Abstract
Background
Experimental evidence supports a role of lipid dysregulation in ovarian cancer progression. We estimated associations with ovarian cancer risk for circulating levels of four lipid groups, previously hypothesized to be associated with ovarian cancer, measured 3–23 years before diagnosis.
Methods
Analyses were conducted among cases (N = 252) and matched controls (N = 252) from the Nurses’ Health Studies. We used logistic regression adjusting for risk factors to investigate associations of lysophosphatidylcholines (LPCs), phosphatidylcholines (PCs), ceramides (CERs), and sphingomyelins (SMs) with ovarian cancer risk overall and by histotype. A modified Bonferroni approach (0.05/4 = 0.0125, four lipid groups) and the permutation-based Westfall and Young approach were used to account for testing multiple correlated hypotheses. Odds ratios (ORs; 10th–90th percentile), and 95% confidence intervals of ovarian cancer risk were estimated. All statistical tests were two-sided.
Results
SM sum was statistically significantly associated with ovarian cancer risk (OR = 1.97, 95% CI = 1.16 to 3.32; P = .01/permutation-adjusted P = .20). C16:0 SM, C18:0 SM, and C16:0 CERs were suggestively associated with risk (OR = 1.95–2.10; P = .004–.01; permutation-adjusted P = .08–.21). SM sum, C16:0 SM, and C16:0 CER had stronger odds ratios among postmenopausal women (OR = 2.16–3.22). Odds ratios were similar for serous/poorly differentiated and endometrioid/clear cell tumors, although C18:1 LPC and LPC to PC ratio were suggestively inversely associated, whereas C18:0 SM was suggestively positively associated with risk of endometrioid/clear cell tumors. No individual metabolites were associated with risk when using the permutation-based approach.
Conclusions
Elevated levels of circulating SMs 3–23 years before diagnosis were associated with increased risk of ovarian cancer, regardless of histotype, with stronger associations among postmenopausal women. Further studies are required to validate and understand the role of lipid dysregulation in ovarian carcinogenesis.
Ovarian cancer is the fifth leading cause of cancer death for US women, with four out of five ovarian cancer patients diagnosed with advanced disease that has spread throughout the abdominal cavity (1). Identifying new risk factors for ovarian cancer is important to discover new opportunities for prevention. One area of interest is lipid metabolism (2).
Laboratory evidence supports a role of lipid dysregulation in ovarian cancer progression and metastasis (3,4). Several prospective human studies reported suggestive associations with complex lipids such as total cholesterol (positive) (5) or high-density lipoprotein (inverse) (6). However, there have been few comprehensive prospective studies of other lipids, such as lysophosphatidylcholines (LPCs), phosphatidylcholines (PCs), ceramides (CERs), and sphingomyelins (SMs), which appear to be different in ovarian cancer patients, as compared with healthy women (7–9).
LPCs act as signaling molecules involved in upregulating cell proliferation, angiogenesis, migration, inflammation, and wound healing (3,4,10–15). Phospholipase A2 converts PCs to LPCs, and may influence cell proliferation, invasion, and migration (3). In multiple case-control studies, ovarian cancer patients had higher plasma or urine LPC levels compared with controls (14,16–24). However, because of the retrospective design of these studies, it is unclear whether alterations in these biomarkers preceded or followed the appearance of ovarian cancer.
Another class of lipids that may play a role in ovarian carcinogenesis is SMs that have a phosphocholine head group and CER backbone (3). CERs are proapoptotic signaling molecules in oocytes and ovarian tumors (25–28) with potential metastasis- suppressing properties (29). The primary source of CERs in ovarian tumors is via SM metabolism. CER levels are very low in ovarian tumors (25,28,30), whereas SMs appear to be higher in ovarian tumors vs normal tissue (31).
We leveraged novel metabolomics assays to measure four circulating lipid groups that have been previously hypothesized to be associated with ovarian cancer, LPCs, PCs, CERs, and SMs, in plasma samples in a study of ovarian cancer risk within two large prospective cohorts with 23 years of follow-up.
Methods
Study Population
This analysis was based on data from nested case-control studies in the Nurses Health Studies (NHS (32) and NHSII (33). The NHS was established in 1976 among 121 700 US female nurses aged 30–55 years, and NHSII was established in 1989 among 116 429 female nurses aged 25–42 years. Participants have been followed biennially by questionnaire to update information on exposure status and disease diagnoses. In 1989–1990, 32 826 NHS participants provided blood samples and completed a short questionnaire (32). Between 1996 and 1999, 29 611 NHSII participants provided blood samples and completed a short questionnaire. Details are provided in the Supplementary Materials (available online). Participants provided implied consent to participate by return of questionnaires and biologic specimens, and the study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T. H. Chan School of Public Health, and those of participating registries as required.
Metabolite Profiling
Plasma metabolites were profiled at the Broad Institute of MIT and Harvard (Cambridge, MA) using a liquid chromatography tandem mass spectrometry (LC-MS) method described previously (34–37). Details are provided in the Supplementary Materials (available online).
Thirty-nine individual metabolites belonging to four metabolite classes (11 LPC, 17 PC, 6 SM, and 5 CER) together with the sum of the measured metabolite values within each class (LPC sum, PC sum, SM sum, and CER sum) and the ratios of LPC to PC (LPC:PC) and SM to CER (SM:CER) were analyzed in this study. Metabolite class sums and ratios were calculated based on raw values. The ratio of two metabolite classes was calculated as the ratio of the class sums. Metabolite values, including sums and ratios, were transformed to probit scores for all subsequent analyses to reduce the influence of skewed distributions and heavy tails on the results and to scale the measured metabolite values to the same range.
