Introduction
Sickle cell disease (SCD) is a complex and life-threatening inherited blood disorder and one of most common genetic diseases worldwide.1 Pregnancy in SCD has been associated with increased maternal and fetal complications and a recent meta-analysis showed a strong association between SCD pregnancies and pulmonary complications.2
The COVID-19 infection, caused by the SARS-CoV-2 coronavirus, can lead to an acute respiratory distress syndrome (ARDS) and multiple organ failure.3 Few reports have been published regarding the COVID-19 and pulmonary manifestations in SCD patients to date.4, 5, 6, 7, 8 To our knowledge, no case of a pregnant woman with SCD and COVID-19 infection has been related until now.
Case report
This was a 35-year-old woman, with history of SCA, 28 weeks pregnant. Her prior history was acute chest syndrome (ACS) in 2012, pulmonary thromboembolism in 2017 with pulmonary hypertension (estimated PASP 47 mmHg), leg ulcers in 2012 and 2 previous pregnancies (the last one in 2017, complicated with preeclampsia). She had received the influenza vaccine on April 2, 2020.
The patient presented to the emergency room on May 4, 2020, referring to myalgia and fever for the last 10 days and cough and dyspnea for the last seven days. At the presentation, dyspnea and hypoxia (SpO2 84%) were observed and corrected with 3 L/min of supplemental oxygen. She denied pain.
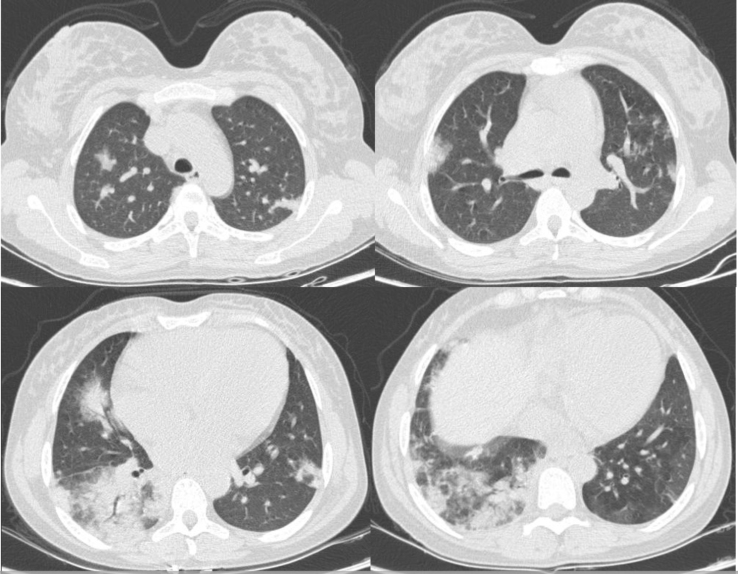
Her initial blood exams were: red blood cells (RBCs) 1.7 × 1012/L; hemoglobin 6.4 g/dL; hematocrit 18.2%; WBC 14.5 × 109/L; neutrophils 11.2 × 109/L; lymphocytes 2.1 × 109/L; platelets 347 × 109/L; reticulocytes 308.9 × 109/L; reactive C-protein 100 mg/L, total bilirubin 2.6 mg/dL (unconjugated 1.55 mg/dL); lactate dehydrogenase 853 U/L; dimer-d 4620 mg/mL; creatinine 0.6 mg/dL; ferritin 4741 ng/mL. The chest CT showed bilateral pulmonary parenchyma ground-glass and consolidative pulmonary opacities with peripheral lung distribution (Figure 1), which was consistent with viral infection in the acute chest syndrome.
Figure 1.
Patient's CT showed typical findings of SARS-CoV-2 infection: bilateral pulmonary parenchyma ground-glass and consolidative pulmonary opacities with peripheral lung distribution.
The SARS-CoV-2 PCR nasopharyngeal swab testing was collected at admission (day 10 of symptoms) and she was conducted to the respiratory isolated intensive care unit (ICU). She was treated with ceftriaxone and azithromycin and received blood transfusion.
She remained with the cough and fever for 2 days, but improvement in dyspnea and hypoxia was observed in this initial moment (SpO2 96% with 2 L/min supplemental oxygen). On the third day of hospitalization, she received positive testing for COVID-19 and was discharged from ICU, given her better clinical condition, being then transferred to the respiratory clinical unit.
On ninth day of hospitalization (day 18 of symptoms), the SARS-CoV-2 PCR nasopharyngeal swab testing was repeated and resulted negative. At this time, the only symptom left was a minimal cough. She remained in blood transfusion therapy during hospitalization. Obstetric monitoring was provided at admission and during the entire hospitalization, with no complications detected, and maintaining good fetal mobility.
