Abstract
Background
Potential benefits of screening mammography among women ages 75 years and older remain unclear.
Methods
We evaluated 10-year cumulative incidence of breast cancer and death from breast cancer and other causes by Charlson Comorbidity Index (CCI) and age in the Medicare-linked Breast Cancer Surveillance Consortium (1999–2010) cohort of 222 088 women with no less than 1 screening mammogram between ages 66 and 94 years.
Results
During median follow-up of 107 months, 7583 were diagnosed with invasive breast cancer and 1742 with ductal carcinoma in situ; 471 died from breast cancer and 42 229 from other causes. The 10-year cumulative incidence of invasive breast cancer did not change with increasing CCI but decreased slightly with age: ages 66–74 years (CCI0 = 4.0% [95% CI = 3.9% to 4.2%] vs CCI ≥ 2 = 3.9% [95% CI = 3.5% to 4.3%]); ages 75–84 years (CCI0 = 3.7% [95% CI = 3.5% to 3.9%] vs CCI ≥ 2 = 3.4% [95% CI = 2.9% to 3.9%]); and ages 85–94 years (CCI0 = 2.7% [95% CI = 2.3% to 3.1%] vs CCI ≥ 2 = 2.1% [95% CI = 1.3% to 3.0%]). The 10-year cumulative incidence of other-cause death increased with increasing CCI and age: ages 66–74 years (CCI0 = 10.4% [95% CI = 10.3 to 10.7%] vs CCI ≥ 2 = 43.4% [95% CI = 42.2% to 44.4%]), ages 75–84 years (CCI0 = 29.8% [95% CI = 29.3% to 30.2%] vs CCI ≥ 2 = 61.7% [95% CI = 60.2% to 63.3%]), and ages 85 to 94 years (CCI0 = 60.3% [95% CI = 59.1% to 61.5%] vs CCI ≥ 2 = 84.8% [95% CI = 82.5% to 86.9%]). The 10-year cumulative incidence of breast cancer death was small and did not vary by age: ages 66–74 years = 0.2% (95% CI = 0.2% to 0.3%), ages 75–84 years = 0.29% (95% CI = 0.25% to 0.34%), and ages 85 to 94 years = 0.3% (95% CI = 0.2% to 0.4%).
Conclusions
Cumulative incidence of other-cause death was many times higher than breast cancer incidence and death, depending on comorbidity and age. Hence, older women with increased comorbidity may experience diminished benefit from continued screening.
Because of the heterogeneity in health status and life expectancy among older women, defined as age 65 years and older, the margin of benefit from screening mammography varies widely (1–3). Evidence exists that women with multiple consequential comorbidities are in fact more likely to undergo screening mammography than their counterparts without comorbidity—an indication of greater access to care—even though they may not experience a net benefit from screening (4,5). In a systematic review of observational studies and decision analyses, we have found that healthy older women with favorable life expectancies may indeed benefit from continued screening mammography unlike those with major comorbidity (2).
Given the long natural history of breast cancer in older women and increased risk of non–breast cancer death with aging, there may be a point when older women may not live long enough to benefit from screening mammography (1,2,6–8). Moreover, because rates of slow-growing tumors increase with age, older women with limited life expectancies may experience less benefit from screening and a greater likelihood of harms from overdiagnosis and overtreatment (1,9–11), including a potential inability to tolerate or complete treatment regimens (12,13). Perhaps reflecting this uncertainty, professional guidelines vary in their recommendations about upper age limits for screening cessation. For example, the American Cancer Society (ACS) recommends stopping screening when life expectancy is less than 10 years (14), whereas the US Preventive Services Task Force (USPSTF) states that there is insufficient evidence to recommend for or against screening women ages 75 years and older (15); most European screening programs stop inviting women for screening between ages 69 and 74 years (16) .
Overall, there is a paucity of empirical evidence on long-term risk of breast cancer juxtaposed with death from breast cancer and other non–breast cancer-related causes according to comorbidity and age among older women undergoing screening mammography (1,2). As a critical step toward informing clinical decisions in this area, our objective in this study was to determine the 10-year cumulative incidence of breast cancer vs death from causes other than breast cancer (hereafter referred to as other-cause death) according to comorbidity and age in the Medicare-linked Breast Cancer Surveillance Consortium (BCSC), a population-based cohort of women undergoing breast imaging in US clinical practice settings.
The institutional review board at Georgetown University approved this study.
Methods
Inclusion and Exclusion Criteria
The sample included women ages 66–94 years without a history of breast cancer who underwent screening mammography between 1999 and 2010 in one of the five mammography registries participating in the Medicare-linked BCSC database: Carolina Mammography Registry, Kaiser Permanente Washington Registry, New Hampshire Mammography Network, San Francisco Mammography Registry, and Vermont Breast Cancer Surveillance System (https://www.bcsc-research.org/). We used the first eligible screening mammogram for each woman included in the sample. Claims data from Kaiser Permanente Washington and Medicare claims data from the other four registries were linked to mammography data. Because comorbidity is computed from claims data within the year prior to the screening mammogram, and Medicare eligibility begins at age 65 years, women included in our sample were at least age 66 years at the time of the screen. Based on previously described methods (17,18), we included women with continuous enrollment in fee-for-service Medicare (parts A and B) for 12 months before and after mammography with no enrollment in a Medicare Advantage plan during this period, which allows for longitudinal assessment of outpatient claims to better understand potential patient comorbidity burden.