Statistical Analysis
Conditional logistic regression was used to evaluate metabolite associations with risk of overall ovarian cancer among all participants (252 cases and 252 controls), and separately by menopausal status at blood draw (82 premenopausal cases and 82 premenopausal controls; 137 postmenopausal cases and 137 postmenopausal controls). Unconditional logistic regression was used to evaluate metabolite associations with ovarian cancer risk by tumor subtype (176 serous/poorly differentiated [PD] cases; 34 endometrioid/clear cell[CC] cases). Metabolite values were used as continuous variables to calculate linear trends. We estimated the odds ratios (OR) and 95% confidence intervals for an increase from the 10th to 90th percentile in metabolite levels (OR = eβ*2.5, β represents the effect estimate from the logistic regression with metabolites modeled as continuous variables and transformed to probit scores). Furthermore, we estimated OR of ovarian cancer and 95% confidence interval for the SM sum measure across quartiles (based on the distribution in controls). Additional details are provided in the Supplementary Materials (available online).
We used a modified Bonferroni approach to account for testing multiple correlated hypotheses by defining the adjusted P value threshold for statistical significance as .01 (.05/4; four measured lipid groups). This approach is used to define statistical significance when assessing the sum of all markers in each of the four metabolite groups: SM sum, CER sum, LPC sum, and PC sum. We also calculated permutation-based (N = 1000 permutations) adjusted P values using the Westfall and Young approach (38), which accounts for testing multiple correlated hypotheses, to define statistical significance (adjusted P < .05) for individual metabolites. We had 88% power to identify an odds ratio of 1.5 at alpha equal to 0.001 (.05/45, Bonferroni threshold) and 97% power at alpha equal to 0.01 (.05/4, modified Bonferroni threshold) in our main analysis (39).
Results
Study Population
Of the 252 cases analyzed, 176 were diagnosed with serous/PD tumors, whereas 34 were classified as having endometrioid/CC tumors (Table 1). The remaining cases were of mucinous or other types. Mean follow-up time was 12.3 years. Distributions of ovarian cancer risk factors were generally in the expected directions for cases and controls.
Table 1.
Characteristics of overall, serous/poorly differentiated (PD) and endometrioid/clear cell (CC) ovarian cancer (OC) cases, and all controls at time of blood collection
| Characteristic | All controls | Overall OC | Serous/PD OC | Endometrioid/CC OC | Other histotypes |
|---|---|---|---|---|---|
| (N = 252) | (N = 252) | (N = 176) | (N = 34) | (N = 42) | |
| Age at blood draw*, mean (SD), y | 55.6 (7.8) | 55.5 (7.9) | 55.3 (7.9) | 54 (8.1) | 57.8 (7.5) |
| Time from blood draw to diagnosis, mean (SD), y | – | 12.3 (5.2) | 12.8 (5.3) | 12.1 (5.1) | 10.9 (4.7) |
| Fasting >8h, No. (%) | 172 (68.3) | 153 (60.7) | 100 (56.8) | 23 (67.6) | 30 (71.4) |
| Tumor morphology, No. (%) | |||||
| Invasive | – | 227 (90.1) | 163 (92.6) | 33 (97.1) | 31 (73.8) |
| Borderline | – | 22 (8.7) | 13 (7.4) | 1 (2.9) | 8 (19.0) |
| Unknown | – | 3 (1.2) | 0 (0) | 0 (0) | 3 (7.1) |
| Menopausal status blood draw*, No. (%) | |||||
| Premenopausal | 82 (32.5) | 82 (32.5) | 56 (31.8) | 16 (47.1) | 10 (23.8) |
| Postmenopausal, no HT use | 71 (28.2) | 68 (27.0) | 47 (26.7) | 5 (14.7) | 16 (38.1) |
| Postmenopausal, HT use | 66 (26.2) | 69 (27.4) | 48 (27.3) | 8 (23.5) | 13 (31.0) |
| Unknown | 33 (13.1) | 33 (13.1) | 25 (14.2) | 5 (14.7) | 3 (7.1) |
| Cohort*, No. (%) | |||||
| NHS | 212 (84.1) | 212 (84.1) | 147 (83.5) | 27 (79.4) | 38 (90.5) |
| NHS II | 40 (15.9) | 40 (15.9) | 29 (16.5) | 7 (20.6) | 4 (9.5) |
| Oral contraceptive use duration, No. (%) | |||||
| None or <3 months | 123 (48.8) | 118 (46.8) | 81 (46.0) | 18 (52.9) | 19 (45.2) |
| 3 months to 3 years | 33 (13.1) | 32 (12.7) | 22 (12.5) | 3 (8.8) | 7 (16.7) |
| 3 to 5 years | 45 (17.9) | 63 (25.0) | 46 (26.1) | 8 (23.5) | 9 (21.4) |
| 5+ years | 51 (20.2) | 39 (15.5) | 27 (15.3) | 5 (14.7) | 7 (16.7) |
| Parity, No. (%) | |||||
| No children | 12 (4.8) | 24 (9.5) | 16 (9.1) | 5 (14.7) | 3 (7.1) |
| 1 child | 11 (4.4) | 13 (5.2) | 8 (4.5) | 1 (2.9) | 4 (9.5) |
| 2 children | 72 (28.6) | 89 (35.3) | 60 (34.1) | 15 (44.1) | 14 (33.3) |
| 3 children | 77 (30.6) | 65 (25.8) | 45 (25.6) | 9 (26.5) | 11 (26.2) |
| 4+ children | 80 (31.7) | 61 (24.2) | 47 (26.7) | 4 (11.8) | 10 (23.8) |
| Tubal ligation, No. (%) | |||||
| Yes | 43 (17.1) | 39 (15.5) | 30 (17.0) | 5 (14.7) | 4 (9.5) |
Matching factor; HT = hormone therapy; NHS = Nurses’ Health Study.
