Abstract
Background
The COVID-19 pandemic has radically shifted healthcare operations within hospitals and universities across the globe. However, the effect of the COVID-19 pandemic on research endeavors and clinical trials is unclear.
Objective
This study investigates the impact of the COVID-19 pandemic on basic science and clinical research within the rhinology community.
Methods
A cross-sectional study was designed utilizing an 8-question survey to identify changes to rhinology research. Questions evaluated the impact of the COVID-19 pandemic on administrative research support and staffing, basic science research, clinical trials and resident research involvement.
Results
Seventy-one participants responded to the survey (8.5% response rate). Most respondents noted changes in IACUC/IRB approval (faster, 33%; slower, 31%). Of those who employed laboratory personnel, 64% were able to continue staff employment with full salary. The majority of animal research and in vitro studies were halted (64% and 56%, respectively), but animal care and cell line maintenance were allowed to continue. Clinical trial enrollment was most commonly limited to COVID derived studies (51%). Forty-seven percent of respondents noted increased resident research participation.
Conclusion
The rapid spread of the SARS-CoV-2 virus has markedly impacted rhinology-related research. Maintaining safe workplace practices as restrictions are lifted will hopefully mitigate the spread of the virus and allow research productivity to resume.
Keywords: COVID-19, SARS-CoV-2, Coronavirus, Pandemic, Basic science, Clinical trial, Resident education, Research, Rhinology
1. Introduction
The coronavirus disease 19 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to spread across the globe. As of 7 June 2020, nearly 7 million people have contracted the virus worldwide with over 400,000 succumbing to the disease [1]. Social distancing measures have been suggested or mandated in an effort to slow the spread of the disease and ease the burden on hospitals and other healthcare facilities. As part of the response, non-essential businesses have closed, universities have cancelled classes, and large-scale international travel restrictions are in place [2]. Within healthcare systems, the focus has shifted away from elective, routine care as the infrastructure has braced for the influx of COVID-19 patients.
The nose and nasopharynx harbor high SARS-CoV-2 viral loads in infected patients [3]. Within otolaryngology, comprehensive examinations often involve instrumentation of the upper aerodigestive tract. Therefore, concerns have been raised that otolaryngologists carry a significant risk of contracting COVID-19 from asymptomatic or presymptomatic carriers undergoing aerosol generating procedures, such as nasal endoscopy or pharyngolaryngoscopy [4]. Thus, many hospitals have suspended elective procedures, particularly those involving the sinonasal and aerodigestive tract [5].
While the COVID-19 pandemic has significantly impacted the routine operations of healthcare systems worldwide, the effect on ongoing research endeavors and clinical trial enrollment has yet to be elucidated. A national shift toward investigation of COVID-19 related pathogenesis, epidemiology, prevention and treatment has created a surge in COVID-19 publications with over 19,000 new publications related to COVID-19 within the past 5 months [6]. An abundance of recommendations and editorials within the field of otolaryngology detail how groups around the world are implementing safe strategies for performing necessary interventions during the pandemic [[7], [8], [9], [10], [11], [12]]. Clinicians who participate in research may have discovered ample time to focus on research projects or ideas. However, in a nationwide crisis with limited resources and the need to provide safe, distanced, working environments for personnel, this has not necessarily resulted in productive research.
In the current study, we sought to investigate the impact of the COVID-19 pandemic upon research practices, both basic science and clinical, within the rhinologic community.
2. Methods
This cross-sectional study was approved for an exemption by the University of Alabama at Birmingham Institutional Review Board. A survey was designed to identify how the COVID-19 pandemic has affected rhinology research. An invitation to participate in the study was distributed electronically via the American Rhinologic Society membership services to all regular and fellow members. The 8-question survey (Supplement 1) was hosted on the SurveyMonkey platform, and answers were collected and stored in a Microsoft Excel database. Percentages of each response to survey questions were calculated. Participants who answered “not applicable” to any question were removed from the denominator for percentage calculations.