Discussion
The ACS is one of the leading causes of death in SCD adults.1 It can be triggered by bacterial infection and, although rare in adults, by viral infection.9 There are only a few studies about these infections causing ACS during the previous epidemics of H1N1 and SARS-CoV in the last decades, mainly in the pediatric population.10, 11 Strouse et al. showed that H1N1 virus had more chance of evolving into ACS with the incidence of as high as 34%.11 The literature is also scant on pulmonary infection by SARS-CoV-2 in SCD, considering its short time of history. Few case reports showed that the symptoms could be respiratory or even unspecific, presenting only with vaso-occlusive crisis. Overall, these results suggest a favorable evolution of the viral infection, even in the presence of SCD comorbidities.4, 12
A recent review of COVID-19 in pregnant women showed some cases of severe maternal morbidity and perinatal deaths, although the majority of the cases had a good outcome.13 On the other hand, a recent paper showed that severe pulmonary complications are common in SCD pregnant women.2 In our report, however, the patient presented with respiratory symptoms and typical radiographic images of COVID-19, raising the hypothesis that the novel coronavirus infection was the trigger of the respiratory manifestation. Despite her previous history of pulmonary comorbidities and the current pregnancy, her clinical evolution was very favorable. As far as we know, not one case of ACS by the novel coronavirus in pregnant SCD patients was found in the literature.
Despite the fact that it is a single-case observation, the evolution of the patient seems to corroborate the proposal of Hussain et al. that anemia, hemolysis and chronic inflammation, physiopathological characteristics of SCD, might have a favorable influence in the clinical course of COVID-19 infection in these patients.6 To enhance the knowledge of the scientific community in this field, we believe that physicians should be encouraged to access and submit their experience in data platforms such as ‘Secure SCD Registry’, coordinated by Panepinto et al., at the Medical College of Wisconsin, USA.14
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Ware R.E., de Montalembert M., Tshilolo L., Abboud M.R. Sickle cell disease. Lancet. 2017;390(10091):311–323. doi: 10.1016/S0140-6736(17)30193-9. [DOI] [PubMed] [Google Scholar]
- 2.Inparaj S., Buckingham M., Oakley L., Seed P.T., Lucas S., Oteng-Ntim E. Pulmonary complications for women with sickle cell disease in pregnancy: systematic review and meta-analysis. Thorax. 2020 doi: 10.1136/thoraxjnl-2019-213796. [DOI] [PubMed] [Google Scholar]
- 3.Down B., Kulkarni S., Ahmed Khan A.H., Barker B., Tang I. Novel coronavirus (COVID-19) infection: what a doctor on the frontline needs to know. Ann Med Surg (Lond) 2020;55:24–29. doi: 10.1016/j.amsu.2020.05.014. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odievre M.H., de Marcellus C., Ducou Le Pointe H., Romain A.S., Youn J., Taytard J. Dramatic improvement after tocilizumab of severe COVID-19 in a child with sickle cell disease and acute chest syndrome. Am J Hematol. 2020;1 doi: 10.1002/ajh.25855. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Luna G., Habibi A., Deux J.F., Colard M., Pham Hung d’Alexandry d’Orengiani A.L., Schlemmer F. Rapid and severe Covid-19 pneumonia with severe acute chest syndrome in a sickle cell patient successfully treated with tocilizumab. Am J Hematol. 2020;13 doi: 10.1002/ajh.25833. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain F.A., Njoku F.U., Saraf S.L., Molokie R.E., Gordeuk V.R., Han J. COVID-19 infection in patients with sickle cell disease. Br J Haematol. 2020;189(5):851–852. doi: 10.1111/bjh.16734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beerkens F., John M., Puliafito B., Corbett V., Edwards C., Tremblay D. COVID-19 pneumonia as a cause of acute chest syndrome in an adult sickle cell patient. Am J Hematol. 2020 doi: 10.1002/ajh.25809. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Nur E., Gaartman A.E., van Tuijn C.F., Tang M.W., Biemond B.J. Vaso-occlusive crisis and acute chest syndrome in sickle cell disease due to 2019 novel coronavirus disease (COVID-19) Am J Hematol. 2020;95(6):725–726. doi: 10.1002/ajh.25821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rees D.C., Williams T.N., Gladwin M.T. Sickle-cell disease. Lancet. 2010;376(9757):2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 10.George A., Benton J., Pratt J. The impact of the 2009 H1N1 influenza pandemic on pediatric patients with sickle cell disease. Pediatr Blood Cancer. 2011;57(4):648–653. doi: 10.1002/pbc.23030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strouse J.J., Reller M.E., Bundy D.G., Amoako M., Cancio M., Han R.N. Severe pandemic H1N1 and seasonal influenza in children and young adults with sickle cell disease. Blood. 2010;116(18):3431–3434. doi: 10.1182/blood-2010-05-282194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCloskey K.A., Meenan J., Hall R., Tsitsikas D.A. COVID-19 infection and sickle cell disease: a UK centre experience. Br J Haematol. 2020 doi: 10.1111/bjh.16779. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Zaigham M., Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020;7 doi: 10.1111/aogs.13867. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panepinto J.A., Brandow A.M., Singh A. Medical College of Wisconsin; Wisconsin, USA: 2020. Secure-SCD Registry. Surveillance epidemiology of coronavirus (COVID-19) under research exclusion. https://covidsicklecell.org/ [accessed 06.08.20] [Google Scholar]