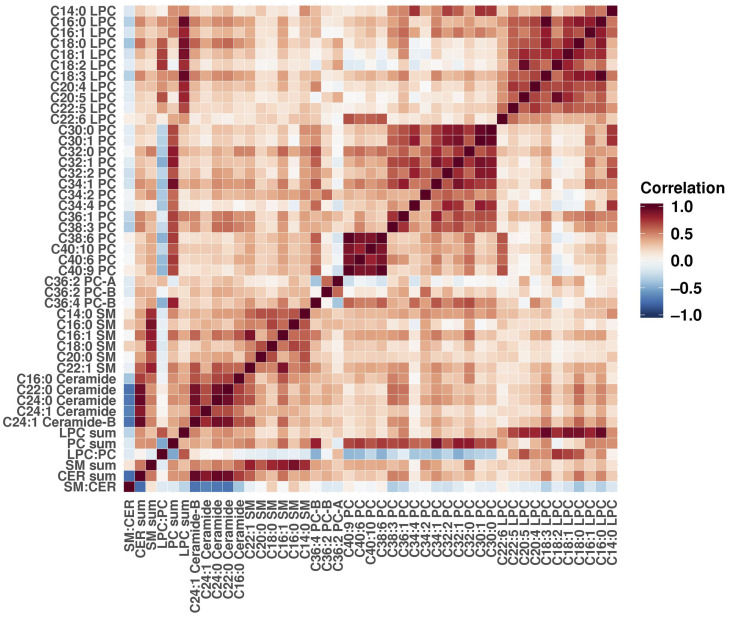
Correlations between Metabolites
Metabolites, metabolite sums, and metabolite ratios belonging to the same class were highly correlated with each other among controls (Figure 1), although the correlation among the SMs was slightly weaker than for the other classes. We observed somewhat lower correlations in postmenopausal women compared with premenopausal at blood draw (Supplementary Figures 1 and 2, available online).
Figure 1.
Metabolite correlations among controls. Pearson correlation was calculated for all pairs of individual metabolites, metabolite sums, and metabolite ratios. Positive correlation coefficients are shown in shades of red, and negative coefficients are shown in shades of blue.
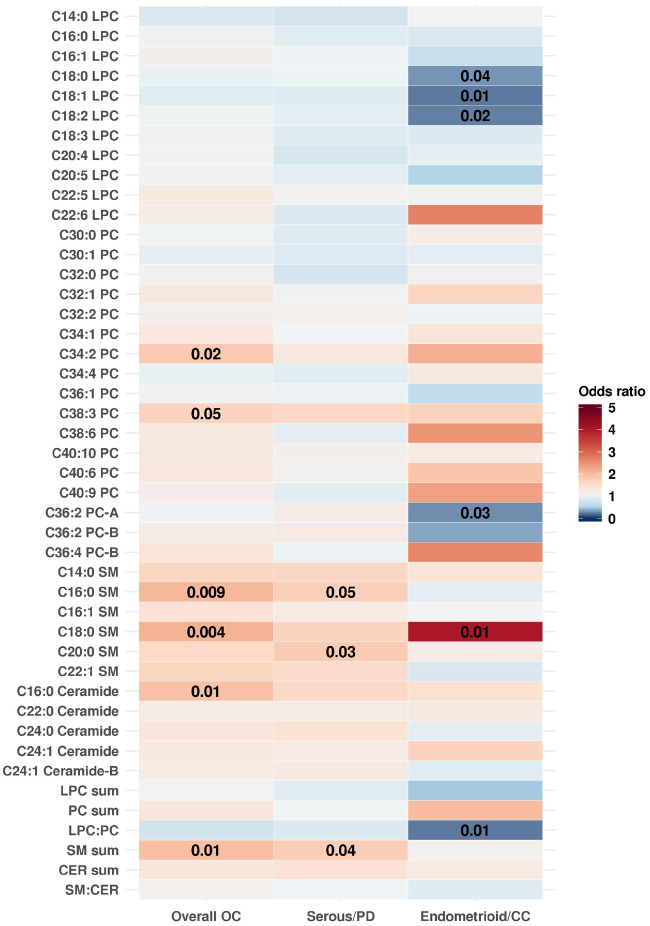
Metabolites Associated With Risk of Ovarian Cancer, Serous/PD and Endometrioid/CC Tumors
The SM sum (OR [95% CI] =1.97 [1.16 to 3.32]; P = .01) was statistically significantly positively associated with risk of overall ovarian cancer when using the modified Bonferroni threshold (Figure 2; Supplementary Table 2, available online); the permutation-adjusted P value was .20. Two individual SMs, C18:0 SM (OR [95% CI] = 2.10 [1.26 to 3.49]; P = .004, adjusted P = .08), and C16:0 SM (OR [95% CI] = 2.06 [1.19 to 3.56]; P = .009, adjusted P = .17), as well as C16:0 CER (OR [95% CI] = 1.95 [1.16 to 3.30]; P = .01, adjusted P = .21) were suggestively positively associated with risk of overall ovarian cancer (Figure 2; Supplementary Table 2, available online). No individual metabolites were statistically significantly associated with ovarian cancer risk overall when using the Westfall and Young approach for multiple comparison testing. Further, no metabolites were statistically significantly associated with risk of serous/PD disease after the Westfall and Young permutation testing, although the risk estimates were similar to the overall results. Notably, C20:0 SM (OR [95% CI] = 1.80 [1.06 to 3.08]), C16:0 SM (OR [95% CI] = 1.73 [1.00 to 3.01]) and the sum of all SMs (OR [95% CI] = 1.77 [1.02 to 3.10]) were positively associated with risk of serous/PD ovarian cancer on the nominal scale (P ≤ .05). For endometrioid/CC disease, C18:1 LPC (OR [95% CI] = 0.24 [0.07 to 0.69]; P = .01, adjusted P = .15) and LPC:PC (OR [95% CI] = 0.24 [0.08 to 0.71]; P = .01, adjusted P = .15) were suggestively inversely associated, whereas C18:0 SM (OR [95% CI] = 4.06 [1.44 to 12.60]; P = .01, adjusted P = .15) was suggestively positively associated with risk. SM sum (and SMs) showed similar odds ratios when comparing tumors diagnosed soon after blood collection (3–11 years) with tumors diagnosed later (12–23 years; Supplementary Table 4, available online) and when comparing rapidly fatal (defined as death occurring within 3 years of diagnosis) to less-aggressive tumors (defined as death occurring at least 3 years after diagnosis; Supplementary Table 5, available online).
