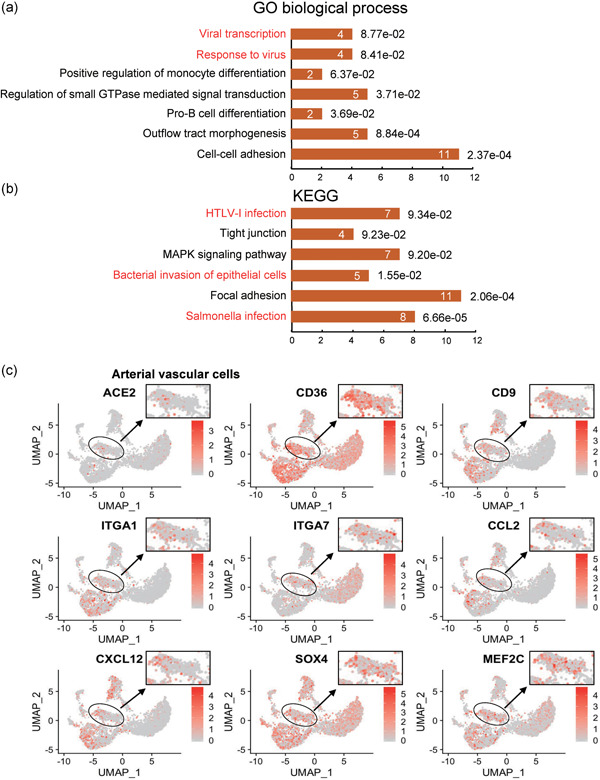
Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) analysis of artery vascular cells in failed hearts. (a) GO term (biological process) analysis of enriched Marker genes of cluster 5. (b) KEGG pathway analysis of enriched marker genes of cluster 5. (c) Feature plot showing the distribution of expression levels of ACE2, CD36, CD9, ITGA1, ITGA7, CCL2, CXCL12, SOX4, and MEF2C in failed hearts
3.3. Higher gene expression of ACE2 in cells infected by RSV or MERS‐CoV
Given that hospital‐related transmission of SARS‐CoV‐2 was suspected in 41% of patients (D. Wang, Hu, et al., 2020), we speculated that patients simultaneously infected by other viruses may be more susceptible to SARS‐CoV‐2 infection. We then determined whether other viral infections might affect the expression of ACE2. We analyzed ACE2 expression in cells infected by various viruses, using published RNA‐Seq data (GSE140226 and GSE139516; McAllister et al., 2020). As expected, ACE2 expression was significantly upregulated when lung carcinoma cells were infected with the respiratory syncytial virus and MERS‐CoV (Figure 6a,b). Moreover, Ace2 expression in pulmonary endothelial cells of mice was upregulated 72 hr after LPS injection (Jambusaria et al., 2020; Figure 6c). Together, these results indicate that patients with a virus infection or inflammation might be more susceptible to SARS‐CoV‐2 infection.
Figure 6.