The BCSC was developed as a collaborative research network of mammography registries to characterize the population of women undergoing screening mammography in the United States. BCSC registries collect information on demographics, risk factors, clinical history, pathology reports, and mammography indication and results (19). Breast cancer diagnoses and tumor characteristics are obtained by linking BCSC data to pathology services, regional Surveillance, Epidemiology, and End Results (SEER) programs, and/or state tumor registries. Data are pooled at a central Statistical Coordinating Center (SCC). Each registry and the SCC have received institutional review board approval for active or passive consenting processes or a waiver of consent to enroll participants, link data, and perform analytic studies. All procedures are Health Insurance Portability and Accountability Act (HIPAA) compliant, and all registries and the SCC have received a Federal Certificate of Confidentiality and other protection for the identities of women, physicians, and facilities that are subjects of this research.
Exposure and Outcomes of Interest
The primary exposure of interest was the Charlson Comorbidity Index (CCI), which is a weighted index that predicts 1-year risk of death using Medicare Part B procedure and diagnostic claims data (20,21). CCI is derived from the sum of weighted conditions (given scores of 1, 2, or 3) based on diagnoses with any of 16 disease conditions using hospital and physician claims data (22). We used the 2000 National Cancer Institute’s Comorbidity Index developed by Klabunde et al. (22).
CCI scores were computed using SAS macros provided by the National Cancer Institute (https://healthcaredelivery.cancer.gov/seermedicare/considerations/comorbidity.html), based on claims data during the year before the screening mammogram. We categorized CCI scores into 0, 1, or no less than 2. The methods for identifying disease conditions and scoring using CCI in the BCSC-Medicare linked data have been described in prior literature (23).
The primary outcomes of interest were incident breast cancer (invasive or ductal carcinoma in situ [DCIS]) diagnoses and other-cause death. Incident invasive breast cancer and DCIS were treated as separate outcomes of incident breast cancer. We modeled time to first breast cancer diagnosis or other-cause death; thus, the other events were treated as competing risks. DCIS was treated as a competing event given that women with a history of DCIS undergo surveillance mammography, not screening mammography. Breast cancer–related death was examined in a secondary analysis. Vital status and cause of death were obtained from SEER and state cancer registries and state vital records.
Statistical Analysis
Demographic (age, race and/or ethnicity, self-reported education level, first-degree family history of breast cancer) and mammography characteristics (time since last mammogram, breast density using Breast Imaging Reporting and Data System classification) were compared by CCI category and outcome. The 10-year cumulative incidence functions for breast cancer incidence and death as well as other-cause death were estimated while accounting for competing risks (24). In the primary analysis, women were followed from date of first screening mammogram at ages 66 years and older or until the earliest of the following outcomes: breast cancer diagnosis (first diagnosis of invasive breast cancer or DCIS), other-cause death, end of complete cancer and vital status data, or 10 years after screening. In a secondary analysis of breast cancer death, we did not treat breast cancer diagnosis as a competing risk, and we followed women from date of first screening mammogram to breast cancer death, other-cause death (competing risk), end of complete cancer or vital status data, or 10 years after screening. Models were estimated separately for each outcome and age group. Fine and Gray proportional subdistribution hazards models for competing risks were also fitted for each outcome and age group including CCI as a covariate (24). Sensitivity analyses that adjusted for BCSC registry as a fixed effect in the Fine and Gray regression models and estimated cumulative incidence functions weighted by the overall frequency distribution of the BCSC registries were also conducted. Confidence intervals (CI) were calculated via bootstrapping by using the 2.5th and 97.5th percentiles from 1000 random bootstrap samples.
Results
We identified 222 088 women ages 66 to 94 years who underwent screening mammography and were eligible for analysis (Table 1 and Figure 1). We compared participant characteristics across CCI categories. Overall, participants were mostly white (84.8%), had a high school diploma or less (50.7%), reported no family history of breast cancer (82.5%), and had scattered fibroglandular density in their breast tissue (54.6%). Approximately 25.9% of the women in the study had a CCI score of no less than 1. Women with a CCI score of 0 were proportionately more likely to be white and a college graduate and have received a mammogram 1–2 years prior to study entry. Women with a CCI score of no less than 2 were proportionately more likely to be older, non-Hispanic black and have less than high school diploma and a prior mammogram no less than 2 years ago.
Table 1.