Figure 2.
Odds ratios (OR) of overall, serous/poorly differentiated (PD) and endometrioid/clear cell (CC) OC for an increase from the 10th to the 90th percentile in metabolite levels. Odds ratios greater than one are shown in shades of red, and odds ratios less than 1 are shown in shades of blue. P values of .05 or less are overlaid on the plot. Estimates were adjusted for risk factors (duration of oral contraceptive use [none or <3 months, 3 months to 3 years, 3 years to 5 years, more than 5 years], tubal ligation [yes/no], and parity [no children, one child, two children, three children, or four or more children]) and additionally for matching factors (cohort [NHS, NHSII]; menopausal status and hormone therapy use at blood draw [premenopausal, postmenopausal and hormone therapy use, postmenopausal and no hormone therapy use, and missing/unknown]; menopausal status at diagnosis [premenopausal, postmenopausal, or unknown]; age [±1 year]; date of blood collection [±1 month]; time of day of blood draw [±2 hours];and fasting status [>8 hours or ≤8 hours]) in subtype analyses. CER = ceramide; NHS = Nurses' Health Study; NHSII = Nurses' Health Study II; LPC = lysophosphatidylcholine; OC = ovarian cancer; PC = phosphatidylcholine; SM = sphingomyelin.
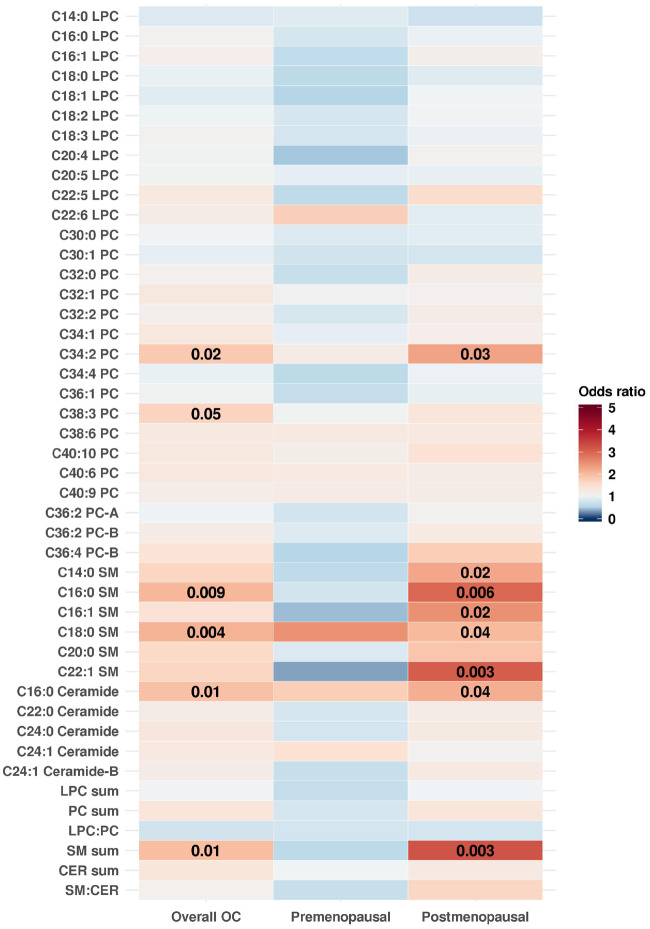
Metabolites Associated with Risk of Ovarian Cancer by Menopausal Status at Blood Draw
The SM sum (OR [95% CI] = 3.22 [1.51 to 6.86], P = .003) was statistically significantly associated with ovarian cancer risk among postmenopausal women at blood collection when using the modified Bonferroni threshold. Two individual markers were nominally positively associated with risk of ovarian cancer among postmenopausal women at blood collection (Figure 3; Supplementary Table 3, available online): C22:1 SM (OR [95% CI] = 3.11 [1.46 to 6.62]; P = .003), and C16:0 SM (OR [95% CI] = 2.98 [1.37 to 6.51]; P = .006), but did not meet the Westfall and Young threshold. SM sum, C22:1 SM, C16:0 SM, and C16:0 CER had stronger associations with risk of ovarian cancer among postmenopausal women (OR = 2.16–3.22) compared with overall ovarian cancer risk (OR = 1.65–2.06). No metabolites were associated with risk of overall ovarian cancer among premenopausal women at blood collection. However, five out of six SMs and SM sum had odds ratios below 1.0 among premenopausal women and risk estimates above one among postmenopausal women at blood collection.
Figure 3.
Odds ratios (odds ratios) of overall OC and by menopausal status at blood draw for an increase from the 10th to the 90th percentile in metabolite levels. Odds ratios greater than one are shown in shades of red and odds ratios less than one are shown in shades of blue. P values of .05 or less are overlaid on the plot. Estimates were adjusted for risk factors (duration of oral contraceptive use [none or <3 months, 3 months to 3 years, 3 years to 5 years, or more than 5 years], tubal ligation [yes/no], and parity [no children, one child, two children, three children, or four or more children]) and additionally for matching factors (cohort [NHS, NHSII]; menopausal status and hormone therapy use at blood draw [premenopausal, postmenopausal and hormone therapy use, postmenopausal and no hormone therapy use, and missing/unknown]; menopausal status at diagnosis [premenopausal, postmenopausal, or unknown]; age [±1 year]; date of blood collection [± month]; time of day of blood draw [±2 hours]; and fasting status [>8 hours or ≤8 hours]) in stratified analyses. CER = ceramide; NHS = Nurses' Health Study; NHSII = Nurses' Health Study II; LPC = lysophosphatidylcholine; OC = ovarian cancer; PC = phosphatidylcholine; SM = sphingomyelin.