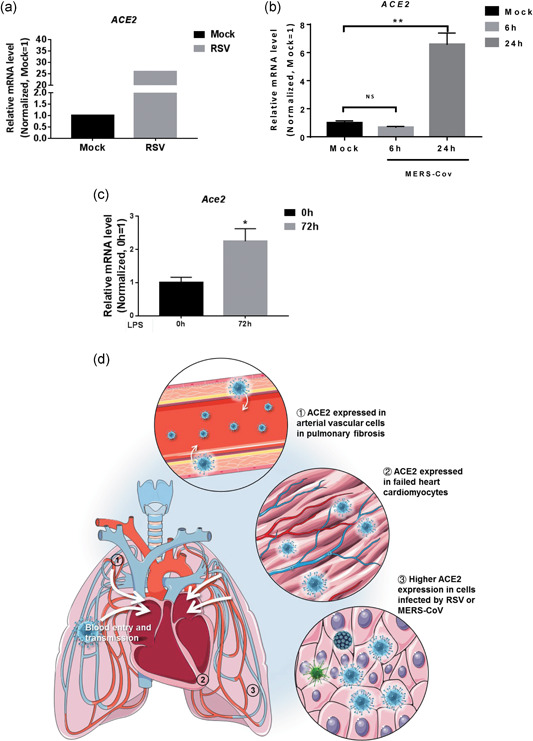
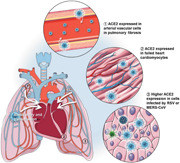
Expression of angiotensin converting enzyme II (ACE2) under other infections. (a) Expression of ACE2 in H929 cells treated with mock virus or respiratory syncytial virus (RSV). (b) Expression of ACE2 in Calu‐3 cells treated with mock or Middle East respiratory syndrome coronavirus (MERS‐CoV) 6hpi and 24hpi. (n = 3). Significance is determined with one‐way analysis of variance (ANOVA) test, **p < .01. (c) Expression of Ace2 in lung endothelial cells 0 and 72 hr after lipopolysaccharide (LPS) injection in mice (n = 10 in 0 hr and n = 12 in 72 hr). Significance is determined with two‐tailed unpaired Student's t test, *p < .05. (d) Schematic review of ACE2 expression providing insights into severe acute respiratory syndrome coronavirus (SARS‐CoV‐2) blood entry and heart injury
4. DISCUSSION
SARS‐CoV‐2 has been reported to exist in human feces, urine, and blood (W. Wang, Xu, et al., 2020). Given that very few viruses can survive gastric acid, the SARS‐CoV‐2 found in feces is less likely to be transmitted through the digestive tract than through the bloodstream. Urine is also directly filtered from blood. Therefore, blood entry of SARS‐CoV‐2 is a pivotal step for its spread to other organs, body fluids and excreta, but how this virus enters the bloodstream has not been fully elucidated. ACE2 was shown to be a receptor for SARS‐CoV‐2, whose expression pattern might provide possible routes for SARS‐CoV‐2 entry. In the present study, we demonstrated that ACE2 was mainly expressed in arterial vascular cells in fibrotic lungs, which might cause blood transmission of SARS‐CoV‐2. Patients with COPD bear a high mortality rate after being infected with SARS‐CoV‐2 (W. Wang, Xu, et al., 2020), and the lungs of COVID‐19 patient displayed characteristics of pulmonary fibrosis (Pan et al., 2020). Therefore, we speculate that pulmonary artery vascular cells may be the main target of SARS‐CoV‐2 attack in patients with pulmonary fibrosis. SARS‐CoV‐2 might invade arterial vascular cells, recruit immune cells, leading to subsequent inflammatory storms, then enter the bloodstream through the pulmonary artery and cause multiple organs injury in critical patients or patients with underlying lung diseases. Further and more detailed work will be needed to explore whether and how SARS‐CoV‐2 invades pulmonary artery vascular cells in COVID‐19 patients. We also found that FURIN but not TMPRSS2 was highly expressed in pulmonary artery vascular cells, indicating that blocking FURIN may help prevent SARS‐CoV‐2 invading the pulmonary artery. Thus, our study has provided new insights into SARS‐CoV‐2 transmission between organs in critical patients or patients with underlying lung diseases.
The first destination of pulmonary circulation outflow is the heart. Therefore, we assume that SARS‐CoV‐2 may attack the heart through the blood flow, which may explain the high incidence of cardiac injury in critical patients (W. Wang, Xu, et al., 2020). Also, heart failure samples displayed a more substantial proportion of ACE2‐positive cells in cardiomyocytes and higher overall expression of ACE2 than the normal heart, which might explain why patients with underlying heart diseases were correlated with higher mortality. Thus, preventing SARS‐CoV‐2 from entering the bloodstream may reduce the chance of myocardial damage in patients with underlying heart diseases. In contrast, the lower proportion of ACE2‐expressing cells in arterial vascular cells of failed hearts might be correlated with weaker anti‐infection ability, along with the activation of the inflammatory response induced by SARS‐CoV‐2 infection.
Finally, we observed a higher gene expression of ACE2 in cells infected by RSV or MERS‐CoV. We speculated that patients simultaneously infected by other viruses may have a higher expression of ACE2, indicating those who bear a coinfection are possibly at a higher risk and are more susceptible to SARS‐CoV‐2 infection. This may explain the high incidence of hospital‐related transmission/infection of SARS‐CoV‐2 (D. Wang, Hu, et al., 2020). A recent study also found that ACE2 expression is highly associated with innate immune genes (Sungnak et al., 2020), and this is an interferon‐stimulated gene (Ziegler et al. 2020). Further study will be needed to confirm whether interferon therapy can increase the risk of patients’ organ injury.
