Abstract
Sipuleucel-T is an autologous cellular immunotherapy that induces an immune response targeted against prostatic acid phosphatase (PAP) to treat asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. In the phase III IMPACT study, sipuleucel-T was associated with a statistically significantly increased overall survival (OS) (median = 4.1 months) vs placebo. Patients with baseline prostate-specific antigen levels in the lowest quartile (≤22.1 ng/mL) exhibited a 13-month improvement in OS with sipuleucel-T. Together, this led sipuleucel-T to be approved and recommended as first-line therapy in various guidelines for treatment of metastatic castration-resistant prostate cancer. This review discusses the varied findings about the mechanisms of action of sipuleucel-T, bringing them together to form a more coherent picture. These pieces include inducing a statistically significant increase in antigen-presenting cell activation; inducing a peripheral immune response specific to the target (PAP) and/or immunizing (PA2024) antigens; stimulating systemic cytotoxic T-lymphocyte activity; and mediating antigen spread (ie, increased antibody responses to secondary proteins in addition to PAP and PA2024). Each of these pieces individually correlates with OS. Sipuleucel-T also traffics T cells to the prostate and is associated with long-term immune memory such that a second course of treatment induces an anamnestic immune response. Prostate cancer does not have a strongly inflamed microenvironment, thus its response to immune checkpoint inhibitors is limited. Because sipuleucel-T is able to traffic T cells to the tumor, it may be an ideal combination partner with immunotherapies including immune checkpoint inhibitors or with radiation therapy.
Prostate cancer is the most common type of new cancer diagnosis in men (20%) and the second most common cause of cancer death in men in the United States (10%) after lung cancer (1). It is estimated that 191 930 new cases of prostate cancer will be diagnosed in 2019 in the United States and 33 330 men will die from this disease (1). Although the incidence of prostate cancer has been falling for the last 10 years—an observation attributed, at least in part, to changes in screening and PSA testing recommendations (1)—the absolute number of men with the disease is likely to increase as more treatment options become available to an aging population, with the highest proportional prevalence being in African American men (1). An estimated 3 million men in the United States or more will have prostate cancer by 2020 according to one model (2).
Most men with prostate cancer present with localized disease or regional spread (1). These men have a good prognosis with a mortality rate similar to the all-cause mortality rate for the general population (2). If the disease progresses to metastatic castration-resistant prostate cancer (mCRPC), patients have an annual all-cause mortality rate of approximately 55% (2). The prevalence of mCRPC will likely increase over time because a growing number of men survive long enough that their prostate cancer progresses to mCRPC, with an estimated prevalence of approximately 42 970 men in the United States in 2020 (2). Therefore, treatments for mCRPC are likely to have the greatest impact on mortality among men with advanced prostate cancer (2). Currently, available treatments for mCRPC include androgen receptor and androgen synthesis inhibitors, chemotherapy, radiopharmaceuticals, and immunotherapy (3). In the United States, approved immunotherapies for mCRPC include sipuleucel-T (Provenge®, Dendreon Pharmaceuticals LLC, Seal Beach, CA) and anti-PD-1 for the small fraction (<3%) of patients with documented microsatellite instability (4).
Sipuleucel-T is an autologous cellular immunotherapy that induces an immune response targeted against prostatic acid phosphatase (PAP) (5). It was the first FDA-approved immunotherapy for the treatment of asymptomatic or minimally symptomatic mCRPC (5). Sipuleucel-T is manufactured by isolating autologous peripheral blood mononuclear cells through leukapheresis and then culturing them ex vivo with PA2024 (a recombinant fusion protein composed of PAP linked to granulocyte-macrophage colony-stimulating factor), resulting in antigen-presenting cell (APC) activation (6). Sipuleucel-T, comprising cultured peripheral blood mononuclear cells that contain the activated APCs, is infused into the patient, with the full treatment regimen consisting of three infusions at approximately 2-week intervals (5). In the phase III IMPACT trial (NCT01133704), sipuleucel-T statistically significantly reduced the risk of death vs placebo in men with mCRPC, with a 13-month overall survival (OS) benefit among men with PSAs in the lowest quartile (<22 ng/mL) (7).
The nature of the antitumor immune response seen with sipuleucel-T treatment is multifaceted. Sipuleucel-T induces T-cell and B-cell trafficking to the tumor margin when administered before prostatectomy in patients with localized prostate cancer (8) and evokes sustained immune responses in patients with either biochemically recurrent, nonmetastatic androgen-dependent prostate cancer (9,10) or mCRPC (7,11–13). Plus, APC activation observed with sipuleucel-T treatment was much higher in earlier stages of prostate cancer (9,10). The trafficking and APC activation observations are the basis for the currently ongoing company-sponsored study ProVent (NCT03686683) in the active surveillance setting, the earliest stage of prostate cancer (Table 1).
Table 1.
List of ongoing studies of sipuleucel-T identified in Clinicaltrials.gov*
| NCT identifier (Acronym) | Study title | Outcome measures | Sponsor |
|---|---|---|---|
| NCT03686683 (ProVent) | Open-Label Trial of Sipuleucel-T Administered to Active Surveillance Patients for Newly Diagnosed Prostate Cancer |
|
Dendreon |
| NCT02463799 (J1522 IRB00056435) | Study of Sipuleucel-T w/ or w/o Radium-223 in Men With Asymptomatic or Minimally Symptomatic Bone-MCRPC |
|
Investigator initiated |
| NCT01818986 (STU 102012-026) | Sipuleucel-T and Stereotactic Ablative Body Radiation (SABR) for Metastatic Castrate-resistant Prostate Cancer (mCRPC) |
|
Investigator initiated |
| NCT01804465 (12557 NCI-2014-00318) | Sipuleucel-T With Immediate vs. Delayed Cytotoxic T-Lymphocyte-Associated Protein 4 (CTLA-4) Blockade for Prostate Cancer |
|
Investigator initiated |
| NCT03329742 (IUSCC-06141706081520) | Sipuleucel-T and Low-protein Diet in Patients With Metastatic Castrate-resistant Prostate Cancer |
|
Investigator initiated |
| NCT01833208 (I 223912 NCI-2013-00633 P30CA016056) | Radiation Therapy in Treating Patients With Metastatic Hormone-Resistant Prostate Cancer Receiving Sipuleucel-T |
|
Investigator initiated |
| NCT01706458 (CO11816 A534260 SMPH/MEDICINE/MEDICINE*H NCI-2012-02026 2012-0352) | Provenge With or Without pTVG-HP DNA Booster Vaccine in Prostate Cancer |
|
Investigator initiated |
Source: clinicaltrials.gov.
Sipuleucel-T has undergone and continues to undergo extensive clinical evaluation (see Tables 1 and 2 for lists of completed and ongoing registered human studies, respectively). The initial indication is for use in men with asymptomatic or minimally symptomatic mCRPC. Both company-sponsored and investigator-initiated studies have evaluated or are currently evaluating sipuleucel-T in either combination with approved agents for the treatment of mCRPC (radium 223 dichloride) or experimental treatments, such as ipilimumab, atezolizumab, indoximod, IL-7, and radiation treatments (Tables 1 and 2). Other studies explored additional aspects of the mechanisms of action of sipuleucel-T, for example, the trafficking of sipuleucel-T to lymph nodes (NCT02036918, Table 2) and the relationship between circulating tumor cells and disease status (NCT02456571, Table 2).
Table 2.