Sphingomyelins
To better understand the dose-response relationship between SM (analyzed as the sum of all measured SMs) and risk of ovarian cancer, we calculated odds ratios for the three highest quartiles compared with the first quartile (Table 2). We observed a dose-response association for overall ovarian cancer among postmenopausal women at blood draw with an OR of 1.68 (95% CI = 0.77 to 3.64) for quartile 2, 1.89 (95% CI = 0.87 to 4.12) for quartile 3, and 2.92 (95% CI = 1.36 to 6.27) for quartile 4 compared with the lowest quartile, with a P trend equal to .003. Suggestive trends (OR, top vs bottom quartile) were observed for serous/PD disease (OR = 1.58, P trend = .05) and overall ovarian cancer risk (OR = 1.67, P trend = .01).
Table 2.
Odds ratio (OR) and 95% confidence intervals of ovarian cancer (OC), according to histotype, and by menopausal status at blood draw by quartiles (based on the distribution in controls) of the sum of all sphingomyelins
| OC | Quartile (range) |
P trend* | |||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| (−2.88, −0.74) | (−0.74, −0.12) | (−0.12, 0.54) | (0.54, 2.88) | ||
| Overall OC | |||||
| OR (95% CI) | 1.00 (referent) | 0.95 (0.53 to 1.7) | 1.45 (0.83 to 2.51) | 1.67 (0.96 to 2.92) | .01 |
| No. of controls | 63 | 63 | 63 | 63 | |
| No. of cases | 54 | 44 | 68 | 84 | |
| Serous/PD OC | |||||
| OR (95% CI) | 1.00 (referent) | 1.02 (0.54 to 1.91) | 1.40 (0.77 to 2.57) | 1.58 (0.87 to 2.89) | .05 |
| No. of controls | 63 | 63 | 63 | 63 | |
| No. of cases | 38 | 32 | 48 | 56 | |
| Endometrioid/clear cell OC | |||||
| OR (95% CI) | 1.00 (referent) | 1.35 (0.43 to 4.26) | 1.76 (0.61 to 5.29) | 0.88 (0.25 to 2.98) | .83 |
| No. of controls | 63 | 63 | 63 | 63 | |
| No. of cases | 8 | 8 | 12 | 6 | |
| Premenopausal OC | |||||
| OR (95% CI) | 1.00 (referent) | 0.68 (0.24 to 1.95) | 1.05 (0.38 to 2.87) | 0.42 (0.14 to 1.24) | .35 |
| No. of controls | 21 | 20 | 20 | 21 | |
| No. of cases | 23 | 20 | 24 | 14 | |
| Postmenopausal OC | |||||
| OR (95% CI) | 1.00 (referent) | 1.68 (0.77 to 3.64) | 1.89 (0.87 to 4.12) | 2.92 (1.36 to 6.27) | .003 |
| No. of controls | 35 | 34 | 34 | 34 | |
| No. of cases | 21 | 28 | 33 | 54 | |
P trend was calculated with a two-sided Wald test as part of a logistic regression model of OC, with the SM sum as a continuous exposure, adjusted for risk factors (duration of oral contraceptive use [none or <3 months, 3 months to 3 years, 3 years to 5 years, or more than 5 years], tubal ligation [yes/no] and parity [no children, one child, two children, three children, four or more children]) and additionally for matching factors (cohort [NHS, NHSII]; menopausal status and hormone therapy use at blood draw [premenopausal, postmenopausal, and hormone therapy use; postmenopausal and no hormone therapy use; and missing or unknown]; menopausal status at diagnosis [premenopausal, postmenopausal, or unknown]; age [±1 year], date of blood collection [±1 month]; time of day of blood draw [±2 hours]; fasting status [>8 hours or ≤8 hours]) in subtype analyses. CI = confidence interval; NHS = Nurses' Health Study; NHSII = Nurses' Health Study II; OC = ovarian cancer; PD = poorly differentiated; SM = sphingomyelin.
Discussion
This is the first large-scale prospective study to examine the relationship of circulating LPCs, PCs, SMs, and CERs, previously hypothesized to be associated with ovarian cancer in experimental studies and retrospective studies comparing ovarian cancer patients to controls (3,4,7–24), with risk of ovarian cancer. Higher levels of SMs (SM sum) were statistically significantly (modified Bonferroni threshold) associated with a two-fold increased risk of ovarian cancer for an increase from the 10th to 90th percentile of the distribution. Whereas no individual metabolites met the Westfall and Young permutation threshold for statistical significance, higher levels of two specific SMs and a CER were suggestively associated with about a two-fold increased risk of ovarian cancer overall, with nominal P values ranging from .004 to .01. No statistically significant differences in association were observed by histotype, although, similar odds ratios were observed for serous/PD tumors as for overall ovarian cancer risk. One SM was suggestively associated with a 4.06-fold increased risk of endometrioid/CC tumors, whereas an LPC and the LPC:PC ratio were suggestively associated with a 0.2-fold lower risk of endometrioid/CC tumors for an increase in levels from the 10th to the 90th percentile, although the overall sample size was limited for this subtype. SMs and SM sum showed similar odds ratios when comparing tumors diagnosed soon after blood collection with tumors diagnosed later and when comparing rapidly fatal with less-aggressive tumors. Notably, more associations were observed among women who were postmenopausal at blood draw, with a nearly three-fold increased risk for the top vs bottom quartile of the SM sum, representing a potentially novel and strong risk factor.