Characteristics of 222 088 older women who underwent screening mammography between 1999 and 2010 in the Breast Cancer Surveillance Consortium by Charlson Comorbidity Index
| Characteristics | Overall, No. (%)* | Charlson Comorbidity Index, No. (%)* |
||
|---|---|---|---|---|
| 0 | 1 | ≥2 | ||
| Total number of women | 222 088 | 164 576 (74.1)† | 42 234 (19.0)† | 15 278 (6.9)† |
| Age group, y | ||||
| 66–74 | 154 784 (69.7) | 117 299 (75.8) | 28 143 (18.2) | 9342 (6.0) |
| 75–84 | 57 859 (26.1) | 40 824 (70.6) | 12 109 (20.9) | 4926 (8.5) |
| 85–94 | 9445 (4.3) | 6453 (68.3) | 1982 (21.0) | 1010 (10.7) |
| Race/ethnicity | ||||
| White, non-Hispanic | 171 189 (84.8) | 130 428 (76.2) | 30 391 (17.8) | 10 370 (6.1) |
| Black, non-Hispanic | 16 218 (8.0) | 9498 (58.6) | 4631 (28.6) | 2089 (12.9) |
| Hispanic | 3754 (1.9) | 2672 (71.2) | 794 (21.2) | 288 (7.7) |
| Asian/Pacific Islander | 7965 (3.9) | 5705 (71.6) | 1739 (21.8) | 521 (6.5) |
| Other | 2645 (1.3) | 1809 (68.4) | 580 (21.9) | 256 (9.7) |
| Missing | 20 317 (9.1) | 14 464 (71.2) | 4099 (20.2) | 1754 (8.6) |
| Education | ||||
| <High school graduate | 30 337 (16.9) | 19 256 (63.5) | 7877 (26.0) | 3204 (10.6) |
| High school graduate or GED | 60813 (33.8) | 44 558 (73.3) | 12 138 (20.0) | 4117 (6.8) |
| Some college or technical school | 44 143 (24.5) | 34 143 (77.3) | 7509 (17.0) | 2491 (5.6) |
| College graduate | 44 530 (24.8) | 36 501 (82.0) | 6122 (13.7) | 1907 (4.3) |
| Missing | 42 265 (19.0) | 30 118 (71.3) | 8 588 (20.3) | 3559 (8.4) |
| Time since prior mammogram | ||||
| No prior mammogram | 6300 (3.1) | 4244 (67.4) | 1406 (22.3) | 650 (10.3) |
| <2 years | 169 787 (82.4) | 128 650 (75.8) | 31 016 (18.3) | 10 121 (6.0) |
| ≥2 years | 30 111 (14.6) | 20 616 (68.5) | 6454 (21.4) | 3041 (10.1) |
| Missing | 15 890 (7.2) | 11 066 (69.6) | 3358 (21.1) | 1466 (9.2) |
| BI-RADs breast density | ||||
| Almost entirely fat | 22 008 (11.1) | 15 312 (69.6) | 4860 (22.1) | 1836 (8.3) |
| Scattered fibroglandular densities | 107 890 (54.6) | 78 660 (72.9) | 21 477 (19.9) | 7753 (7.2) |
| Heterogeneously dense | 61 370 (31.1) | 47 013 (76.6) | 10 591 (17.3) | 3 766 (6.1) |
| Extremely dense | 6284 (3.2) | 4993 (79.5) | 959 (15.3) | 332 (5.3) |
| Missing | 24 536 (11.0) | 18 598 (75.8) | 4347 (17.7) | 1591 (6.5) |
| First-degree family history of breast cancer | ||||
| No | 145 928 (82.5) | 108 147 (74.1) | 27 654 (19.0) | 10 127 (6.9) |
| Yes | 30 990 (17.5) | 23 055 (74.4) | 5697 (18.4) | 2238 (7.2) |
| Missing | 45 170 (20.3) | 33 374 (73.9) | 8 883 (19.7) | 2913 (6.4) |
| Mode of detection‡ | ||||
| Screen detected | 6049 (75.0) | 4626 (76.5) | 1047 (17.3) | 376 (6.2) |
| Non-screen detected | 2014 (25.0) | 1468 (72.9) | 405 (20.1) | 141 (7.0) |
| Missing | 1262 (13.5) | 966 (76.5) | 235 (18.6) | 61 (4.8) |
Percentages are based on the row unless otherwise indicated. BI-RADS = Breast Imaging Reporting and Data System.
Percentages are based on the column.
Based on women with a diagnosis of invasive breast cancer or ductal carcinoma in situ during follow-up.
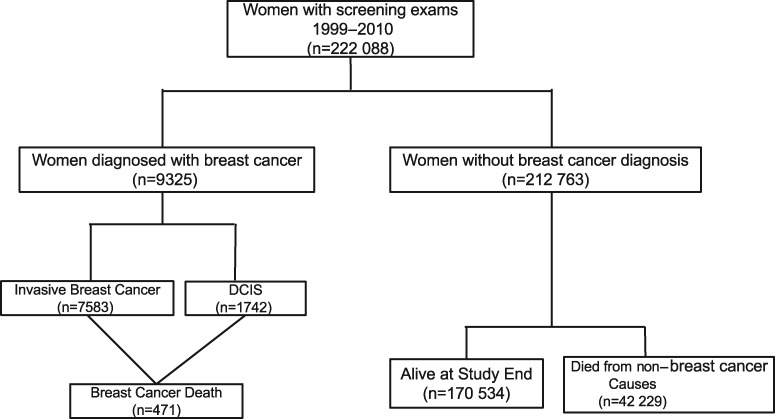
Figure 1.
Flowchart of study population. In the primary analysis, end of follow-up occurred at the earliest of breast cancer diagnosis (first diagnosis of invasive BC or DCIS), non–breast cancer-related death, last date of complete capture of cancer or vital status data, or 10 years after the screening mammogram. In the secondary analysis, end of follow-up occurred at the earliest of breast cancer death, non–breast cancer-related death, last date of complete capture of cancer or vital status data, or 10 years after the screening mammogram. BC = breast cancer; DCIS = ductal carcinoma in situ.