List of completed studies of sipuleucel-T Identified in Clinicaltrials.gov*
| NCT identifier (Acronyms) | Study title | Sponsor |
|---|---|---|
| NCT01727154 (PRIME) | Immune Monitoring Protocol in Men With Prostate Cancer Enrolled in a Clinical Trial of Sipuleucel-T | Dendreon |
| NCT01477749 | Sipuleucel-T Manufacturing Demonstration Study | Dendreon |
| NCT01306890 (PROCEED) | A Registry of Sipuleucel-T Therapy in Men With Advanced Prostate Cancer | Dendreon |
| NCT00901342 | Open Label Study of Sipuleucel-T in Metastatic Prostate Cancer | Dendreon |
| NCT01338012 | Sipuleucel-T in Metastatic Castrate Resistant Prostate Cancer | Dendreon |
| NCT01981122 | A Study of Sipuleucel-T With Administration of Enzalutamide in Men With Metastatic Castrate-Resistant Prostate Cancer | Dendreon |
| NCT01431391 | Sequencing of Sipuleucel-T and ADT in Men With Non-metastatic Prostate Cancer | Dendreon |
| NCT00779402 (PROTECT) | Provenge Treatment and Early Cancer Treatment | Dendreon |
| NCT01487863 | Concurrent vs. Sequential Sipuleucel-T & Abiraterone Treatment in Men With Metastatic Castrate Resistant Prostate Cancer | Dendreon |
| NCT00715078 | To Evaluate Sipuleucel-T Manufactured With Different Concentrations of (PA2024) Antigen | Dendreon |
| NCT01133704 | Immunotherapy With APC8015 (Sipuleucel-T, Provenge) for Asymptomatic, Metastatic, Hormone-Refractory Prostate Cancer | Dendreon |
| NCT00065442 | Provenge (Sipuleucel-T) Active Cellular Immunotherapy Treatment of Metastatic Prostate Cancer After Failing Hormone Therapy | Dendreon |
| NCT00849290 | Immunotherapy for Men With Objective Disease Progression on Protocol D9902 Part B (NCT00065442) | Dendreon |
| NCT00027599 | APC8015 and Bevacizumab in Treating Patients With Prostate Cancer | Dendreon |
| NCT00005947 | Vaccine Therapy in Treating Patients With Metastatic Prostate Cancer That Has Not Responded to Hormone Therapy | Dendreon |
| NCT00715104 (NeoACT) | Sipuleucel-T as Neoadjuvant Treatment in Prostate Cancer | Dendreon |
| NCT02729103 | Treatment Patterns in Metastatic Prostate Cancer | Investigator initiated |
| NCT01560923 (2011LS109) | Phase II Study of Sipuleucel-T and Indoximod for Patients With Refractory Metastatic Prostate Cancer | Investigator initiated |
| NCT01832870 (SIPIPI 2013) | Sipuleucel-T and Ipilimumab for Advanced Prostate Cancer | Investigator initiated |
| NCT02237170 (GCO 11-1689) | Immune Monitoring on Sipuleucel-T | Investigator initiated |
| NCT02353715 (Pro00058229 PEAX) | Men With Metastatic Castrate-Resistant Prostate Cancer Treated With Either Sipuleucel-T (Provenge), Abiraterone Acetate (Zytiga) or Enzalutamide (Xtandi) Undergoing Cardiopulmonary Exercise Testing | Investigator initiated |
| NCT01420965 (11C0231) | Sipuleucel-T, CT-011, and Cyclophosphamide for Advanced Prostate Cancer | Investigator initiated |
| NCT02793219 (GU-15-103 HSC-MS-15-0882) | Provenge Followed by Docetaxel in Castration-Resistant Prostate Cancer | Investigator initiated |
| NCT02793765 (GU-15-104 HSC-MS-15-0883) | Docetaxel Followed by Provenge in Metastatic Prostate Cancer | Investigator initiated |
| NCT02036918 (Pro00047231) | Dendreon Lymph Node Biopsy in Metastatic Castrate-Resistant Prostate Cancer | Investigator initiated |
| NCT01174368 | Efficacy Trial of the Implantation of Mouse Renal Adenocarcinoma Macrobeads in Subjects With Castration-Resistant Prostate Cancer Resistant to Taxanes (Docetaxel, Cabazitaxel) and Evidence of Disease Progression on Androgen-axis Inhibition and/or Immunotherapy in the Form of Sipuleucel-T | Investigator initiated |
| NCT01274572 (MC 10-11) | Blood for Immune Response to Provenge in HRPC | Investigator initiated |
| NCT03024216 (Rosser-2015-4) | Clinical Study of Atezolizumab (Anti-PD-L1) and Sipuleucel-T in Patients With Asymptomatic or Minimally Symptomatic Metastatic Castrate Resistant Prostate Cancer | Investigator initiated |
| NCT02232230 (21C-2013-02) | A Multicenter Trial Enrolling Men With Advanced Prostate Cancer Who Are to Receive Combination Radiation and Sipuleucel-T | Investigator initiated |
| NCT02042053 (NYU S12-03902) | PET/MR Assessment of Sipuleucel T Treatment for Metastatic Castration Resistant Prostate Cancer | Investigator initiated |
| NCT02456571 (Pro00063296) | CTC Immune Checkpoint | Investigator initiated |
| NCT01807065 (12367 NCI-2013-00542) | Sipuleucel-T With or Without Radiation Therapy in Treating Patients With Hormone-Resistant Metastatic Prostate Cancer | Investigator initiated |
| NCT01881867 (CITN12-03 NCI-2013-00998 CITN12-03 IL7 P30CA015704 P50CA097186 U01CA154967) |
CYT107 After Vaccine Treatment (Provenge) in Patients With Metastatic Castration-Resistant Prostate Cancer | Investigator initiated |
| NCT02159950 (I 250813 NCI-2014-01184 P30CA016056) | Sipuleucel-T With or Without Tasquinimod in Treating Patients With Metastatic Hormone-Resistant Prostate Cancer | Investigator initiated |
Source: clinicaltrials.gov.
The purpose of this review is to collate and present published data and findings that when reviewed together, reveal a coherent, cogent mechanism of action for sipuleucel-T.
Differences Between Therapeutic Vaccines and Other Therapies for Advanced Prostate Cancer
Understanding the mechanism and unique characteristics of different therapeutic options for mCRPC is critical when considering rational combinatorial approaches, akin to assessing how individual pieces will fit together to form a complete puzzle. Here, we discuss distinguishing characteristics of therapeutic vaccines compared with conventional therapies and checkpoint inhibitors.
Comparing Therapeutic Vaccines vs Conventional Systemic Anticancer Therapy
Current conventional anticancer therapies such as docetaxel have several key limitations, including causing damage to normal cells and tissues that results in long-term side effects including the ability for some tumors to develop resistance to certain treatments (14). Immunotherapy, in contrast, is an adaptive approach to cancer treatment. By harnessing the body’s own immune system to target tumor cells, therapeutic vaccines may overcome some of the limitations of current conventional anticancer therapies.
Therapeutic cancer vaccines differ from conventional anticancer therapies in several distinct ways (Table 3) (15–17). First, they direct the immune system to target the cancer, rather than targeting the cancer directly. As a result, it may take weeks to months to mount a clinically significant immune response following immunotherapy (16,17,19). Yet, in contrast to the conventional options, the effect of these therapeutic vaccines can be durable because they may induce the development of long-lived antigen-specific memory cells, which may lead to the slowing of a tumor’s growth by providing prolonged immunologic intervention (15–17). Another difference between these types of treatment is that the evolution of tumor genetics can result in resistance to conventional anticancer therapies, whereas the immune system can often adapt to these changes, such that therapeutic vaccines can continue to provide an antitumor response (15–17).
Table 3.
Differences between systemic anticancer therapy and therapeutic vaccines
| Category | Conventional therapies | Therapeutic vaccines |
|---|---|---|
| Site of action | Specific targets at tumor or its microenvironment | Immune system |
| Pharmacodynamics | Action often immediate | Delayed onset of therapeutic action |
| Involves immunologic memory response | No | Yes |
| Tumor response to treatment (evolution and/or mutations) | Develops resistance to therapy | Develops new immunogenic targets |
| Limitation related to activity | Toxicity, including damage to healthy cells* | Requires adequate immune function systemically and at tumor site to act and react |
Many systemic chemotherapies target pathways involved in multiple biologic processes. Based on information described in multiple sources (15–18).
Comparing Therapeutic Vaccines vs Other Immunotherapies
Cancer immunotherapy can involve several different approaches to harness and direct the immune system against cancer (20). First, therapeutic vaccination primes the immune system to mount a response against tumor-associated or tumor-specific antigens (21). Second, immune checkpoint inhibition blocks negative costimulatory molecules on effector T cells, thus preventing tumor-directed suppression of antitumor effector cells (22). Third, chimeric antigen receptor (CAR)-T immunotherapy involves the binding of antigen-specific CAR-T cells to antigen-expressing tumor cells to eliminate them (23).
