Abstract
Renal glucose reabsorption was measured with the stepped hyperglycemic clamp in 15 subjects with type 2 diabetes mellitus (T2DM) and 15 without diabetes after 2 days and after more chronic (14 days) treatment with empagliflozin. Patients with T2DM had significantly greater maximal renal glucose transport (TmG) compared with subjects without diabetes at baseline (459 ± 53 vs. 337 ± 25 mg/min; P < 0.05). Empagliflozin treatment for 48 h reduced the TmG in both individuals with and without diabetes by 44 ± 7 and 53 ± 6%, respectively (both P < 0.001). TmG was further reduced by empagliflozin in both groups on day 14 (by 65 ± 5 and 75 ± 3%, respectively). Empagliflozin reduced the plasma glucose concentration threshold for glucose spillage in the urine similarly in individuals with T2DM and without diabetes to <40 mg/dL, which is well below the normal fasting plasma glucose concentration. In summary, sodium-glucose transporter-2 inhibition with empagliflozin reduces both TmG and threshold for glucose spillage in the urine in patients with T2DM and those without diabetes.
Introduction
The sodium-glucose transporter-2 inhibitors (SGLT2i) represent the newest class of oral antidiabetic agents approved for the treatment of patients with type 2 diabetes mellitus (T2DM) (1). Unbound SGLT2i is filtered by the glomerulus and binds to the SGLT2 in the S1 segment of the proximal tubule, resulting in glucosuria and decline in the plasma glucose concentration (1,2). The resultant decline in plasma glucose concentration secondarily leads to amelioration of insulin resistance in muscle and improved β-cell function (3–5). The kidney filters ∼180 L of plasma each day, which in healthy individuals without diabetes contains ∼180 g of glucose. In healthy individuals, all of the filtered glucose is reabsorbed in the early proximal tubule. A total of 80–90% of the filtered glucose is taken up in the S1 segment of the proximal tubule via a sodium-dependent mechanism by SGLT2, whereas the remaining 10–20% is taken up in the S3 segment by SGLT1 (1,2). Thus, inhibition of renal SGLT2, as well as SGLT1, provides a therapeutic target to reduce glycemia in individuals with T2DM.
Three previous studies have examined the effect of SGLT2i on the kinetics of renal glucose transport in man (6–8). In the study by DeFronzo et al. (6) using the stepped hyperglycemic clamp technique, dapagliflozin was shown to reduce the maximum renal glucose reabsorptive capacity (TmG) at which glucose appeared in the urine in both individuals with T2DM and normal glucose tolerant (NGT) individuals. In subjects with T2DM, the plasma glucose concentration at which the TmG was reached was ∼150 mg/dL, which is well above the fasting plasma glucose (FPG) concentration in NGT subjects. Nonetheless, when given to NGT individuals in the postabsorptive state, dapagliflozin causes marked glucosuria (6,9), indicating that reduction in TmG cannot explain the increase in renal glucose excretion. Rather, the induction of glucosuria was explained by a marked decrease in the renal threshold for glucose spillage into the urine to <40 mg/dL (6). In the study by Sha et al. (7), subjects consumed three standardized meals, and plasma glucose was determined frequently throughout the day as urine was collected over 24 h after a single dose of canagliflozin. From these measurements, the renal threshold for glucose was estimated to be ∼60–70 mg/dL. However, from the SD of the mean, it is clear that the renal threshold must have been >80 mg/dL in many individuals. Although this would explain the glucosuric effect of the SGLT2i in patients with T2DM, it cannot explain the marked glucosuria observed in NGT individuals, many of whom have a fasting glucose concentration <80 mg/dL. In a subsequent publication (8), the same authors compared this method (measurement of urine glucose excretion with frequent plasma glucose determinations following a standardized meal) with the stepped hyperglycemic clamp in 14 patients with T2DM before and after 8 days of canagliflozin. The renal threshold, determined with the stepped hyperglycemic clamp, could not be determined in two subjects and averaged 49 mg/dL in the remaining 12. The correlation between the renal threshold determined by the two methods in the same subject was poor (r2 = 0.24). Using the stepped hyperglycemic clamp in Zucker Diabetic Fatty rats (10), the same authors reported that canagliflozin reduced the renal threshold to 94 mg/dL.
The purpose of the current study was twofold. First was to use the stepped hyperglycemic clamp to determine the TmG and threshold for glucose spillage into the urine in subjects with T2DM and without diabetes before and after empagliflozin administration. Second was to examine the acute and more chronic effects of empagliflozin on the Tm and renal glucose threshold in subjects with T2DM and without diabetes. The study is registered at ClinicalTrials.gov (NCT01867307).
Research Design and Methods
Subjects
Fifteen subjects with T2DM and 15 subjects without diabetes participated in the study. The patient characteristics are shown in Supplementary Table 1. The HbA1c in subjects with T2DM and without diabetes was 7.8 ± 0.2 and 5.6 ± 0.1%, respectively. All subjects without diabetes had an HbA1c <6.5%; 8 of the 16 subjects without diabetes had an FPG ≥100 mg/dL. Other than having diabetes, subjects were in good general health as determined by medical history, physical exam, screening laboratory tests, and electrocardiogram. Body weight was stable (±3 pounds) in all subjects for 3 months prior to study, and no subject participated in any excessively heavy exercise program. Other than metformin and/or sulfonylureas, no subjects were taking any medications known to effect glucose metabolism.