3. Results
Seventy-one participants responded to the 8-question survey for a total response rate of approximately 8.5%. The questions in the survey were designed to evaluate the effect of the SARS-CoV-2 pandemic on administrative research support and staffing, basic science laboratories, clinical trials, and resident research involvement.
-
i.
Administrative Research Support and Staffing
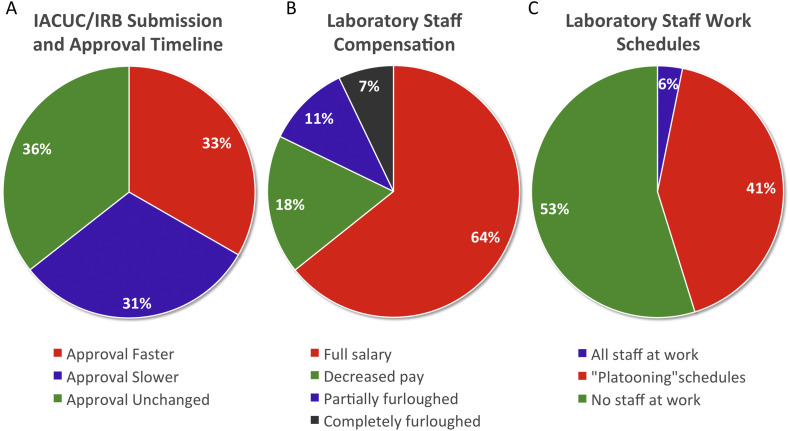
Approximately 33% and 31% (n = 15 and n = 14) of respondents reported a change in the IACUC/IRB approval process to be faster or slower, respectively at their institution. While ~36% (n = 16) reported no change in the approval process. (Fig. 1A).
Fig. 1.
The effect of COVID-19 pandemic on research support and staff: IACUC/IRB submission and approval timeline (A), Laboratory staff compensation (B), Laboratory staff work schedules (C).
Of those who employ laboratory personnel, two-thirds (64.3%; n = 18) have continued to pay their staff a full salary. Salary changes (17.9%; n = 5), partial dismissal of staff (10.7%; n = 3), and complete employee furloughs (7.1%; n = 2) were reported less frequently (Fig. 1B). The vast majority of laboratories had alterations to personnel schedules: 53.1% (n = 17) of respondents indicated employees were unable to return to the lab and 40.6% (n = 13) reported alternating schedules, as a “platooning” format. Only 6.3% (n = 2) reported normal lab staff work schedules (Fig. 1C).
-
ii.
Laboratory Based Research
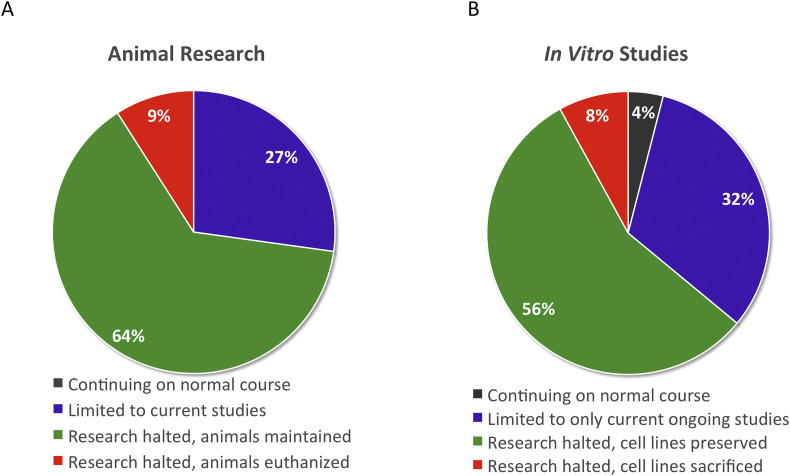
Of those respondents who participated in animal based research, none were able to continue normal activities in place prior to the pandemic. The majority (63.6%; n = 14) were forced to halt all animal-based research, but were permitted to continue animal care, 27.3% (n = 6) were limited to currently ongoing studies, and a small percentage (9.1%; n = 2) were forced to euthanize research animals (Fig. 2A).