Immune checkpoint inhibitors target negative costimulatory molecules that are upregulated in certain forms of cancer (22). Immune checkpoint inhibitors, however, have immune-related toxicities as a result of disrupting the immunological balance between tolerance and autoimmunity. Toxicities associated with immune checkpoint inhibitors, which can limit their use, include the following: fatigue, rash and other skin disorders, gastrointestinal events, endocrinopathies, pneumonitis, colitis, hepatitis, encephalitis, and other more rare events (24).
CAR-T immunotherapy involves isolating T cells from a patient and genetically modifying the T cells to recognize a target surface antigen, therefore they are particularly complex to manufacture. The resultant autologous CAR-T cells are then infused into the patient (23). The development of this technology led to encouraging progress in difficult-to-treat cancers such as pediatric acute lymphoblastic leukemia and adult relapse or refractory non-Hodgkin lymphoma (25). CART-T immunotherapy treatments have clinically significant adverse event profiles, such as cytokine-release syndrome and neurotoxicities (25), and have yet to be proven to be safe or effective in solid tumors.
Newer treatments such as bispecific and multispecific antibodies that have multiple targets are being developed to target cancer (26). Eventually, these may offer additional benefits to patients given how they target disease and how they are manufactured.
The Clinical Impact of Sipuleucel-T
Sipuleucel-T acts as a therapeutic cancer vaccine. The phase III IMPACT study showed that sipuleucel-T treatment of men with mCRPC was associated with a statistically significant relative risk reduction in death of 22% (P = .03), with median OS being 4.1 months longer in the sipuleucel-T group compared with placebo (25.8 vs 21.7 months, respectively) (7). Most adverse events associated with sipuleucel-T treatment are low grade, with only 6.8% of patients experiencing grade 3 adverse events with sipuleucel-T (7). The most common adverse events within 1 day of sipuleucel-T infusion were chills (51.2%), fever (22.5%), fatigue (16.0%), nausea (14.2%), and headache (10.7%) (7). When considering use of sipuleucel-T, additional aspects of the clinical profile of sipuleucel-T should be considered: 1) delayed therapeutic effect, 2) greater benefit when employed in early vs more advanced mCRPC, and 3) enhanced OS observed for African Americans vs white men treated with sipuleucel-T, possibly related to different disease characteristics in the two populations. The latter observation is observed despite the low enrollment of African Americans in the clinical trial (27,28).
Delayed Effects With Treatment
The delayed treatment effect of sipuleucel-T on clinical outcomes is now recognized as an aspect known to be associated with certain immunotherapies (15,29,30). This delayed effect may explain why proximal endpoints such as PSA levels, objective disease progression, and onset of disease-related pain were not altered in IMPACT. Rather, there was an improvement in distal endpoints, including a statistically significant prolongation of the time-to-first use of opioids (31). In a pooled analysis of three phase III studies, although time-to-disease–related pain was not statistically significantly different between groups, 39% of sipuleucel-T-treated patients compared with 19% of those on placebo were pain-free at 12 months (31). Time-to-first opioid analgesic use was 12.6 months in the sipuleucel-T arm compared with 9.7 months in the placebo arm (hazard ratio [HR] = 0.755, 95% confidence interval [CI] = 0.579 to 0.985, P = .038) (31). These findings are important because pain often becomes a dominant symptom in advancing disease and has a statistically and clinically significant impact on patients’ ability to continue to carry out their daily activities (32–34). Interestingly, the Kaplan-Meier curves for time-to-disease–related pain and time-to-first opioid analgesic use with sipuleucel-T vs placebo diverge after approximately 6 months, consistent with a delayed effect of immunotherapy (31). These changes in late-occurring outcomes reflect the time taken for sipuleucel-T immunotherapy to begin to impact the disease.
Impact of Earlier Use of Sipuleucel-T
Another feature of sipuleucel-T as an immunotherapy is the potential for greater impact when used earlier in the disease course, when immunosuppressive pressures may be less. Further analysis of the IMPACT trial has shown that baseline prostate-specific antigen (PSA) is a strong predictor of treatment effect with sipuleucel-T (P < .0001). The estimated improvement in median OS ranged from 13.0 months in the lowest baseline PSA quartile (ie, PSA <22 ng/mL) to 2.8 months in the highest quartile. The estimated 3-year survival in the lowest PSA quartile was 62% for patients treated with sipuleucel-T and 42% with placebo, a 50% relative increase with sipuleucel-T (35). PSA is an indicator of disease volume (36); therefore, patients with a lower PSA level are likely to be earlier in their disease process, potentially giving them more time to benefit from sipuleucel-T.
Differential Benefit by Race
Interestingly, sipuleucel-T appears to impart greater OS benefit in African American men than in white men with mCRPC (27,28,37). A pooled analysis of phase III studies reported a median OS of 45.3 months in the African American population as compared with 24.7 months in matched (using Halabi-predicted survival) white patients (P = .02; both groups receiving sipuleucel-T) (28). This advantage extended to a comparison with control patients, who had a 14.6-month OS (P = .003) (27). In an independent dataset from the PROCEED registry (NCT01306890), median OS was greater in African Americans as compared with whites(35.2 and 29.9 months, respectively; HR = 0.81, 95% CI = 0.68 to 0.97, P = .03) (37). Because baseline PSA, an important predictor of OS for sipuleucel-T (35), was statistically significantly higher in African American patients in PROCEED, a case-matched analysis was undertaken. In PSA-matched cohorts, median OS was 35.3 and 25.8 months in African American and whitemen, respectively (HR = 0.70, 95% CI = 0.57 to 0.86, P < .001) (37). Race was found to be an independent predictor of OS after sipuleucel-T on multivariable and sensitivity analyses (37).
These differences in response in these two populations may be explained, at least in part, by observed differences in their immune systems. There are known differences in the relative proportions of various immune cells between African American men and white men (38–40), even in men with prostate cancer (41). Expression profiling of white blood cells revealed biologically significant differences in the levels of transcripts relevant to immune cell function in African Americans compared with other races (42), and racial differences have been observed in B-cell and T-cell signaling (43,44). Furthermore, gene expression analyses found differences in immune response pathways in the aggressive prostate cancer experienced by African Americans (45–47). Additional research on the association between immune responses and OS outcomes after sipuleucel-T is needed to explain these differences and to provide insights aimed at improving future treatments.
Pieces of the Sipuleucel-T Puzzle
The antitumor immune response to sipuleucel-T is multifaceted with data showing that sipuleucel-T induces sustained peripheral T-cell and B-cell responses to target antigens PAP and PA2024 (7,9–13), resulting in downstream responses to secondary, nontarget antigens. When administered before prostatectomy in localized prostate cancer, sipuleucel-T causes T cells and B cells to traffic to the tumor margin (8). Furthermore, the cytotoxic potential of activated T cells has also been documented (48) and demonstrated via video microscopy (49).
Explaining the clinical impact of immunotherapy, including sipuleucel-T, requires an understanding of the effect of anticancer immunotherapy on the peripheral immune system and the tumor microenvironment. Certain cancers, such as melanoma, bladder cancer, and non–small cell lung cancer, have an inflamed microenvironment with notable T-cell infiltration, increased expression of PD-L1, and high tumor mutation burden (50). These tumors are particularly suited to treatment with immune checkpoint inhibitors. In these cancers, the PD-1/PD-L1 pathway is an important regulator of T-cell activity and may contribute to tumor development and progression (51). In addition, tumor-infiltrating lymphocytes secrete inflammatory cytokines, particularly interferon-gamma (IFN-γ), that trigger tumor cells to express PD-1, which binds to PD-1 on T cells to inhibit antitumor T-cell responses (52,53). Patients may benefit from being tested for PD-L1 expression before being treated with PD-L1–blocking antibody therapeutics (54), although current assays lack sufficient sensitivity and specificity to accurately identify treatment responders and nonresponders (55).