The only other prospective study investigating these four groups of lipids assessed their associations with risk of three cancers (40). Similar to our results for ovarian cancer risk, positive associations, although not statistically significant, were observed for several SMs with risk of breast cancer (C16:0 SM, C18:0 SM, C20:2 SM, and C24:0; 4/7 measured SM) (40). Additionally, two of seven measured SMs were nominally positively associated with risk of prostate cancer, and five of seven SMs were nominally positively associated with colorectal cancer.
SMs are the most abundant class of sphingolipids in the cell, essential elements of the cell membrane, and critical players in cell function. SM are de novo synthesized from CERs and hydrolyzed into CERs, which are involved in cellular proliferation, growth, and apoptosis (41). SMs were higher in ovarian tumors compared with healthy tissue (31,42), and increased levels of SMs were also reported in taxol-resistant human ovarian cancer cell lines (43). Additionally, acid sphingomyelinase (the enzyme that converts SMs into CERs) has been associated with cisplatin resistance in ovarian tumors (44) and was lower in human ovarian cancer cells compared to human primary ovarian cells (45). A recent study showed that sphingomyelin in microdomains of the plasma membrane regulates amino acid–stimulated mTOR signal activation (46), which is essential for cell growth and proliferation (47). Another link between SMs and ovarian cancer is that statins, which can decrease circulating levels of SM (48), reduced serous intraepithelial carcinoma (STIC) development in ovarian cancer mouse models (49). Although a large study of the Danish cancer registry found no association of statin use with ovarian cancer risk overall, a suggestive inverse relationship was noted for mucinous tumors (50). However, statin use has been associated with improved survival (51). Our results did not change when excluding participants (n = 16) reporting statin or other cholesterol-lowering drugs from the main analysis (data not shown). Our data, in conjunction with the already existing literature, strongly support further examination of SMs as a risk factor for ovarian cancer, including larger nested case-control studies and biologic work to assess if this is a causal relationship or if SM levels reflect other biological processes involved in ovarian carcinogenesis.
Additionally, we observed several similar patterns of associations by lipid group, although not statistically significant, comparing our results with results from Kühn et al. (40). In addition to SMs, LPCs were nominally associated with lower risk of breast, colorectal, and prostate in that study and with both endometrioid/CC ovarian tumors and serous/PD ovarian tumors in our study; C18:0 LPC was a specific marker that was inversely associated with risk across multiple cancers. PCs were nominally associated with higher risk of colorectal, breast, and endometrioid ovarian tumors. These findings suggest that systematic lipid changes, many years before diagnosis, may be related to increased risk of several cancers; notably cancer cell lines and tumors have altered lipid profiles (52).
The current model for ovarian carcinogenesis distinguishes two tumor types. Type I carcinomas develop from benign precursor lesions (borderline or atypical proliferative tumors), whereas type II tumors develop from a STIC in the fallopian tube (53). Endometrioid/CC tumors are characterized as type I tumors and are associated with endometriosis (53). Interestingly, in a recent lipidomic study of endometrial fluid in women with ovarian endometriosis, lysophospholipids, calculated as the sum of LPCs and LPEs, were lower in endometriosis cases compared to healthy controls (54). Additionally, the lipidomics study found CERs to be down-regulated in endometriosis cases. In our analysis, two of the five measured CERs were associated with lower risk of endometrioid/CC and three CERs were associated with lower risk of ovarian cancer in premenopausal women, who were younger at blood collection, and represent the most similar subgroup to the women in the endometriosis study. Additionally, several LPCs were inversely associated with type I tumors in our study. Larger studies are needed to assess CERs and LPCs as potential risk biomarkers for endometrioid tumors, particularly in younger women.
Our study has several strengths and limitations. To the best of our knowledge, this is the first study assessing the associations of these lipids with ovarian cancer risk overall, by histotype and by menopausal status at blood collection. Given the low incidence rate of ovarian cancer, our study has a relatively large sample size with 252 ovarian cancer cases and 252 matched controls. However, we were still limited in some analyses, including examining primary associations for linear effects only and having limited power in the stratified analyses, possibly leading to an overestimation of effects. Strengths include the long follow-up time and detailed covariate information. Another limitation is represented by the one-point-in-time blood samples analyzed here. To address this limitation, we conducted a pilot study showing that the majority of the measured metabolites have a high within-person stability over time (intraclass correlation coefficient or Spearman correlations were higher than 0.65) (37).
In this study we found SMs to be associated with increased risk of ovarian cancer, particularly among postmenopausal women. We observed elevated levels of SMs in ovarian cancer cases 3–23 years before diagnosis. Additionally, C18:1 LPC and LPC:PC ratio were associated with lower risk of endometrioid/CC tumors. These results provide new and promising candidates for risk biomarkers. Experimental studies may help identify the mechanisms through which a dysregulated lipid metabolism supports carcinogenesis, potentially leading to the development of new therapeutic targets for prevention and treatment of ovarian cancer. Population studies are needed to validate these findings and further characterize their potential as risk biomarkers. If confirmed, these markers may be used to identify high-risk women who can benefit from preventive interventions. Additional studies are needed to identify biological predictors of circulating SMs, the underlying biological relationship with ovarian tumors and the ovaries, and, ultimately, how higher levels of circulating SMs may be involved in ovarian carcinogenesis.
Funding
This work was supported by the National Institutes of Health (P01 CA087969, U01 CA176726, UM1 CA186107) and the Department of Defense (W81XWH-12–1-0561).
Notes
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The authors have no conflicts of interest to report. The authors assume full responsibility for analyses and interpretation of these data.
We thank the participants and staff of the NHS and NHSII for their valuable contributions, as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, and WY.