Fig. 2.
The effect of COVID-19 pandemic on laboratory-based research: Animal research (A) and in vitro studies (B).
In vitro studies were similarly impacted with merely 4% (n = 1) able to continue in normal pre-COVID in vitro research. The majority of researchers (56%; n = 14) were required to halt their studies, but allowed to preserve cell lines, while 32% (n = 8) were able to continue current studies. Two researchers (8%) were required to halt research and were not permitted to preserve cell lines (Fig. 2B).
-
iii.
Clinical Trials
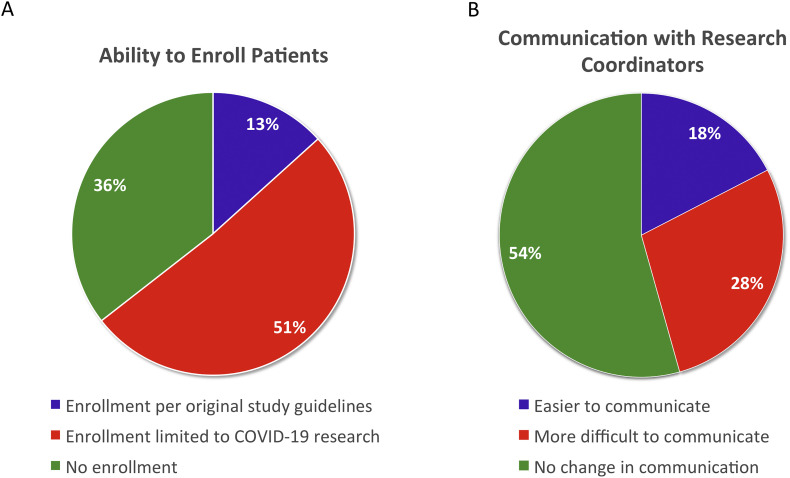
For clinical trials, 51.1% (n = 23) were allowed to enroll patients into COVID derived studies only. Approximately, 36% (n = 16) reported an inability to enroll patients in any studies, while 13.3% (n = 6) selected that they were permitted to continue enrolling in all clinical trials (Fig. 3A). Finally, the pandemic's impact on communication between coordinators and investigators was assessed in the new age of working remotely. Most respondents reported no change (54.3%, n = 25) with communication. While 17.4% (n = 8) indicated improved communication with their research coordinators, 28.2% (n = 13) conveyed more difficulty (Fig. 3B).
-
iv.
Resident Research Involvement
Fig. 3.
The effect of COVID-19 pandemic on clinical trials: Ability to enroll patients in clinical trials (A) and communication with research coordinators (B).
For respondents responsible for resident education and research activities, there were definite changes to involvement in either clinical or basic science research. Most reported an increase in resident involvement (46.8%, n = 22). The remainder reported a decrease in involvement (36.1%, n = 17) or no change (17%, n = 8) (Fig. 4 ).
Fig. 4.