In contrast, prostate cancer is generally not T-cell “inflamed” and is, therefore, associated with limited response to single-agent immune checkpoint inhibition (56,57). In defining tumor types based on their immunity profile, prostate cancer is defined as having an immune-desert phenotype (58). This description is characterized by a paucity of CD8+ T cells. These findings may indicate an absence of preexisting antitumor immunity, suggesting that the presence of tumor-specific T cells is the rate-limiting step if using checkpoint inhibitors. This concept is supported by recent studies demonstrating that vaccination (targeting the same PAP antigen as sipuleucel-T), in combination with PD-1 blockade, elicited PSA declines and objective responses in patients with advanced prostate cancer and an infiltration of tumors with CD8+ T cells (59). In contrast, studies conducted with nivolumab (PD-1 inhibitor) (56,60), ipilimumab (cytotoxic T lymphocytes [CTLA] 4 inhibitor) (56,61), and pembrolizumab (62) as monotherapies in patients with prostate cancer demonstrated limited clinical activity.
Defining Immune Responses to Sipuleucel-T Piece by Piece
The immune responses to sipuleucel-T are multifactorial. First, there is a statistically significant increase in APC activation. Second, peripheral cellular and humoral immune responses specific to PAP and PA2024 are then induced. Third, this is followed by stimulation of local and systemic cytotoxic T-lymphocyte activity. Fourth, the immune system mounts a secondary response to additional antigens expressed by the tumor, the so-called phenomenon of antigen spread. Last, this secondary response yields increasing cytotoxic T-cell activity.
Antigen-Presenting Cell Activation
APC activation is a measure of product potency and immune activation, and increasing cumulative APC activation (across the three doses of sipuleucel-T) is statistically significantly correlated with improved OS in mCRPC (11). In three phase III, randomized, double-blind, multicenter trials of sipuleucel-T in men with mCRPC (including IMPACT), sipuleucel-T statistically significantly increased APC activation as compared with placebo, with a median cumulative APC activation of 26.7 (Table 4) (11). Increases in APC activation were seen with each subsequent infusion of sipuleucel-T, indicating that the first infusion primes the immune system and subsequent infusions boost the response in a classical vaccine-mediated memory response.
Table 4.
Cumulative APC activation in phase II and III studies with sipuleucel-T in patients with mCRPC
| Study | Design | Treatments | No.* | Median cumulative APC activation† |
|---|---|---|---|---|
| IMPACT‡, D9901§, D9902A¶ (11) | Phase III, multicenter, randomized, double-blind trials |
|
476 | 26.7 |
| STAMP# (31) | Phase II, randomized, open-label trial | Sipuleucel-T + concurrent AA + P | 35 | 33.65 |
| Sipuleucel-T + sequential AA + P** | 34 | 38.24 |
Sipuleucel-T recipients in APC activation analysis. AA = abiraterone acetate; APC = antigen-presenting cell; P = prednisone.
APC activation is the increase in surface CD54 expression on APCs expressed as an upregulation ratio of average number of molecules on postculture vs preculture cells. Cumulative APC activation is the sum of APC activation after all three sipuleucel-T injections.
NCT00065442.
NCT00005947.
NCT01133704.
NCT01487863.
Started 10 weeks after the first infusion of sipuleucel-T.
Additionally, there are data supporting an anamnestic APC activation with sipuleucel-T; these data come from the phase II study P10-1 (NCT01338012, PROTECT II) (63). Here, nine patients with castration-sensitive prostate cancer, initially treated with three infusions of sipuleucel-T or placebo in the phase III trial PROTECT (NCT00779402), received a booster infusion (9) after progressing to mCRPC. These patients were retreated after a median of 9.5 years after the end of PROTECT. Activation of APCs was higher in those patients who were retreated with sipuleucel-T than in treatment-naïve patients, indicating that memory T cells were interacting with APCs because the latter have no memory (63). Accordingly, cumulative APC activation (CD54 upregulation), which was correlated with OS in the IMPACT study (11), was seen to be much higher in P10-1 than in IMPACT (63).
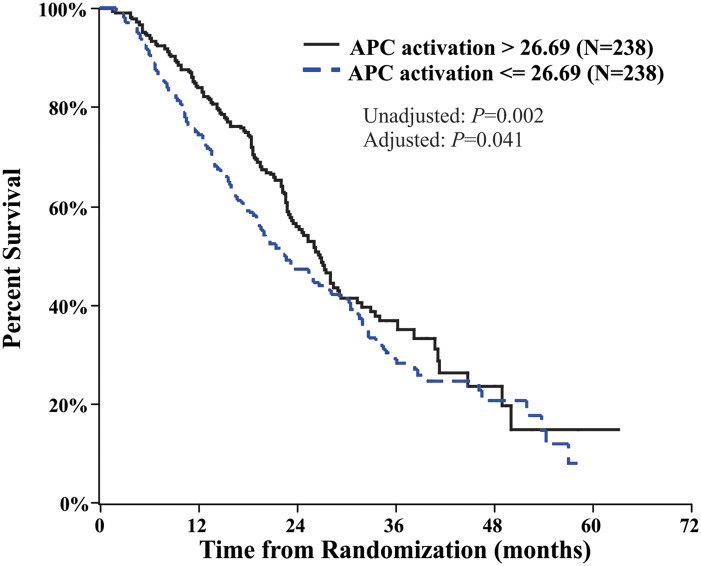
The phase II STAND (NCT01431391) study in men with biochemically recurrent prostate cancer following prostatectomy and/or radiotherapy found no difference in APC activation when sipuleucel-T was administered before or after androgen-deprivation therapy; however, APC activation in general was higher than that observed in IMPACT (10). Interestingly, cumulative APC activation was approximately 37% higher in STAND vs IMPACT (ie, when sipuleucel-T was administered to patients with less-advanced disease) (7,10). This difference may be a consequence of the STAND patient population being younger and therefore having a more robust immune system or having an earlier prostate cancer disease stage with less tumor burden and associated immune suppression than patients in IMPACT. As described above, sipuleucel-T APC activation was shown to correlate with OS in the IMPACT study (Figure 1) (11).
Figure 1.
Survival in men with mCRPC who received sipuleucel-T in the IMPACT study, stratified by above and below the median cumulative APC activation after treatment. Cumulative APC activation value (hazard ratio = 0.76, 95% confidence interval = 0.58 to 0.99). This figure comes from figure 5, panel A of Sheikh et al. (11), which was distributed under the terms of the Creative Commons Attribution License that permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited. APC = antigen presenting cell; mCRPC = metastatic castration-resistant prostate cancer.
Peripheral Cellular and Humoral Immune Responses
Sipuleucel-T induces peripheral immune responses specific to PAP, the target antigen, and PA2024, the immunizing antigen. These responses are measured by assessing a range of cellular and humoral immune parameters such as T-cell proliferation and the IFN-γ enzyme-linked immunosorbent spot for cellular immune responses and levels of antibodies for humoral responses. In support of the mode of action of sipuleucel-T, peripheral immune responses to PA2024 and/or PAP also correlate with OS (11).
Data from clinical trials with sipuleucel-T also show that most subjects develop peripheral antigen-specific immune responses. For example, in IMPACT, sipuleucel-T induced peripheral immune responses (either T cell or humoral) to PA2024 and/or PAP in 79% of treated patients compared with 13% of patients in the control group (11). Similar peripheral immune responses were reported in a number of phase II trials of sipuleucel-T (10,12,13). Moreover, the P10-1 study, in which patients received a second course of sipuleucel-T almost 10 years after their initial treatment, showed that peripheral PA2024 and PAP cellular and humoral responses were present before retreatment and boosted after the first infusion of sipuleucel-T (63).
A study in which patients with mCRPC receiving sipuleucel-T underwent lymph node biopsies to determine the magnitude of sipuleucel-T–induced leukocyte activation in tumor-affected lymph nodes (NCT02036918) was recently completed. A secondary aim of this study was to examine the relationship between the lymph node immune response to sipuleucel-T and the peripheral immune response. Results are forthcoming.