In conclusion, our findings indicate that patients with pulmonary fibrosis, heart failure, and virus infection have a higher risk and are more susceptible to SARS‐CoV‐2 infection. SARS‐CoV‐2 might spread into the bloodstream to attack other organs (Figure 6d). This study provides new insights into how the virus transmits between organs. We also propose a potential therapeutic strategy to mitigate SARS‐CoV‐2 transmission to the heart and to prevent development into severe cases in COVID‐19 patients.
CONFLICT OF INTERESTS
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
M. D. conceived and designed the project. G. J. analyzed most experiments. W. X. performed immunofluorescence staining of ACE2. L. L., Y. Z., S. Y., Q. Y., Z. X., W. X., and Z. X. provided reagents, advice, and discussed results. M. D., G. J., and L. Q. wrote the manuscript. All authors discussed results and commented on the manuscript.
Supporting information
Supporting information
ACKNOWLEDGMENTS
This study was supported by the Shanghai Science and Technology Commission of China (19JC1411300 to D. Meng), General Programs (81873469, 81670450 to D. Meng, 81873536 to X. Wang, 81572713 to X. Zhi), and the Great Program (91639103 to D. Meng) of the National Natural Science Foundation of China, and the China Postdoctoral Science Foundation (2019M651371 and 2019T120303 to X. Wei).
Guo J, Wei X, Li Q, et al. Single‐cell RNA analysis on ACE2 expression provides insights into SARS‐CoV‐2 potential entry into the bloodstream and heart injury. J Cell Physiol. 2020;235:9884–9894. 10.1002/jcp.29802
Jieyu Guo, Xiangxiang Wei, and Qinhan Li contributed equally to this study.
DATA AVAILABILITY STATEMENT
Data availability Public data for normal and fibrotic lungs scRNA‐seq is available in GSE132771. Public data for healthy hearts scRNA‐seq was available in GSE109816, failed hearts scRNA‐seq in GSE121893. Public data for respiratory syncytial virus infection RNA‐seq is available in GSE140226, MERS‐CoV in GSE139516, and LPS injection in GSE136848.
REFERENCES
- Donoghue, M. , Hsieh, F. , Baronas, E. , Godbout, K. , Gosselin, M. , Stagliano, N. , & Acton, S. (2000). A novel angiotensin‐converting enzyme‐related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1‐9. Circulation Research, 87(5), E1–9. 10.1161/01.res.87.5.e1 [DOI] [PubMed] [Google Scholar]
- Hamming, I. , Timens, W. , Bulthuis, M. L. , Lely, A. T. , Navis, G. , & van Goor, H. (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. Journal of Pathology, 203(2), 631–637. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Krüger, N. , Herrler, T. , Erichsen, S. , & Pöhlmann, S. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181, 271–280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , & Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 395(10223), 497–506. 10.1016/s0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambusaria, A. , Hong, Z. , Zhang, L. , Srivastava, S. , Jana, A. , Toth, P. T. , & Rehman, J. (2020). Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. eLife, 9, 9. 10.7554/eLife.51413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan, J. , Ge, J. , Yu, J. , Shan, S. , Zhou, H. , Fan, S. , & Wang, X. (2020). Structure of the SARS‐CoV‐2 spike receptor‐binding domain bound to the ACE2 receptor. Nature, 10.1038/s41586-020-2180-5 [DOI] [PubMed] [Google Scholar]
- Li, W. , Moore, M. J. , Vasilieva, N. , Sui, J. , Wong, S. K. , Berne, M. A. , & Farzan, M. (2003). Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature, 426(6965), 450–454. 10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister, C. S. , Ansaldi, D. , Growcott, E. J. , Zhong, Y. , Quackenbush, D. , Wolff, K. C. , & Kuhen, K. L. (2020). Dexamethasone inhibits respiratory syncytial virus‐driven mucus production while increasing viral replication without altering antiviral interferon signaling. Virology, 540, 195–206. 10.1016/j.virol.2019.10.007 [DOI] [PubMed] [Google Scholar]