Table 2 compares baseline demographic characteristics and CCI by outcome status at the end of study follow-up. Women were followed for a median of 107 months (interquartile range [IQR] = 65–120). By the end of follow-up, 9325 women were diagnosed with breast cancer (7583 invasive and 1742 DCIS), 471 women died from breast cancer, and 42 229 from other causes (Figure 1). Women diagnosed with invasive breast cancer were older than those alive at the end of follow-up. Women who died from causes other than breast cancer were older, had a higher CCI score, and were more likely to have less than a high school education compared to women alive at the end of follow-up without a breast cancer diagnosis.
Table 2.
Characteristics of 222 088 older women at index screening mammogram in the BCSC (1999–2010) by status at the end of follow-up
| Characteristics | Overall, No. (%)* | Status at end of follow-up, No. (%)* |
|||
|---|---|---|---|---|---|
| Alive | Died from other causes | Invasive breast cancer | DCIS | ||
| Total | 222 088 | 170 534 | 42 229 | 7583 | 1742 |
| Follow-up time in months (median, IQR) | 107.0 (65.00, 120.0) | 120.0 (83.00, 120.0) | 65.00 (37.00, 91.00) | 38.00 (12.00, 71.00) | 36.00 (12.00, 64.00) |
| Characteristics at screen | |||||
| Charlson Comorbidity Index | |||||
| 0 | 164 576 (74.1) | 133 173 (78.1) | 24 343 (57.6) | 5685 (75.0) | 1375 (78.9) |
| 1 | 42 234 (19.0) | 29 191 (17.1) | 11 356 (26.9) | 1416 (18.7) | 271 (15.6) |
| ≥2 | 15 278 (6.9) | 8170 (4.8) | 6530 (15.5) | 482 (6.4) | 96 (5.5) |
| Age group, y | |||||
| 65–69 | 103 979 (46.8) | 90 648 (53.2) | 9031 (21.4) | 3406 (44.9) | 894 (51.3) |
| 70–74 | 50 805 (22.9) | 39 661 (23.3) | 8782 (20.8) | 1937 (25.5) | 425 (24.4) |
| 75–79 | 37 385 (16.8) | 25 373 (14.9) | 10 422 (24.7) | 1315 (17.3) | 275 (15.8) |
| 80–84 | 20 474 (9.2) | 11 296 (6.6) | 8382 (19.8) | 682 (9.0) | 114 (6.5) |
| 85–94 | 9445 (4.3) | 3556 (2.1) | 5612 (13.3) | 243 (3.2) | 34 (2.0) |
| Race/ethnicity | |||||
| White, non-Hispanic | 171 189 (84.8) | 132 353 (84.7) | 31 343 (84.9) | 6142 (87.6) | 1351 (83.9) |
| Black, non-Hispanic | 16 218 (8.0) | 11 943 (7.6) | 3613 (9.8) | 524 (7.5) | 138 (8.6) |
| Hispanic | 3754 (1.9) | 3049 (2.0) | 581 (1.6) | 97 (1.4) | 27 (1.7) |
| Asian/Pacific Islander | 7965 (3.9) | 6778 (4.3) | 951 (2.6) | 162 (2.3) | 74 (4.6) |
| Other | 2645 (1.3) | 2095 (1.3) | 443 (1.2) | 87 (1.2) | 20 (1.2) |
| Missing | 20 317 (9.1) | 14 316 (8.4) | 5298 (12.5) | 571 (7.5) | 132 (7.6) |
| Education | |||||
| <High school graduate | 30 337 (16.9) | 20 888 (15.0) | 8312 (25.5) | 954 (15.1) | 183 (12.3) |
| High school graduate or GED | 60 813 (33.8) | 46 301 (33.2) | 11 959 (36.7) | 2049 (32.4) | 504 (34.0) |
| Some college or technical school | 44 143 (24.5) | 35 342 (25.4) | 6781 (20.8) | 1659 (26.2) | 361 (24.3) |
| College graduate | 44 530 (24.8) | 36 870 (26.4) | 5565 (17.1) | 1659 (26.2) | 436 (29.4) |
| Missing | 42 265 (19.0) | 31 133 (18.3) | 9612 (22.8) | 1262 (16.6) | 258 (14.8) |
| Time since prior mammogram | |||||
| No prior mammogram | 6300 (3.1) | 4011 (2.5) | 1985 (5.2) | 262 (3.7) | 42 (2.6) |
| <2 years | 169 787 (82.4) | 133 803 (84.0) | 28 736 (75.0) | 5852 (82.6) | 1396 (85.4) |
| ≥2 years | 30 111 (14.6) | 21 379 (13.4) | 7565 (19.8) | 969 (13.7) | 198 (12.1) |
| Missing | 15 890 (7.2) | 11 341 (6.7) | 3943 (9.3) | 500 (6.6) | 106 (6.1) |
| BI-RADS breast density | |||||
| Almost entirely fat | 22 008 (11.1) | 17 148 (11.3) | 4356 (11.6) | 396 (5.9) | 108 (7.1) |
| Scattered fibroglandular densities | 107 890 (54.6) | 82 902 (54.6) | 20 686 (55.2) | 3504 (52.4) | 798 (52.3) |
| Heterogeneously dense | 61 370 (31.1) | 47 096 (31.0) | 11 179 (29.9) | 2533 (37.9) | 562 (36.8) |
| Extremely dense | 6284 (3.2) | 4746 (3.1) | 1229 (3.3) | 250 (3.7) | 59 (3.9) |
| Missing | 24 536 (11.0) | 18 642 (10.9) | 4779 (11.3) | 900 (11.9) | 215 (12.3) |
| First-degree family history of breast cancer | |||||
| No | 145 928 (82.5) | 115 193 (82.9) | 25 088 (82.1) | 4557 (76.8) | 1090 (77.3) |
| Yes | 30 990 (17.5) | 23 818 (17.1) | 5476 (17.9) | 1376 (23.2) | 320 (22.7) |
| Missing | 45 170 (20.3) | 31 523 (18.5) | 11 665 (27.6) | 1650 (21.8) | 332 (19.1) |
Percentages are column percentages based on nonmissing values. BCSC = Breast Cancer Surveillance Consortium; BI-RADS = Breast Imaging Reporting and Data System; DCIS = ductal carcinoma in situ; GED = General Educational Development; IQR = interquartile range.