Antigen Spread
There is growing evidence that part of the mechanism of action of sipuleucel-T involves antigen spread (ie, the broadening of the immune response to additional antigens expressed by the tumor) after the initial immune response to the specific, target antigen (15,64). In this process, tumor cells targeted by antigen-specific T cells are lysed, releasing a range of tumor-specific, secondary antigens that APCs present back to the mobilized immune system leading to further action.
An analysis of data from IMPACT and ProACT (NCT00715078), a randomized, single-blind trial of sipuleucel-T in patients with advanced mCRPC, showed that sipuleucel-T induces antibody responses to secondary antigens (65). These antigens include PSA, KLK2, LGALS3, and LGALS8 (65), which have been shown to be expressed at elevated levels in prostate cancer and/or to play a role in prostate cancer development. Additionally, responses were also observed to K-RAS and E-RAS, which have functional relevance in cancer (66–72). Antigen spread was observed beginning 2 weeks after sipuleucel-T treatment and persisted for at least 6 months, the last immune monitoring time point in the IMPACT study (65).
Most patients who exhibited a response to secondary antigens also had an IgG response to PAP (65). In addition, there was considerable overlap between responses to secondary antigens, with fewer than 30% of patients having a response to only a single secondary antigen. For example, 9% of patients treated with sipuleucel-T had an IgG response to PSA with E-RAS, LGALS8, and LGALS3, and 25% had a response to at least three of these four antigens (65). An antibody response to PSA or LGALS3 was associated with improved survival in patients receiving sipuleucel-T as compared with placebo in IMPACT (P ≤ .05). The extent of antigen spread was also associated with improved OS. These data suggest that antigen spread has a role in the mechanism of action by which sipuleucel-T exerts its survival benefit (65).
Results from two phase II studies have also shown antigen spread with sipuleucel-T. In mCRPC patients receiving concurrent vs sequential abiraterone with sipuleucel-T in STAMP and STRIDE (NCT01487863 and NCT01981122), IgG levels to all secondary antigens were statistically significantly increased from baseline in both arms at weeks 6, 10, and 14 (P < .01) (31). Importantly, antigen spread was considered to be a response to sipuleucel-T treatment and not to abiraterone, because there were no differences in antigen spread between the concurrent and sequential arms in STAMP (31). In the trial STAND that enrolled patients with biochemically recurrent prostate cancer, sipuleucel-T treatment resulted in IgG responses to the secondary antigens E-RAS, KLK2, K-RAS, LGALS3, and LGALS8, which were similar between the study arms (ie, sipuleucel-T then androgen-deprivation therapy and androgen-deprivation therapy followed by sipuleucel-T) (10). Moreover, in STAND, in patients with an early disease state, the magnitude of antigen spread to each antigen at week 2 was statistically significantly higher (P < .01) than in mCRPC patients in the IMPACT and STAMP trials, as was the number of subjects who exhibited antigen spread (10).
T-Cell Trafficking to the Prostate and Cytotoxic T-Cell Activity
The NeoACT (NCT00715104) study of patients with untreated, localized prostate cancer examined the effect of neoadjuvant sipuleucel-T prior to radical prostatectomy (8). Here, immune infiltrates in tumor specimens removed during surgery were compared with pretreatment biopsy specimens. These analyses showed recruitment of activated effector T cells into the prostate tumor microenvironment concurrent with a systemic antigen-specific T-cell response to sipuleucel-T (8). Postoperative tissue specimens showed a threefold or greater increase in CD3+, CD4+ (helper T cell), and CD8+ T cells (CTLs infiltration at the interface between the tumor and healthy tissue compared with biopsy specimens taken before sipuleucel-T treatment (binomial proportions: all Ps < .001) (8).
Next-generation sequencing of peripheral blood and prostate tissue specimens from NeoACT showed that after infusion of sipuleucel-T, peripheral blood T cells become less diverse (73). In addition, the T-cell clones observed in the prostate tissue had greater commonality with peripheral blood clones after the first infusion, implying that the priming infusion of sipuleucel-T programs peripheral T cells to traffic to the prostate tissue. Compared with prostate cancer patients who had not received sipuleucel-T, T cells in prostate cancer tissue from sipuleucel-T–treated patients had greater diversity, suggesting that specific T-cell clones are recruited from the peripheral blood to the prostate tumor microenvironment, which could enhance immunological containment of the tumor.
CTL Activity
One of the ways in which cancer cells evade immune destruction is by suppressing the natural role of CTLs (74). There is evidence that sipuleucel-T is able to generate antigen-specific CTLs (as measured by cell surface CD107a) in patients with prostate cancer (48,49) with greater CTL activity correlating to improved OS (48).
An analysis of T-cell activity in the STAMP and STRIDE trials demonstrated that sipuleucel-T induces a marked increase in the proliferation of both PA2024-specific CD4+ and CD8+ T cells (48). Because CD4 helper T cells facilitate the differentiation and expansion of CTLs, their induction is an essential part of the immune response to sipuleucel-T. Most important, these analyses demonstrated a statistically significant correlation between OS and PAP- and PA2024-specific CTL responses at month 6 (P = .0134 and P = .0006, respectively).
Furthermore, the cytolytic activity of CTLs against PAP-expressing target cells was recently documented by confocal microscopy, providing visual verification of CTL action after sipuleucel-T treatment at week 6 and month 6 (49). Taken together, these data indicate that the induction of tumor lysis via antigen-specific CD8+ cells is an important component of the mechanism of action of sipuleucel-T (48).
Completing the Mechanism of Action Jigsaw
Combination Therapies
Previous research has shown that antiandrogen therapy has an effect on local and systemic immune responses in prostate cancer (75), making immunotherapies an attractive option for patients who develop resistance to androgen-directed therapy. For prostate cancer treatment, immune checkpoint inhibitors may have a role in combination with agents that enhance the recruitment of effector T cells to the prostate cancer microenvironment (57). For example, PD-L1 expression is increased in APCs and T cells in patients with enzalutamide-resistant, castration-resistant prostate cancer, suggesting that anti-PD-L1 therapy may be a logical next step when a patient develops enzalutamide resistance (76). Therefore, a key focus in immunotherapy for prostate cancer is how best to sequence or combine immunotherapies to enhance the immune response as the disease progresses and the immunogenic environment changes (59,77).
Combination With Agents Targeting Negative Costimulatory Molecules
The NeoACT studies showed that, following sipuleucel-T treatment, both CD3+ T cells recruited to the tumor (8) and T cells isolated from peripheral blood (78) expressed PD-1. Moreover, CTLA-4 (CD152) expression on T-cells was observed before and after sipuleucel-T infusion in both NeoACT and ProACT subjects (Dendreon, data on file); however, subjects in ProACT exhibited statistically higher levels of CTLA-4 prior to sipuleucel-T treatment. It was also found that PD-L1 expression increases on prostate-circulating tumor cells following sipuleucel-T treatment (79). Collectively, these data provide a biologic rationale for studying sipuleucel-T in combination with agents targeting the negative costimulatory molecules. Studies are currently underway to assess the combination of sipuleucel-T with other immunotherapies, such as the PD-L1 inhibitor atezolizumab (NCT03024216) and the CTLA-4 inhibitor ipilimumab (NCT01804465). A preliminary, phase I study (NCT01832870) of sipuleucel-T and ipilimumab (n= 9) indicated the potential synergistic effects of this combination, with higher levels of PAP- and PA2024-specific antibody titers after the combination than would be expected with sipuleucel-T alone (80). Of the nine surviving men, six have had a follow-up of more than 50 months and received a range of subsequent therapies (81).
Combination With Radiation
Sipuleucel-T is also being studied in combination with stereotactic ablative body radiation (NCT01818986) and the addition of radium-223 (NCT02463799). These latter studies are being conducted because inflammation and immunomodulatory cytokines are increased following radiation therapy, and radiation-induced cell death releases antigens that may be targeted through antigen spread, following activation of the immune system with sipuleucel-T (82).
Combination With Agents Stimulating Tumor Inflammation
Studies are also underway to assess combinations of immunotherapies and potential stimulants of tumor inflammation. One such study is KEYNOTE-365, a nonrandomized phase Ib/II study that assessed different treatment combinations in patients with mCRPC (NCT02861573); preliminary data have been presented (83–89). Final results are forthcoming.