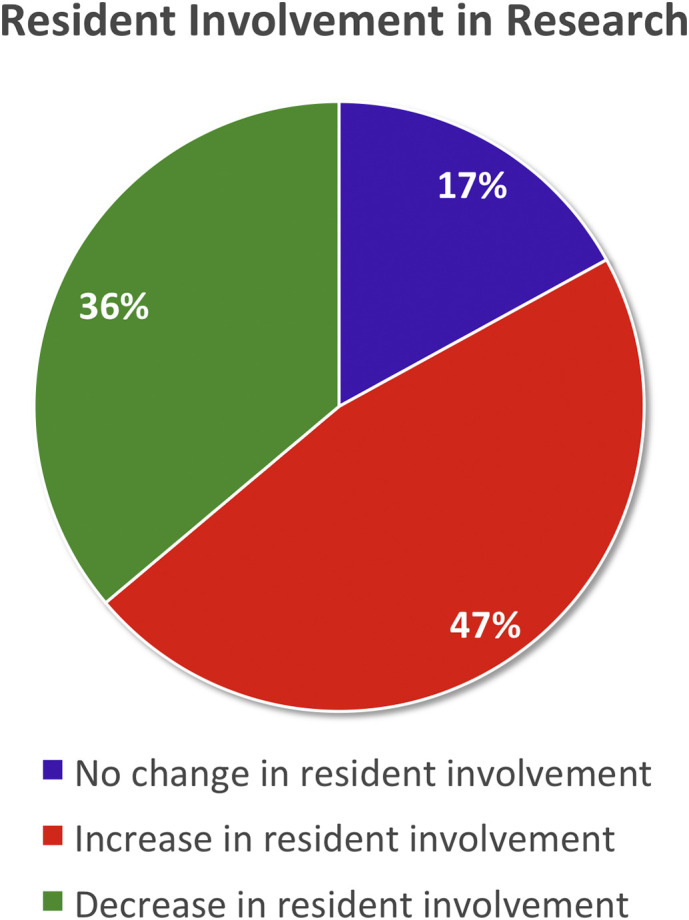
Resident participation in research activities since the COVID-19 pandemic.
4. Discussion
The COVID-19 pandemic has markedly impacted rhinology-related research productivity according to this cross-sectional survey study. As expected, the majority of respondents that were involved in animal research had limitations to both ongoing and new projects. A few respondents were even required to sacrifice research animals. Given the potential need for medications, personal protective equipment (PPE), lab personnel, and supplies to complete studies, even regional specific shut-downs can limit or halt studies in other areas of the country. With schools and child care centers closed, maintaining a full time staff to care for large numbers of research animals is also prohibitive [13]. Therefore, these findings are not surprising for animal-based research and in vitro studies.
Similarly, clinical research patient enrollment was markedly affected. Possible reasons include research coordinators working from home, lack of supplies to perform studies, and/or lack of patients presenting for elective procedures or outpatient visits thereby decreasing the pool of potential subjects. For some institutions, patient enrollment into clinical trials has ceased when elective cases and outpatient follow-up visits were stopped because they were considered “non-essential” to the care of patients. The long-term impact of the pandemic may include loss of publishing productivity, grant writing, and grant funding. For most investigators, the consequences will include loss of salary support and bonuses. Despite limitations in enrolling patients in trials, most respondents indicated easier or similar communication with research coordinators. This may be due to decreased scheduling conflicts by the principal investigator or fewer active trials run by the research coordinator.
Hospitals and academic medical centers across the country are losing millions of dollars due to the decrease in elective cases and inpatient demand for diagnoses other than SARS-CoV-2 viral illnesses [14]. This is particularly pronounced in hospitals that altered operative and clinic schedules in preparation for the surge of SARS-CoV-2 patients. While this is welcome news for patients, citizens, and healthcare workers within these facilities, it has resulted in significant loss of revenue. Some hospitals are having to furlough “non-essential” employees, which can include critical support personnel for research labs. This has a direct impact on current studies in the laboratory, animal care, and cell line preservation as well as the livelihood of our co-researchers [15]. Thankfully, most investigators were still capable of remunerating staff at full salary. However, there were laboratories that were forced to dismiss or furlough personnel. It is possible that we will see more deleterious effects in the coming months due to delayed data acquisition and loss of potential funding.
In regards to resident participation in research, there was a varied response rate that could be related to geographic differences in virus impact. Nearly 50% of those involved in resident research reported an increase in resident participation. Because regional differences were not assessed in this survey, it is possible that residents in areas less impacted by the COVID-19 had platooned schedules allowing more time to complete clinical research projects. Residents in harder hit regions may have been more likely re-deployed to other units or hospitals to care for critically ill patients, and probably represent those centers with decreased resident research involvement [16].
The authors recognize the major limitations of this study. A larger response rate may be more representative of the entire rhinologic community. Also, the lack of geographic data hampers our ability to make inferences, particularly about changes in resident participation in research. Similarly, we are unable to measure differences in research impact based upon type of practice (i.e. private versus academic).