The 10-year cumulative incidence of breast cancer (both invasive breast cancer and DCIS), death from causes other than breast cancer, and breast cancer death stratified by age and CCI group are shown in Tables 3 and 4, respectively. Overall, the 10-year cumulative incidence of invasive breast cancer following a screening mammogram decreased with increasing age: Specifically, cumulative incidence decreased from 4.0% (95% CI = 3.9% to 4.1%) in women ages 66–74 years to 3.6% (95% CI = 3.5% to 3.8%) in women ages 75–84 years and finally to 2.7% (95% CI = 2.4% to 3.0%) in those ages 85–94 years. Notably, cumulative incidence of invasive breast cancer did not vary by levels of CCI among those ages 66–74 years (CCI0 = 4.0% [95% CI = 3.9% to 4.2%] vs CCI ≥ 2 = 3.9% [95% CI = 3.5% to 4.3%]); ages 75–84 years (CCI0 = 3.7% [95% CI = 3.5% to 3.9%] vs CCI ≥ 2 = 3.4% [95% CI = 2.9% to 3.9%]); and ages 85–94 years (CCI0 = 2.7% [95% CI = 2.3% to 3.1%] vs CCI ≥ 2 = 2.1% [95% CI = 1.3% to 3.0%]).
Table 3.
Ten-year cumulative incidence of incident breast cancer, non–breast cancer-related death, and breast cancer death stratified by age*
| Outcome | No. of events | 10-year risk by age group, % (95% CI) |
||
|---|---|---|---|---|
| 66–74 y | 75–84 y | 85–94 y | ||
| Invasive breast cancer | 7583 | 4.0 (3.9 to 4.1) | 3.6 (3.5 to 3.8) | 2.7 (2.4 to 3.0) |
| DCIS | 1742 | 1.0 (0.9 to 1.0) | 0.7 (0.6 to 0.8) | 0.4 (0.3 to 0.5) |
| Death from non–breast cancer causes | 42 229 | 14.5 (14.3 to 14.8) | 35.7 (35.3 to 36.1) | 65.4 (64.3 to 66.5) |
| Death from breast cancer | 471 | 0.2 (0.2 to 0.3) | 0.29 (0.25 to 0.34) | 0.3 (0.2 to 0.4) |
A separate model was fit for each age group and outcome. CI = confidence interval; DCIS = ductal carcinoma in situ.
Table 4.
Ten-year cumulative incidence of incident invasive breast cancer, non–breast cancer-related death, and breast cancer death, stratified by age and Charlson Comorbidity Index*
| Outcome, CCI level | No. of events | 10-year risk by age group, % (95% CI) |
||
|---|---|---|---|---|
| 66–74 y | 75–84 y | 85–94 y | ||
| 10-year risk of invasive breast cancer† | 7583 | |||
| CCI = 0 | 4.0 (3.9 to 4.2) | 3.7 (3.5 to 3.9) | 2.7 (2.3 to 3.1) | |
| CCI = 1 | 4.0 (3.7 to 4.2) | 3.4 (3.1 to 3.7) | 2.9 (2.2 to 3.7) | |
| CCI ≥ 2 | 3.9 (3.5 to 4.3) | 3.4 (2.9 to 3.9) | 2.1 (1.3 to 3.0) | |
| 10-year risk of non–breast cancer-related death | 42 229 | |||
| CCI = 0 | 10.4 (10.3 to 10.7) | 29.8 (29.3 to 30.2) | 60.3 (59.1 to 61.5) | |
| CCI = 1 | 22.5 (21.9 to 23.1) | 46.0 (45.1 to 47.0) | 72.8 (70.6 to 74.7) | |
| CCI ≥ 2 | 43.4 (42.2 to 44.4) | 61.7 (60.2 to 63.3) | 84.8 (82.5 to 86.9) | |
| 10-year risk of breast cancer death | 471 | |||
| CCI = 0 | 0.2 (0.2 to 0.3) | 0.3 (0.2 to 0.3) | — | |
| CCI = 1 | 0.3 (0.2 to 0.4) | 0.4 (0.3 to 0.5) | — | |
| CCI ≥ 2 | 0.3 (0.2 to 0.4) | 0.3 (0.2 to 0.5) | — | |
Based on models that adjust for Charlson Comorbidity Index (CCI), a separate model was fit for each age group and outcome of interest. CI = confidence interval; — = not computed because of small sample size.