Discussion
Sipuleucel-T has been and continues to be explored as part of various treatment regimens for prostate cancer (Tables 1 and 2). It has been shown that sipuleucel-T drives T cells to the tumor periphery in the prostate (8), which results in APC activation that is higher in earlier stages of prostate cancer (10). Additional research suggests a role for antigen spread in its actions. Although the role of the tumor microenvironment in the antitumor immune response in prostate cancer has not been fully elucidated, insights into the immunogenic mechanisms are rapidly evolving, including the identification of new treatment targets. Recent data published by Gao and colleagues (90) indicate that V-type immunoglobulin domain-containing suppressor of T-cell activation may be an important immune-regulatory mechanism in prostate cancer. Thus, increasing knowledge of the tumor microenvironment, as well as developing combination treatment options, should improve prostate cancer management. A deeper understanding of the tumor microenvironment and immune-relevant changes (cells, proteins, and transcription) in response to immunotherapy will inform effective combination of therapies to improve patient outcomes.
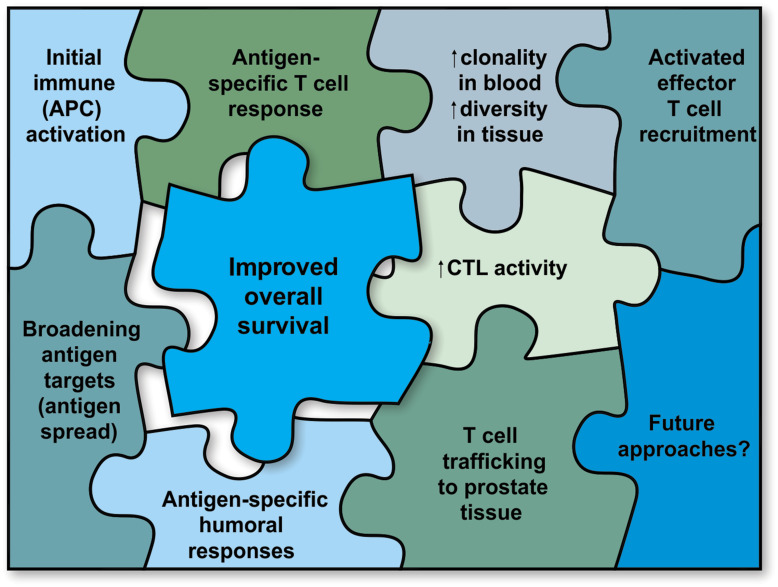
Sipuleucel-T prolongs OS in men with mCRPC, with most adverse events being consistent with infusion-related events and mild to moderate in severity. As to the mechanism of action of sipuleucel-T, enough pieces of the jigsaw puzzle are in place to obtain a clear picture (Figure 2), with a wealth of data indicating a mechanism similar to that of a vaccine. Sipuleucel-T, through APC activation, generates strong and persistent antigen-specific humoral and T-cell responses, as well as T-cell trafficking to tumor tissues, stimulation of local and systemic CTL activity, and antigen spread. All of these effects may be the key to turning a cold, immune-desert tumor into a hot, immune-enriched one, thus facilitating an immunological cascade against the tumor. The activation and spread of immune responses following treatment with sipuleucel-T, along with its persistent efficacy and safety profile, raise the prospect of prolonged anticancer effects that can be boosted and may be potentiated by appropriate combination therapy.
Figure 2.
The jigsaw puzzle of the mechanism of action of sipuleucel-T. APC = antigen presenting cell; CTL = cytotoxic T lymphocytes.
Funding
This work was funded by Dendreon Pharmaceuticals LLC, including both provision of medical writing support and sponsoring many of the sipuleucel-T studies described herein.
Notes
The funder contributed to the writing of this review, its contents, and the decision to submit it for publication in partnership with the rest of the author body in accordance with the principles of Good Publication Practices.
Medical writing assistance, in the form of developing the draft outline and manuscript first draft in consultation with the authors, revising draft versions, assembling tables and figures, collating author comments, copy editing, fact checking, referencing, and providing graphic services was provided by Catherine Lee and Jackie Phillipson of Zoetic Science (Macclesfield, UK), which was funded by Dendreon Pharmaceuticals LLC. Additional medical writing support was provided by Helen M. Wilfehrt, PhD, CMPP, of Dendreon Pharmaceuticals LLC.
Conflicts of interest: Dr Madan has nothing to disclose. Dr Antonarakis reports grants and personal fees from Janssen, Sanofi, Dendreon, AstraZeneca, Clovis, and Merck; personal fees from Astellas, Medivation, Eli Lilly and Co, Amgen, Qiagen, and ESSA; and grants from Johnson & Johnson, Genentech, Novartis, Bristol Myers-Squibb, Tokai, and Celgene, outside the submitted work. Dr Drake reports consulting fees from Bristol Myers Squibb, Brooklyn Therapeutics, Compugen, EMD Serono, F-Star, Ferring, Genocea, Harpoon, Kleo, Merck, Pfizer, Roche-Genentech, Sanofi Aventis, Shattuck Labs, Tizona, and Werewolf, as well as equity interest in Compugen, Harpoon, Kleo, Tizona, and Werewolf. Dr Fong reports grants from Dendreon, Abbvie, Bavarian Nordic, BMS, Janssen, Merck, and Roche/Genentech during the conduct of the study. Dr Yu reports personal fees from Amgen, Clovis, Astrazeneca, Janssen, Pharmacyclics, QED, Tolmar, Incyte, EMD Serono, and Churchill; and grants and personal fees from Bayer, Dendreon, Merck, Seattle Genetics, outside the submitted work. Dr McNeel reports grants, personal fees, and other from Madison Vaccines, Inc; other from Janssen Pharmaceuticals and Novartis; nonfinancial support from BMS; and grants and other from Merck, outside the submitted work. In addition, Dr McNeel has several patents related to vaccines for prostate cancer, none specific to sipuleucel-T, licensed to Madison Vaccines, Inc. Dr Lin has nothing to disclose. Dr Chang reports other from Dendreon, outside the submitted work. She was an employee of Dendreon Pharmaceuticals LLC, makers of sipuleucel-T, when this manuscript was written. Dr Sheikh reports other from Dendreon Pharmaceuticals, outside the submitted work. Dr Sheikh is a current employee of Dendreon Pharmaceuticals LLC, makers of sipuleucel-T. In addition, Dr Sheikh has a patent “Humoral Immune Response Against Tumor Antigens After Treatment With a Cancer Antigen Specific Active Immunotherapy and Its Association With Improved Clinical Outcome,” issued and a patent, “Gene Expression Markers for Predicting Overall Survival in Subjects Treated With Sipuleucel-T,” pending. Dr Gulley has nothing to disclose.
References
- 1. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Scher HI, Solo K, Valant J, Todd MB, Mehra M.. Prevalence of prostate cancer clinical states and mortality in the United States: estimates using a dynamic progression model. PLoS One. 2015;10(10):e0139440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Prostate Cancer: Version 2.2019; 2019. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed June 30, 2019.
- 4. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dendreon Pharmaceuticals LLC. PROVENGE® (sipuleucel-T) Prescribing Information 2017. http://www.provengehcp.com/Portals/5/Provenge-PI.pdf. Accessed July 19, 2018.