Supplementary Material
References
- 1.American Cancer Society. Cancer Facts & Figures 2018. Atlanta: American Cancer Society; 2018. [Google Scholar]
- 2. Röhrig F, Schulze A.. The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. 2016;16(11):732.. [DOI] [PubMed] [Google Scholar]
- 3. Pyragius CE, Fuller M, Ricciardelli C, et al. Aberrant lipid metabolism: an emerging diagnostic and therapeutic target in ovarian cancer. IJMS. 2013;14(4):7742–7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tania M, Khan M, Song Y.. Association of lipid metabolism with ovarian cancer. Curr Oncol. 2010;17(5):6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Helzlsouer KJ, Alberg AJ, Norkus EP, et al. Prospective study of serum micronutrients and ovarian cancer . J Natl Cancer Inst. 1996;88(1):32–37. [DOI] [PubMed] [Google Scholar]
- 6. Melvin JC, Seth D, Holmberg L, et al. Lipid profiles and risk of breast and ovarian cancer in the Swedish AMORIS study. Cancer Epidemiol Biomarkers Prevent. 2012;21(8):1381–1384. [DOI] [PubMed] [Google Scholar]
- 7. Braicu EI, Darb-Esfahani S, Schmitt WD, et al. High-grade ovarian serous carcinoma patients exhibit profound alterations in lipid metabolism. Oncotarget. 2017;8(61):102912.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ke C, Hou Y, Zhang H, et al. Large‐scale profiling of metabolic dysregulation in ovarian cancer. Int J Cancer. 2015;136(3):516–526. [DOI] [PubMed] [Google Scholar]
- 9. Ke C, Li A, Hou Y, et al. Metabolic phenotyping for monitoring ovarian cancer patients. Sci Rep. 2016;6(1):23334.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu Y, Fang X, Casey G, et al. Lysophospholipids activate ovarian and breast cancer cells .Biochem J. 1995;309(3):933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fang X, Gaudette D, Furui T, et al. Lysophospholipid growth factors in the initiation, progression, metastases, and management of ovarian cancer. Ann NY Acad Sci. 2006;905(1):188–208. [DOI] [PubMed] [Google Scholar]
- 12. Fang X, Yu S, Bast RC, et al. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells . J Biol Chem. 2004;279(10):9653–9661. [DOI] [PubMed] [Google Scholar]
- 13. Sawada K, Morishige K-I, Tahara M, et al. Alendronate inhibits lysophosphatidic acid-induced migration of human ovarian cancer cells by attenuating the activation of rho. Cancer Res. 2002;62(21):6015–6020. [PubMed] [Google Scholar]
- 14. Cai Q, Zhao Z, Antalis C, et al. Elevated and secreted phospholipase A2 activities as new potential therapeutic targets in human epithelial ovarian cancer. FASEB J. 2012;26(8):3306–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tokumura A, Kume T, Fukuzawa K, et al. Peritoneal fluids from patients with certain gynecologic tumor contain elevated levels of bioactive lysophospholipase D activity. Life Sci. 2007;80(18):1641–1649. [DOI] [PubMed] [Google Scholar]
- 16. Fan L, Zhang W, Yin M, et al. Identification of metabolic biomarkers to diagnose epithelial ovarian cancer using a UPLC/QTOF/MS platform. Acta Oncol. 2012;51(4):473–479. [DOI] [PubMed] [Google Scholar]
- 17. Okita M, Gaudette DC, Mills GB, et al. Elevated levels and altered fatty acid composition of plasma lysophosphatidylcholine (lysoPC) in ovarian cancer patients. Int J Cancer.1997;71(1):31–34. [DOI] [PubMed] [Google Scholar]
- 18. Sedlakova I, Vavrova J, Tosner J, et al. Lysophosphatidic acid: an ovarian cancer marker. Eur J Gynaecol Oncol. 2008;29(5):511–514. [PubMed] [Google Scholar]
- 19. Sedlakova I, Vavrova J, Tosner J, et al. Lysophosphatidic acid (LPA)–a perspective marker in ovarian cancer. Tumour Biol. 2011;32(2):311–316. [DOI] [PubMed] [Google Scholar]
- 20. Shan L, Chen YA, Davis L, et al. Measurement of phospholipids may improve diagnostic accuracy in ovarian cancer. PLoS One. 2012;7(10):e46846.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sutphen R, Xu Y, Wilbanks GD, et al. Lysophospholipids are potential biomarkers of ovarian cancer. Cancer Epidemiol Biomarkers Prevent. 2004;13(7):1185–1191. [PubMed] [Google Scholar]
- 22. Xiao YJ, Schwartz B, Washington M, et al. Electrospray ionization mass spectrometry analysis of lysophospholipids in human ascitic fluids: comparison of the lysophospholipid contents in malignant vs nonmalignant ascitic fluids. Anal Biochem. 2001;290(2):302–313. [DOI] [PubMed] [Google Scholar]
- 23. Xu Y, Shen Z, Wiper DW, et al. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA. 1998;280(8):719–723. [DOI] [PubMed] [Google Scholar]
- 24. Zhang T, Wu X, Ke C, et al. Identification of potential biomarkers for ovarian cancer by urinary metabolomic profiling. J Proteome Res. 2013;12(1):505–512. [DOI] [PubMed] [Google Scholar]
- 25. Lin HY, Delmas D, Vang O, et al. Mechanisms of ceramide-induced COX-2-dependent apoptosis in human ovarian cancer OVCAR-3 cells partially overlapped with resveratrol. J Cell Biochem. 2013;114(8):1940–1954. [DOI] [PubMed] [Google Scholar]
- 26. Morita Y, Perez GI, Paris F, et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med. 2000;6(10):1109–1114. [DOI] [PubMed] [Google Scholar]
- 27. Tilly JL, Kolesnick RN.. Sphingolipids, apoptosis, cancer treatments and the ovary: Investigating a crime against female fertility. Biochim Biophys Acta. 2002;1585(2-3):135–138. [DOI] [PubMed] [Google Scholar]