Includes women who may die of breast cancer sometime after their diagnosis.
Overall, cumulative incidence of DCIS decreased slightly with increasing age. Specifically, it decreased from 1.0% (95% CI = 0.9% to 1.0%) in women ages 66–74 years to 0.7% (95% CI = 0.6% to 0.8%) in those aged 75–84 years, and finally to 0.4% (95% CI = 0.3% to 0.5%) among their counterparts ages 85–94 years. The association between cumulative incidence of DCIS and CCI could not be examined within each age group because of low numbers of DCIS cases within each age and CCI category.
Overall, among women who were not diagnosed with breast cancer, cumulative incidence of other-cause death increased with increasing age. It increased from 14.5% (95% CI = 14.3% to 14.8%) among women ages 66–74 years to 35.7% (95% CI = 35.3% to 36.1%) in those ages 75–84 years through to 65.4% (95% CI = 64.3% to 66.5%) in those ages 85–94 years. When stratifying by CCI score, cumulative incidence of other-cause death increased with increasing age and comorbidity among women ages 66–74 years (CCI0 = 10.4% [95% CI = 10.3% to 10.7%] vs CCI ≥2 = 43.4% [95% CI = 42.2% to 44.4%]); ages 75–84 years (CCI0 = 29.8% [95% CI = 29.3% to 30.2%] vs CCI ≥2 = 61.7% [95% CI = 60.2% to 63.3%]); and ages 85–94 years (CCI0 = 60.3% [95% CI = 59.1% to 61.5%] vs CCI ≥2 = 84.8% [95% CI = 82.5% to 86.9%]).
Overall, cumulative incidence of breast cancer death was very small and did not vary by age. It increased from 0.24% (95% CI = 0.21% to 0.27%) in women ages 66–74 years to 0.29% (95% CI = 0.25% to 0.34%) in those ages 75–84 years to 0.31% (95% CI = 0.21% to 0.43%) in those ages 85–94 years. Cumulative incidence of breast cancer death increased slightly with increasing comorbidity among women ages 66–74 years (CCI0 = 0.2% [95% CI = 0.2% to 0.3%] vs CCI ≥2 = 0.3% [95% CI = 0.2% to 0.4%]), but not among women ages 75–84 years (CCI0 = 0.3% [95% CI = 0.2% to 0.3%] vs CCI ≥2 = 0.3% [95% CI = 0.2% to 0.5%]).
Sensitivity analyses that further adjusted for mammography registry showed similar results (data shown in Supplementary Tables 2 and 3, available online).
Discussion
We used Medicare-linked data from the population-based BCSC cohort to determine cumulative incidence of invasive breast cancer and death as well as other-cause death among older women following a screening mammogram. Cumulative incidence of other-cause death was many times higher than the breast cancer incidence and death, depending on comorbidity and age. Specifically, women ages 85–94 years have an approximately 24-fold greater likelihood of dying from other causes than being diagnosed with invasive breast cancer. Notably, only about 5% of breast cancer cases died from their breast cancer within 10 years of the screening mammogram. Our findings address a key information gap that was highlighted in a prior systematic review examining harms and benefits of screening mammography in older women by comorbidity and age (2). Our data extend the research conducted by two of the studies within the systematic review (2) by empirically contextualizing the comparative risks of invasive breast cancer diagnosis vs other-cause death and highlighting the diminished potential benefit of screening mammography in older women, particularly among those with major comorbid conditions (5,23).
Our findings are consistent with recent SEER data showing breast cancer incidence decreasing after age 75 years (25). In contrast to prior studies that have identified older age as a key risk factor for developing DCIS (11,26–29), whose incidence increases with aging, our study found a lower risk of DCIS with advancing age. We observed a U-shaped association of advancing age with DCIS and incident invasive breast cancer, as incidence increased to age 75 years and then decreased afterward. This could be the result of examining a study population of older women who have undergone regular routine screening mammography and could be an indication of the capability of screening among older women to reduce absolute risk of breast cancer earlier before competing comorbidities arise.
Our analyses separated invasive and DCIS breast cancer cases to highlight the potential concerns around overdiagnosis and overtreatment when screening older women. Notably, DCIS treatment has not been shown to have a direct survival benefit, despite a similar burden of treatment to invasive breast cancer (30,31). DCIS cases make up about 19% of all cancer cases in our study population, and prior studies have shown that increased screening has led to increased numbers of DCIS cases (29,32).This is particularly concerning when prior studies indicate that women with limited life expectancies are being screened at high rates (4,5). A study of the Screening Mammography Program of British Columbia found that the risk of overdiagnosis of both invasive breast cancer and DCIS was elevated among women ages 60 years and older compared to younger women (33). The prospect of overdiagnosis related to screening mammography when life expectancy is limited highlights the critical need to identify an evidence-based stopping point for screening when no net clinically meaningful margin of benefit is possible.