- 6. Sheikh NA, Jones LA.. CD54 is a surrogate marker of antigen presenting cell activation. Cancer Immunol Immunother. 2008;57(9):1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. [DOI] [PubMed] [Google Scholar]
- 8. Fong L, Carroll P, Weinberg V, et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Inst. 2014;106(11):dju372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beer TM, Bernstein GT, Corman JM, et al. Randomized trial of autologous cellular immunotherapy with sipuleucel-T in androgen-dependent prostate cancer. Clin Cancer Res. 2011;17(13):4558–4567. [DOI] [PubMed] [Google Scholar]
- 10. Antonarakis ES, Kibel AS, Yu EY, et al. Sequencing of sipuleucel-T and androgen deprivation therapy in men with hormone-sensitive biochemically recurrent prostate cancer: a phase II randomized trial. Clin Cancer Res. 2017;23(10):2451–2459. [DOI] [PubMed] [Google Scholar]
- 11. Sheikh NA, Petrylak D, Kantoff PW, et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013;62(1):137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petrylak D, Quinn D, Dreicer R, et al. 2526 Immune responses and clinical data from STRIDE, a randomized, phase 2, open label study of sipuleucel-T with concurrent vs sequential enzalutamide administration in metastatic castration resistant prostate cancer. Eur J Cancer. 2015;51 (Supplement 3):S483. [Google Scholar]
- 13. Small EJ, Lance RS, Gardner TA, et al. A randomized phase II trial of sipuleucel-T with concurrent versus sequential abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2015;21(17):3862–3869. [DOI] [PubMed] [Google Scholar]
- 14. Hwang C. Overcoming docetaxel resistance in prostate cancer: a perspective review. Ther Adv Med Oncol. 2012;4(6):329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gulley JL, Madan RA, Heery CR.. Therapeutic vaccines and immunotherapy in castration-resistant prostate cancer: current progress and clinical applications In: Dizon DS, ed. American Society of Clinical Oncology Educational Book. Alexandria, VA: American Society of Clinical Oncology; 2013:e166–e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gulley JL, Drake CG.. Immunotherapy for prostate cancer: recent advances, lessons learned, and areas for further research. Clin Cancer Res. 2011;17(12):3884–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gulley JL, Madan RA, Schlom J.. Impact of tumour volume on the potential efficacy of therapeutic vaccines. Curr Oncol. 2011;18(3):e150–e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gulley JL. Therapeutic vaccines: the ultimate personalized therapy? Hum Vaccin Immunother. 2013;9(1):219–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Madan RA, Gulley JL, Fojo T, et al. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010;15(9):969–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oiseth SJ, Aziz MS.. Cancer immunotherapy: a brief review of the history, possibilities, and challenges ahead. J Cancer Metastasis Treat. 2017;3(10):250–261. [Google Scholar]
- 21. Guo C, Manjili MH, Subjeck JR, et al. Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res. 2013;119:421–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. May KF Jr, Gulley JL, Drake CG, Dranoff G, Kantoff PW.. Prostate cancer immunotherapy. Clin Cancer Res. 2011;17(16):5233–5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jackson HJ, Rafiq S, Brentjens RJ.. Driving CAR T-cells forward. Nat Rev Clin Oncol. 2016;13(6):370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park JH, Geyer MB, Brentjens RJ.. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood. 2016;127(26):3312–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clift IC. Bispecific, multispecific antibodies grapple with cancer. Genet Eng News. 2019;39(2):46–47. https://www.genengnews.com/insights/bispecific-multispecific-antibodies-grapple-with-cancer/. Accessed May 13, 2019. [Google Scholar]
- 27. McLeod DG, Quinn DI, Whitmore JB, Tabesh M.. Sipuleucel-T in African Americans: a subgroup analysis of three phase III sipuleucel-T trials in advanced prostate cancer. J Clin Oncol. 2011;29(suppl 15):e15148. [Google Scholar]
- 28. Quinn DI, Freedland SJ, Heath EI, et al. Survival outcomes for African-American (AA) vs matched Caucasian (CAU) patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) treated with sipuleucel-T (sip-T). J Clin Oncol. 2017;35(suppl 6):192. [Google Scholar]
- 29. Schlom J. Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst. 2012;104(8):599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singh BH, Gulley JL.. Immunotherapy and therapeutic vaccines in prostate cancer: an update on current strategies and clinical implications. Asian J Androl. 2014;16(3):364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Small EJ, Higano CS, Kantoff PW, et al. Time to disease-related pain and first opioid use in patients with metastatic castration-resistant prostate cancer treated with sipuleucel-T. Prostate Cancer Prostatic Dis. 2014;17(3):259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fitch MI, Gray R, Franssen E, et al. Men’s perspectives on the impact of prostate cancer: implications for oncology nurses. Oncol Nurs Forum. 2000;27(8):1255–1263. [PubMed] [Google Scholar]
- 33. Lindqvist O, Rasmussen BH, Widmark A.. Experiences of symptoms in men with hormone refractory prostate cancer and skeletal metastases. Eur J Oncol Nurs. 2008;12(4):283–290. [DOI] [PubMed] [Google Scholar]
- 34. Lindqvist O, Rasmussen BH, Widmark A, et al. Time and bodily changes in advanced prostate cancer: talk about time as death approaches. J Pain Symptom Manage. 2008;36(6):648–656. [DOI] [PubMed] [Google Scholar]
- 35. Schellhammer PF, Chodak G, Whitmore JB, et al. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology. 2013;81(6):1297–1302. [DOI] [PubMed] [Google Scholar]
- 36. Carter HB. Prostate cancers in men with low PSA levels--must we find them? N Engl J Med. 2004;350(22):2292–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sartor AO, Armstrong AJ, Ahaghotu C, et al. Overall survival (OS) of African-American (AA) and Caucasian (CAU) men who received sipuleucel-T for metastatic castration-resistant prostate cancer (mCRPC): final PROCEED analysis. J Clin Oncol. 2019;37(suppl 15):5035. [Google Scholar]
- 38. Freedman DS, Gates L, Flanders WD, et al. Black/white differences in leukocyte subpopulations in men. Int J Epidemiol. 1997;26(4):757–764. [DOI] [PubMed] [Google Scholar]
- 39. Lim E-M, Cembrowski G, Cembrowski M, et al. Race‐specific WBC and neutrophil count reference intervals. Int J Lab Hematol. 2010;32(6, pt 2):590–597. [DOI] [PubMed] [Google Scholar]
- 40. Hsu JW, Wingard JR, Logan BR, et al. Race and ethnicity influences collection of granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells from unrelated donors, a Center for International Blood and Marrow Transplant Research analysis. Biol Blood Marrow Transplant. 2015;21(1):165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vidal AC, Howard LE, de Hoedt A, et al. Neutrophil, lymphocyte and platelet counts, and risk of prostate cancer outcomes in white and black men: results from the SEARCH database. Cancer Causes Control. 2018;29(6):581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Keller MF, Reiner AP, Okada Y, et al. Trans-ethnic meta-analysis of white blood cell phenotypes. Hum Mol Genet. 2014;23(25):6944–6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Longo DM, Louie B, Mathi K, et al. Racial differences in B cell receptor signaling pathway activation. J Transl Med. 2012;10(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sugimoto K, Stadanlick J, Ikeda F, et al. Influence of ethnicity in the outcome of hepatitis C virus infection and cellular immune response. Hepatology. 2003;37(3):590–599. [DOI] [PubMed] [Google Scholar]
- 45. Kinseth MA, Jia Z, Rahmatpanah F, et al. Expression differences between African American and Caucasian prostate cancer tissue reveals that stroma is the site of aggressive changes. Int J Cancer. 2014;134(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rebbeck TR, Devesa SS, Chang BL, et al. Global patterns of prostate cancer incidence, aggressiveness, and mortality in men of African descent. Prostate Cancer. 2013;2013:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Powell IJ, Bock CH, Ruterbusch JJ, et al. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J Urol. 2010;183(5):1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Antonarakis ES, Small EJ, Petrylak DP, et al. Antigen-specific CD8 lytic phenotype induced by sipuleucel-t in hormone-sensitive or castration-resistant prostate cancer and association with overall survival. Clin Cancer Res. 2018;24(19):4662–4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Inman B, Vu T, Yu Evan Y, et al. PD14-07 Real-time imaging demonstrating T-cell mediated destruction of prostatic acid phosphatase (PAP)-expressing cells in patients (PTS) treated with sipuleucel-T (SIP-T). J Urol. 2018;199(4S):e307. [Google Scholar]