- 28. Prinetti A, Millimaggi D, D’Ascenzo S, et al. Lack of ceramide generation and altered sphingolipid composition are associated with drug resistance in human ovarian carcinoma cells. Biochem J .2006;395(2):311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kitatani K, Usui T, Sriraman SK, et al. Ceramide limits phosphatidylinositol-3-kinase C2β-controlled cell motility in ovarian cancer: potential of ceramide as a metastasis-suppressor lipid. Oncogene. 2016;35(21):2801.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ogretmen B, Hannun YA.. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4(8):604–616. [DOI] [PubMed] [Google Scholar]
- 31. Fong MY, McDunn J, Kakar SS.. Identification of metabolites in the normal ovary and their transformation in primary and metastatic ovarian cancer. PLoS One. 2011;6(5):e19963.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hankinson SE, Willett WC, Michaud DS, et al. Plasma prolactin levels and subsequent risk of breast cancer in postmenopausal women .J Natl Cancer Inst.1999;91(7):629–634. [DOI] [PubMed] [Google Scholar]
- 33. Tworoger SS, Sluss P, Hankinson SE.. Association between plasma prolactin concentrations and risk of breast cancer among predominately premenopausal women. Cancer Res. 2006;66(4):2476–2482. [DOI] [PubMed] [Google Scholar]
- 34. Mascanfroni ID, Takenaka MC, Yeste A, et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat Med. 2015;21(6):638.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O’Sullivan JF, Morningstar JE, Yang Q, et al. Dimethylguanidinovaleric acid is a marker of liver fat and predicts diabetes. J Clin Invest. 2017;127(12):4394–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paynter NP, Balasubramanian R, Giulianini F, et al. Metabolic predictors of incident coronary heart disease in women. Circulation. 2018;137(8):841–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Townsend MK, Clish CB, Kraft P, et al. Reproducibility of metabolomic profiles among men and women in 2 large cohort studies. Clin Chem. 2013;59(11):1657–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Westfall PH, Young SS, Wright SP.. On adjusting P-values for multiplicity. Biometrics. 1993;49(3):941–945. [Google Scholar]
- 39. Gauderman WJ. Sample size requirements for matched case‐control studies of gene–environment interaction. Stat Med. 2002;21(1):35–50. [DOI] [PubMed] [Google Scholar]
- 40. Kühn T, Floegel A, Sookthai D, et al. Higher plasma levels of lysophosphatidylcholine 18:0 are related to a lower risk of common cancers in a prospective metabolomics study. BMC Med .2016;14(1):13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bienias K, Fiedorowicz A, Sadowska A, et al. Regulation of sphingomyelin metabolism. Pharmacol Rep. 2016;68(3):570–581. [DOI] [PubMed] [Google Scholar]
- 42. Kozar N, Kruusmaa K, Bitenc M, et al. Metabolomic profiling suggests long chain ceramides and sphingomyelins as a possible diagnostic biomarker of epithelial ovarian cancer. Clin Chim Acta. 2018;481:108–114. [DOI] [PubMed] [Google Scholar]
- 43. Huang H, Tong T-T, Yau L-F, et al. LC-MS based sphingolipidomic study on A2780 human ovarian cancer cell line and its taxol-resistant strain. Sci Rep. 2016;6(1):34684.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maurmann L, Belkacemi L, Adams N, et al. A novel cisplatin mediated apoptosis pathway is associated with acid sphingomyelinase and FAS proapoptotic protein activation in ovarian cancer. Apoptosis. 2015;20(7):960–974. [DOI] [PubMed] [Google Scholar]
- 45. Dai S, Liu J, Sun X, Wang N. Acid sphingomyelinase, a novel negative biomarker of ovarian cancer. Eur Rev Pharmacol Sci. 2015;19(11):2076–2083. [PubMed] [Google Scholar]
- 46. Zama K, Mitsutake S, Okazaki T, et al. Sphingomyelin in microdomains of the plasma membrane regulates amino acid‐stimulated mTOR signal activation. Cell Biol Int. 2018;42(7):823. [DOI] [PubMed] [Google Scholar]
- 47. Wullschleger S, Loewith R, Hall MN.. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. [DOI] [PubMed] [Google Scholar]
- 48. Schlitt A, Blankenberg S, Yan D, et al. Further evaluation of plasma sphingomyelin levels as a risk factor for coronary artery disease. Nutr Metabol. 2006;3(1):5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kobayashi Y, Kashima H, Wu R-C, et al. Mevalonate pathway antagonist suppresses formation of serous tubal intraepithelial carcinoma and ovarian carcinoma in mouse models. Clin Cancer Res. 2015;21(20):4652–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baandrup L, Dehlendorff C, Friis S, et al. Statin use and risk for ovarian cancer: a Danish nationwide case–control study. Br J Cancer. 2015;112(1):157.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Elmore RG, Ioffe Y, Scoles DR, et al. Impact of statin therapy on survival in epithelial ovarian cancer. Gynecol Oncol. 2008;111(1):102–105. [DOI] [PubMed] [Google Scholar]
- 52. Ray U, Roy SS.. Aberrant lipid metabolism in cancer cells–the role of oncolipid‐activated signaling. FEBS J. 2018;285(3):432–443. [DOI] [PubMed] [Google Scholar]
- 53. Kurman RJ, Shih I-M.. The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am J Pathol. 2016;186(4):733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Domínguez F, Ferrando M, Díaz-Gimeno P, et al. Lipidomic profiling of endometrial fluid in women with ovarian endometriosis. Biol Reprod. 2017;96(4):772–779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.