Whereas cumulative incidence of invasive breast cancer showed no relationship with the CCI score, increased CCI score was associated with other-cause death in our study. The association of CCI with breast cancer death was more complex and depended on age; higher CCI was associated with an increased likelihood of breast cancer death for those ages 66 to 74 years but was unrelated to breast cancer death for those ages 75 years and older. These findings overall align with our prior work that showed no association between higher comorbidity burden and breast cancer stage at diagnosis (23). The pattern of findings observed here could be due to all women in our population having screening mammography, only about 26% of whom had a CCI score no less than 1, which indicates a low comorbidity burden compared to other studies examining cancer screening outcomes in the elderly (34,35), including a study that reported on a 5% random sample of the Medicare population (36). Also, our study looked at overall 10-year breast cancer cumulative incidence and death among women with at least one screening mammogram, rather than by stage of disease, which may be less affected by comorbidity burden. Thus, although the BCSC is a large, diverse population–based sample of women undergoing screening mammography in the United States (19), a fairly low comorbidity burden in this population might lead to underestimating the potential impact of comorbidity on breast cancer outcomes and, possibly, also other-cause death.
Our study had some limitations. Despite our large sample, the relatively small number of DCIS cases limited our ability to perform deeper DCIS-specific analyses and to stratify our findings by CCI. Because we studied a screening population by design, we were unable to compare outcomes between screened vs nonscreened women. Although the BCSC data reflect high-quality ascertainment of screening mammography that minimizes misclassification of screening vs diagnostic mammograms, using an entirely screening population might overrepresent the true risk of breast cancer and underrepresent the risk of other-cause death in the general population, particularly given the relatively low comorbidity burden within this population. Despite these limitations, our study had major strengths. The BCSC is the largest population-based and socioeconomically and ethnically diverse prospective cohort study of women undergoing screening mammography in the United States. Additionally, BCSC registries follow most women for more than 10 years, enabling the ascertainment of cancer diagnoses and mortality. Our design allowed us to estimate cumulative incidence of incident breast cancer juxtaposed with breast cancer and other-cause death within a 100% screening population, which is a primary assumption of models that inform the current breast cancer screening guidelines in the United States (37).
In conclusion, with advancing age, there was a decrease in cumulative incidence of invasive breast cancer and DCIS and no marked change in risk of breast cancer death irrespective of comorbidity. On the other hand, cumulative incidence of other-cause death markedly increased with advancing age and comorbidity. In light of these findings, older women with major comorbidity and advanced age may experience diminished benefit from continuing routine screening mammography. Further research is needed to characterize appropriate stopping ages for screening mammography based on combinations of age and health status (38).
Funding
This research was supported by grant 1R01CA207361-01A1 from the National Cancer Institute (DB). Data collection for this work was additionally supported by the Breast Cancer Surveillance Consortium with funding from the National Cancer Institute (P01CA154292, U54CA163303, R01CA149365).
Notes
All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent views of the National Cancer Institute or National Institutes of Health.
The sponsor did not have a role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The authors have no conflicts of interest to disclose.
We thank the Breast Cancer Surveillance Consortium and the study participants. The collection of cancer and vital status data was supported in part by state public health departments and cancer registries throughout the United States (https://www.bcsc-research.org/about/work-acknowledgement).
Information about the data used in this study is available from the Breast Cancer Surveillance Consortium. Restrictions apply to the availability of these data (https://www.bcsc-research.org/).
Concept and design: JD, DLM, KK, DB. Analysis and interpretation of data: JD, LA, DLM, ESO, DSMB, KK, BLS, DB. Drafting of manuscript: JD, DB. Critical revision of the manuscript for important intellectual content: JD, LA, SA, DLM, BLS, ESO, LMH, TO, DSMB, JTS, LCW, KK, DB. Statistical analysis: LA, DLM. Obtained funding: DB, DLM, KK.
Supplementary Material
References
- 1. Braithwaite D, Demb J, Henderson LM.. Optimal breast cancer screening strategies for older women: current perspectives. Clin Interv Aging. 2016;11:111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braithwaite D, Walter LC, Izano M, Kerlikowske K.. Benefits and harms of screening mammography by comorbidity and age: a qualitative synthesis of observational studies and decision analyses. J Gen Intern Med. 2016;31(5):561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McPherson CP, Swenson KK, Lee MW.. The effects of mammographic detection and comorbidity on the survival of older women with breast cancer. J Am Geriatr Soc. 2002;50(6):1061–1068. [DOI] [PubMed] [Google Scholar]
- 4. Royce TJ, Hendrix LH, Stokes WA, Allen IM, Chen RC.. Cancer screening rates in individuals with different life expectancies. JAMA Intern Med. 2014;174(10):1558–1565. doi: 10.1001/jamainternmed.2014.3895. [DOI] [PubMed] [Google Scholar]
- 5. Yasmeen S, Hubbard RA, Romano PS, et al. Risk of advanced-stage breast cancer among older women with comorbidities. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1510–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wood RY, Giuliano KK, Liu LM.. Challenges in mammography screening for older women. Nurse Pract. 2004;29(11):12–13. [DOI] [PubMed] [Google Scholar]
- 7. Kerlikowske K, Salzmann P, Phillips KA, Cauley JA, Cummings SR.. Continuing screening mammography in women aged 70 to 79 years: impact on life expectancy and cost-effectiveness. JAMA. 1999;282(22):2156–2163. [DOI] [PubMed] [Google Scholar]
- 8. Walter LC, Schonberg MA.. Screening mammography in older women: a review. JAMA. 2014;311(13):1336–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151(10):738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Cancer Society. Breast Cancer Facts and Figures 2017-2018 Atlanta, GA; ACS; 2017. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2017-2018.pdf. Accessed June 28, 2018.