- 50. Gajewski TF. The next hurdle in cancer immunotherapy: overcoming the non-T-cell-inflamed tumor microenvironment. Semin Oncol. 2015;42(4):663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Modena A, Ciccarese C, Iacovelli R, et al. Immune checkpoint inhibitors and prostate cancer: a new frontier? Oncol Rev. 2016;10(1):293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Taube JM, Young GD, McMiller TL, et al. Differential expression of immune-regulatory genes associated with PD-L1 display in melanoma: implications for PD-1 pathway blockade. Clin Cancer Res. 2015;21(17):3969–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Diggs LP, Hsueh EC.. Utility of PD-L1 immunohistochemistry assays for predicting PD-1/PD-L1 inhibitor response. Biomark Res. 2017;5(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mehta K, Patel K, Parikh RA.. Immunotherapy in genitourinary malignancies. J Hematol Oncol. 2017;10(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rijnders M, de Wit R, Boormans JL, Lolkema MPJ, van der Veldt A.. Systematic review of immune checkpoint inhibition in urological cancers. Eur Urol. 2017;72(3):411–423. [DOI] [PubMed] [Google Scholar]
- 58. Chen DS, Mellman I.. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. [DOI] [PubMed] [Google Scholar]
- 59. McNeel DG, Eickhoff JC, Wargowski E, et al. Concurrent, but not sequential, PD-1 blockade with a DNA vaccine elicits anti-tumor responses in patients with metastatic, castration-resistant prostate cancer. Oncotarget. 2018;9(39):25586–25596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. De Bono JS, Goh JCH, Ojamaa K, et al. KEYNOTE-199: pembrolizumab (pembro) for docetaxel-refractory metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2018;36(suppl 15):5007. [Google Scholar]
- 63. Beer TM, Corman J, Lance RS, et al. Boosting long-term immune responses to sipuleucel-T (sip-T) by retreatment of patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2017;35(suppl 6):196. [Google Scholar]
- 64. Gulley JL, Madan RA, Pachynski R, et al. Role of antigen spread and distinctive characteristics of immunotherapy in cancer treatment. J Natl Cancer Inst. 2017;109(4):djw261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. GuhaThakurta D, Sheikh NA, Fan LQ, et al. Humoral immune response against nontargeted tumor antigens after treatment with sipuleucel-T and its association with improved clinical outcome. Clin Cancer Res. 2015;21(16):3619–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Darson MF, Pacelli A, Roche P, et al. Human glandular kallikrein 2 expression in prostate adenocarcinoma and lymph node metastases. Urology. 1999;53(5):939–944. [DOI] [PubMed] [Google Scholar]
- 67. Herrmann E, Bogemann M, Bierer S, et al. The endothelin axis in urologic tumors: mechanisms of tumor biology and therapeutic implications. Expert Rev Anticancer Ther. 2006;6(1):73–81. [DOI] [PubMed] [Google Scholar]
- 68. Kubota E, Kataoka H, Aoyama M, et al. Role of ES cell-expressed Ras (ERas) in tumorigenicity of gastric cancer. Am J Pathol. 2010;177(2):955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nelson JB, Hedican SP, George DJ, et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med. 1995;1(9):944–949. [DOI] [PubMed] [Google Scholar]
- 70. Nelson JB, Udan MS, Guruli G, Pflug BR.. Endothelin-1 inhibits apoptosis in prostate cancer. Neoplasia. 2005;7(7):631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rittenhouse HG, Finlay JA, Mikolajczyk SD, Partin AW.. Human kallikrein 2 (hK2) and prostate-specific antigen (PSA): two closely related, but distinct, kallikreins in the prostate. Crit Rev Clin Lab Sci. 1998;35(4):275–368. [DOI] [PubMed] [Google Scholar]
- 72. Williams SA, Xu Y, De Marzo AM, Isaacs JT, Denmeade SR.. Prostate-specific antigen (PSA) is activated by KLK2 in prostate cancer ex vivo models and in prostate-targeted PSA/KLK2 double transgenic mice. Prostate. 2010;70(7):788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sheikh N, Cham J, Zhang L, et al. Clonotypic diversification of intratumoral T cells following sipuleucel-T treatment in prostate cancer subjects. Cancer Res. 2016;76(13):3711–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hanahan D, Weinberg RA.. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 75. Kalina LJ, Neilson SD, Comber PA, et al. Immune modulation by androgen deprivation and radiation therapy: implications for prostate cancer immunotherapy. Cancers. 2017;9(12):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bishop JL, Sio A, Angeles A, et al. PD-L1 is highly expressed in enzalutamide resistant prostate cancer. Oncotarget. 2015;6(1):234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. O’Sullivan Coyne G, Madan RA, Gulley JL.. Nivolumab: promising survival signal coupled with limited toxicity raises expectations. J Clin Oncol. 2014;32(10):986–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wesley J, Kuan L-Y, Chadwick E, et al. Abstract 5509: evidence of T lymphocyte activation following sipuleucel-T treatment. Cancer Res. 2011;71(suppl 8):5509. [Google Scholar]
- 79. Rekoske BT, Olson BM, McNeel DG.. Antitumor vaccination of prostate cancer patients elicits PD-1/PD-L1 regulated antigen-specific immune responses. Oncoimmunology. 2016;5(6):e1165377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Scholz M, Yep S, Chancey M, et al. Phase I clinical trial of sipuleucel-T combined with escalating doses of ipilimumab in progressive metastatic castrate-resistant prostate cancer. Immuno Targets Ther. 2017;6:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ku J, Wilenius K, Larsen C, et al. Survival after sipuleucel-T (SIP-T) and low-dose ipilimumab (IPI) in men with metastatic, progressive, castrate-resistant prostate cancer (M-CRPC). J Clin Oncol. 2018;36(suppl 6):368. [Google Scholar]
- 82. Raval RR, Sharabi AB, Walker AJ, Drake CG, Sharma P.. Tumor immunology and cancer immunotherapy: summary of the 2013 SITC primer. J Immunother Cancer. 2014;2(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fong PCC, Retz M, Drakaki A, et al. Keynote-365 cohort C: pembrolizumab (pembro) plus enzalutamide (enza) in abiraterone (abi)-pretreated patients (pts) with metastatic castrate resistant prostate cancer (mCRPC). J Clin Oncol. 2019;37(suppl 7):171.30433848 [Google Scholar]
- 84. Fong PCC, Retz M, Drakaki A, et al. Pembrolizumab (pembro) plus enzalutamide (enza) in abiraterone (abi)-pretreated patients (pts) with metastatic castrate resistant prostate cancer (mCRPC): cohort C of the phase 1b/2 KEYNOTE-365 study. J Clin Oncol. 2019;37(suppl 15):5010. [Google Scholar]
- 85. Massard C, Retz M, Hammerer P, et al. Keynote-365 cohort b: pembrolizumab (pembro) plus docetaxel and prednisone in abiraterone (abi) or enzalutamide (enza)-pretreated patients (pts) with metastatic castrate resistant prostate cancer (mCRPC). J Clin Oncol. 2019;37(suppl 7):170.30433849 [Google Scholar]
- 86. Massard C, Retz M, Hammerer P, et al. Pembrolizumab (pembro) plus docetaxel and prednisone in abiraterone (abi) or enzalutamide (enza)-pretreated patients (pts) with metastatic castrate resistant prostate cancer (mCRPC): cohort B of the phase 1b/2 KEYNOTE-365 study. J Clin Oncol. 2019;37(suppl 15):5029. [Google Scholar]
- 87. Yu EY, Massard C, Retz M, et al. Keynote-365 cohort a: pembrolizumab (pembro) plus olaparib in docetaxel-pretreated patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC). J Clin Oncol. 2019;37(suppl 7):145. [Google Scholar]
- 88. Yu EY, Massard C, Retz M, et al. Pembrolizumab (pembro) plus olaparib in docetaxel-pretreated patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC): cohort A of the phase 1b/2 KEYNOTE-365 study. J Clin Oncol. 2019;37(suppl 15):5027. [Google Scholar]
- 89. Yu EY, Wu H, Schloss C.. KEYNOTE-365: phase 1b/2 trial of pembrolizumab combination therapy for metastatic castration-resistant prostate cancer (mCRPC). Eur Urol. 2017;16(3):e360. [Google Scholar]
- 90. Gao J, Ward JF, Pettaway CA, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med. 2017;23(5):551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]