- Pan, Y. , Guan, H. , Zhou, S. , Wang, Y. , Li, Q. , Zhu, T. , & Xia, L. (2020). Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019‐nCoV): A study of 63 patients in Wuhan, China. European Radiology, 10.1007/s00330-020-06731-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungnak, W. , Huang, N. , Becavin, C. , Berg, M. , Queen, R. , Litvinukova, M. , & Barnes, J. L. (2020). SARS‐CoV‐2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nature Medicine, 10.1038/s41591-020-0868-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls, A. C. , Park, Y. J. , Tortorici, M. A. , Wall, A. , McGuire, A. T. , & Veesler, D. (2020). Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell, 181, 281–292.e6. 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, S. , Yi, Q. , Fan, S. , Lv, J. , Zhang, X. , Guo, L. , & Chen, Y. (2020). Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv. 10.1101/2020.02.10.20021832% [DOI]
- Wang, C. , Xie, J. , Zhao, L. , Fei, X. , Zhang, H. , Tan, Y. , … Bian, X. (2020). Aveolar macrophage activation and cytokine storm in the pathogenesis of severe COVID‐19, 25 March, preprint (Version 1) available at Research Square. 10.21203/rs.3.rs-19346/v1 [DOI]
- Wang, D. , Hu, B. , Hu, C. , Zhu, F. , Liu, X. , Zhang, J. , & Peng, Z. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. Journal of the American Medical Association, 323, 1061. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Yu, P. , Zhou, B. , Song, J. , Li, Z. , Zhang, M. , & Hu, S. (2020). Single‐cell reconstruction of the adult human heart during heart failure and recovery reveals the cellular landscape underlying cardiac function. Nature Cell Biology, 22(1), 108–119. 10.1038/s41556-019-0446-7 [DOI] [PubMed] [Google Scholar]
- Wang, W. , Xu, Y. , Gao, R. , Lu, R. , Han, K. , Wu, G. , & Tan, W. (2020). Detection of SARS‐CoV‐2 in different types of clinical specimens. Journal of the American Medical Association, 10.1001/jama.2020.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z. , & McGoogan, J. M. (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) Outbreak in China: Summary of a report of 72 314 cases from the chinese center for disease control and prevention. Journal of the American Medical Association. 10.1001/jama.2020.2648 [DOI] [PubMed]
- Zhao, Y. , Zhao, Z. , Wang, Y. , Zhou, Y. , Ma, Y. , & Zuo, W. (2020). Single‐cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019‐nCov. bioRxiv. 10.1101/2020.01.26.919985 [DOI] [PMC free article] [PubMed]
- Zhou, F. , Yu, T. , Du, R. , Fan, G. , Liu, Y. , Liu, Z. , & Cao, B. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: A retrospective cohort study. Lancet, 395, 1054–1062. 10.1016/s0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , Yang, X. L. , Wang, X. G. , Hu, B. , Zhang, L. , Zhang, W. , & Shi, Z. L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler, C. G. K. , Allon, S. J. , Nyquist, S. K. , Mbano, I. M. , Miao, V. N. , Tzouanas, C. N. , & Ordovas‐Montanes, J. (2020). SARS‐CoV‐2 receptor ACE2 is an interferon‐stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell, 10.1016/j.cell.2020.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
Public data for normal and fibrotic lungs scRNA‐seq is available in GSE132771. Public data for healthy hearts scRNA‐seq was available in GSE109816, failed hearts scRNA‐seq in GSE121893. Public data for respiratory syncytial virus infection RNA‐seq is available in GSE140226, MERS‐CoV in GSE139516, and lipopolysaccharide (LPS) injection in GSE136848.
Data availability Public data for normal and fibrotic lungs scRNA‐seq is available in GSE132771. Public data for healthy hearts scRNA‐seq was available in GSE109816, failed hearts scRNA‐seq in GSE121893. Public data for respiratory syncytial virus infection RNA‐seq is available in GSE140226, MERS‐CoV in GSE139516, and LPS injection in GSE136848.