- 11. Virnig BA, Tuttle TM, Shamliyan T, Kane RL.. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102(3):170–178. [DOI] [PubMed] [Google Scholar]
- 12. Meneses K, Benz R, Azuero A, Jablonski-Jaudon R, McNees P.. Multimorbidity and breast cancer. Semin Oncol Nurs. 2015;31(2):163–169. [DOI] [PubMed] [Google Scholar]
- 13. Schonberg MA, Marcantonio ER, Li D, Silliman RA, Ngo L, McCarthy EP.. Breast cancer among the oldest old: tumor characteristics, treatment choices, and survival. J Clin Oncol. 2010;28(12):2038–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oeffinger KC, Fontham ETH, Etzioni R, et al. Breast cancer screening for women at average risk. JAMA. 2015;314(15):1599.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Siu AL, U.S. Preventive Services Task Force . Screening for breast cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164(4):279–296. [DOI] [PubMed] [Google Scholar]
- 16. Broeders M, Moss S, Nyström L, et al. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14–25. [DOI] [PubMed] [Google Scholar]
- 17. Fenton JJ, Zhu W, Balch S, Smith-Bindman R, Fishman P, Hubbard RA.. Distinguishing screening from diagnostic mammograms using Medicare claims data. Med Care. 2014;52(7):e44–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hubbard RA, Zhu W, Balch S, Onega T, Fenton JJ.. Identification of abnormal screening mammogram interpretation using Medicare claims data. Health Serv Res. 2015;50(1):290–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ballard-Barbash R, Taplin SH, Yankaskas BC, et al. Breast Cancer Surveillance Consortium: a national mammography screening and outcomes database. Am J Roentgenol. 1997;169(4):1001–1008. [DOI] [PubMed] [Google Scholar]
- 20. Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 21. Klabunde CN, Legler JM, Warren JL, Baldwin L-M, Schrag D.. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17(8):584–590. [DOI] [PubMed] [Google Scholar]
- 22. Klabunde CN, Potosky AL, Legler JM, Warren JL.. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. [DOI] [PubMed] [Google Scholar]
- 23. Braithwaite D, Zhu W, Hubbard RA, et al. Screening outcomes in older US women undergoing multiple mammograms in community practice: does interval, age, or comorbidity score affect tumor characteristics or false positive rates? J Natl Cancer Inst. 2013;105(5):334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fine JP, Gray RJ.. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496. [Google Scholar]
- 25. SEER Cancer Statistics Factsheets: Breast Cancer. Bethesda, MD; National Cancer Institute; 2015. http://seer.cancer.gov/statfacts/html/breast.html. Accessed June 7, 2015. [Google Scholar]
- 26. Virnig BA, Wang S-Y, Shamilyan T, Kane RL, Tuttle TM.. Ductal carcinoma in situ: risk factors and impact of screening. JNCI Monogr. 2010;2010(41):113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kerlikowske K, Barclay J, Grady D, Sickles EA, Ernster V.. Comparison of risk factors for ductal carcinoma in situ and invasive breast cancer. J Natl Cancer Inst. 1997;89(1):76–82. [DOI] [PubMed] [Google Scholar]
- 28. Ernster VL, Ballard-Barbash R, Barlow WE, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002;94(20):1546–1554. [DOI] [PubMed] [Google Scholar]
- 29. Smith-Bindman R, Kerlikowske K.. Is there a downside to elderly women undergoing screening mammography? J Natl Cancer Inst. 1998;90(18):1322–1323. [DOI] [PubMed] [Google Scholar]
- 30. Barrio AV, Van Zee KJ.. Controversies in the treatment of ductal carcinoma in situ. Annu Rev Med. 2017;68(1):197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P.. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015;1(7):888. [DOI] [PubMed] [Google Scholar]
- 32. Hong YK, McMasters KM, Egger ME, Ajkay N.. Ductal carcinoma in situ current trends, controversies, and review of literature. Am J Surg. 2018;216(5):998–1003. [DOI] [PubMed] [Google Scholar]
- 33. Coldman A, Phillips N.. Incidence of breast cancer and estimates of overdiagnosis after the initiation of a population-based mammography screening program. CMAJ. 2013;185(10):E492–E498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Klabunde CN, Zheng Y, Quinn VP, et al. Influence of age and comorbidity on colorectal cancer screening in the elderly. Am J Prev Med. 2016;51(3):e67–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guilcher SJT, Lofters A, Glazier RH, Jaglal SB, Voth J, Bayoumi AM.. Level of disability, multi-morbidity and breast cancer screening: does severity matter? Prev Med. 2014;67:193–198. [DOI] [PubMed] [Google Scholar]
- 36. Tan A, Kuo Y-F, Goodwin JS.. Predicting life expectancy for community-dwelling older adults from Medicare claims data. Am J Epidemiol. 2013;178(6):974–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different U.S. Breast Cancer Screening strategies. Ann Intern Med. 2016;164(4):215.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA.. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ. 2014;348:g366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.