This is the first known study to evaluate the impact of the COVID-19 pandemic on rhinology related research. Our findings indicate that the pandemic has negatively impacted a large number of rhinology research practices. As the pandemic progresses, it will be critical to investigate methods in which research can continue with safe workplace practices in an effort to avoid wasting of resources due to postponing or terminating ongoing experiments. While we have observed a large volume of publications related to the SARS-CoV-2 virus and the effect on medical and surgical practices, we will undoubtedly observe a major negative impact on other research endeavors.
5. Conclusion
The SARS-CoV-2 virus has challenged all aspects of medicine over the last several months. The duration of the pandemic and its widespread impact on our daily lives is unclear, but devising a plan to ensure the longevity of our research endeavors is critical to the field of rhinology.
Funding
The authors have no funding source to report for this manuscript.
Declaration of competing interest
The authors have no relevant conflicts of interest to disclose.
References
- 1.John'’s Hopkins Center for Systems Science and Engineering https://coronavirus.jhu.edu/map.html Available at: Accessed 6/7/20.
- 2.Sohrabi C., Alsafi Z., O’Neill N. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou L., Ruan F., Huang M. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel Z.M., Fernandez-Miranda J., Hwang P.H. Letter: precautions for endoscopic transnasal skull base surgery during the COVID-19 pandemic. Neurosurgery. 2020;87(1):E66–E67. doi: 10.1093/neuros/nyaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z., Zhang L. At the center of the COVID-19 pandemic: lessons learned for otolaryngology-head and neck surgery in China. Int Forum Allergy Rhinol. 2020;10(5):584–586. doi: 10.1002/alr.22585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Library of Medicine https://pubmed.ncbi.nlm.nih.gov/?term=covid+19 Available at. [DOI] [PubMed]
- 7.Goldman R.A., Swendseid B., JYK Chan. Tracheostomy management during the COVID-19 pandemic. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820923632. E-pub ahead of print In press. [DOI] [PubMed] [Google Scholar]
- 8.Noel J.E., Orloff L.A., Sung K. Laryngeal evaluation during the COVID-19 pandemic: transcervical laryngeal ultrasonography. Otolaryngol Head Neck Surg. 2020;194599820922984 doi: 10.1177/0194599820922984. E-pub ahead of print In press. [DOI] [PubMed] [Google Scholar]
- 9.Ranasinghe V., Mady L.J., Kim S. Major head and neck reconstruction during the COVID-19 pandemic: the University of Pittsburgh approach. Head Neck. 2020 doi: 10.1002/hed.26207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Setzen M., Svider P.F., Pollock K. COVID-19 and rhinology: a look at the future. Am J Otolaryngol. 2020:102491. doi: 10.1016/j.amjoto.2020.102491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skoog H., Withrow K., Jeyarajan H. Tracheotomy in the SARS-CoV-2 pandemic. Head Neck. 2020 doi: 10.1002/hed.26214. E-pub ahead of print In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Workman A.D., Welling D.B., Carter B.S. Endonasal instrumentation and aerosolization risk in the era of COVID-19: simulation, literature review, and proposed mitigation strategies. Int Forum Allergy Rhinol. 2020 doi: 10.1002/alr.22577. E-pub ahead of print In press. [DOI] [PubMed] [Google Scholar]
- 13.Gewin V. Safely conducting essential research in the face of COVID-19. https://www.nature.com/articles/d41586-020-01027-y Available at: [DOI] [PubMed]
- 14.Grimm C.A. DHHS; 2020. Hospital experiences responding to the COVID-19 pandemic: results of a National Pulse Survey March 23–27, 2020. [Google Scholar]
- 15.Apuzzo M., Kirkpatrick D.D. Covid-19 changed how the world does science, together. https://www.nytimes.com/2020/04/01/world/europe/coronavirus-science-research-cooperation.html Available at:
- 16.Crosby D.L., Sharma A. Insights on otolaryngology residency training during the COVID-19 pandemic. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820922502. E-pub ahead of print In press. [DOI] [PubMed] [Google Scholar]