Research Design
This was an open-label, two-arm (with and without diabetes) study in which all subjects were treated with empagliflozin, 25 mg/day, after completion of baseline studies (Supplementary Fig. 1). On days −5 and −4, a 24-h urine collection was performed for measurement of glucose, creatinine, sodium, potassium, and chloride. The FPG, sodium, potassium, and chloride concentrations were determined on the same day that the 24-h urine was collected.
On day −3, a stepped hyperglycemic clamp was performed in the morning following a 10-h overnight fast. Subjects reported to the Clinical Research Center at 6 a.m. (time = −180 min), a catheter was placed into an antecubital vein, and a prime (30 mg)–continuous (0.18 mL/min) infusion of iohexol (250 mg/mL) was initiated. A second catheter was placed into a vein on the dorsum of the hand, and the hand was placed in a thermoregulated box heated to 70°C. Subjects consumed a water load (20 mL/kg) to ensure frequent voiding. At 7:30 a.m. (−90 min), subjects without diabetes were asked to void, the urine was discarded, and a volume of water equivalent to the voided volume of urine was consumed. Arterialized blood samples were obtained at −90, −30, and 0 min for the measurement of plasma glucose, creatinine, iohexol, sodium, potassium, and chloride concentrations. Urine was collected from −90 to 0 min for determination of glucose, sodium, chloride, potassium, and iohexol concentrations. At time 0 (9 a.m.), a stepped hyperglycemic clamp (11) was performed. The plasma glucose concentration was acutely raised and maintained at 40 mg/dL above the fasting level (i.e., from ∼100 to 140 mg/dL) for 40 min, and urine was collected from 0–40 min and analyzed for glucose, iohexol creatinine, sodium, potassium, and chloride. From 40–80, 80–120, 120–160, 160–200, 200–240, 240–280, 280–320, and 320–360 min, the plasma glucose concentration was acutely raised and maintained at 180, 220, 260, 300, 340, 380, 420, and 460 mg/dL, respectively. Urine was collected during each 40-min period, and the subject consumed an amount of water equal to the voided urine volume to ensure spontaneous voiding. All urine samples were analyzed for glucose, iohexol, sodium, potassium, and chloride. During the hyperglycemic clamp, subjects ingested 15–30 meq KCl to avoid hypokalemia, which can occur in response to the increase in insulin secretion.
The stepped hyperglycemic clamp protocol in subjects with T2DM was similar to that in the subjects without diabetes, with the following exception. Upon arrival at the Clinical Research Center at 6 a.m., a low-dose insulin infusion (0.1–0.2 mU/kg/min) was started to reduce the FPG concentration to ∼100 mg/dL. The insulin infusion then was discontinued for 20 min, at which time the stepped hyperglycemic clamp was performed as described above. The subjects with T2DM did not take their oral antidiabetic medication on the day of the stepped hyperglycemic clamp.
On day −2 and day −1, 24-h urines were again collected for measurement of glucose, creatinine, sodium, potassium, and chloride. The FPG, creatinine, sodium, potassium, and chloride were determined on the same days of collection (Supplementary Fig. 1).
At ∼8 a.m. on day 0, subjects with T2DM and without diabetes were started on empagliflozin, 25 mg/day, which they took in the morning for 14 days (Supplementary Fig. 1). On days 0 (i.e., the day that empagliflozin therapy was started) and 1 (second day of empagliflozin administration), 24-h urines were collected for determination of glucose, creatinine, sodium, potassium, and chloride. FPG, creatinine, sodium, potassium, and chloride were measured on the same days. On the morning of day 2 (i.e., 48 h after the start of empagliflozin), the stepped hyperglycemic clamp was repeated as described above. On days 12 and 13, repeat 24-h urine collections and fasting plasma samples were obtained and analyzed as described above (Supplementary Fig. 1). On day 14, the stepped hyperglycemic clamp was repeated.
Analytical Techniques
Glucose concentration in plasma and urine was determined by glucose oxidase method (Analox; Analox Instruments, Lumenburg, MA). Plasma and urine iohexol concentrations were determined by high-performance liquid chromatography (6). Plasma and urine creatinine concentrations were determined with the Dimension Xpand Plus chemistry analyzer (Siemens, Deerfield, IL). Plasma sodium and chloride were determined with the Dimension Xpand Plus chemistry analyzer. Urine sodium and chloride were determined with the Dimension Vista 1500 Analyzer (Siemens).
Calculations
Glomerular filtration rate (GFR) was determined from the clearance of iohexol as the iohexol infusion rate divided by the steady state plasma iohexol concentration during each step of the hyperglycemic clamp. Urinary glucose excretion (UGE) during each step of the hyperglycemic clamp was calculated as the product of urinary volume during that step and urinary glucose concentration. The filtered glucose load at each hyperglycemic clamp step was calculated as the GFR (mL/min) × mean plasma glucose concentration (mg/mL) during the same period. The percentage of the filtered glucose load excreted during each time period was calculated as the UGE during each step of the hyperglycemic clamp divided by the filtered glucose load during the same period. To calculate the Tm, the pharmacodynamic model (GReab = Tm × [1 − e−k × PG]) described by DeFronzo et al. (6), which describes the relationship among glucose reabsorption rate (GReab), plasma glucose concentration (PG), and Tm, was used. Glucose reabsorption (GReab) was calculated as the difference between the amount of glucose filtered and the amount of glucose excreted during each hyperglycemic clamp step.
The plasma glucose concentration at which glucose first appeared in the urine was calculated as: (Tm/GFR) × (1 − e−k×PG) × 100 (6). We also estimated the threshold by extrapolating the regression line relating UGE and plasma glucose concentration during the hyperglycemic clamps. These values agreed within 10%, and the pharmacodynamic calculation is provided in this study to be consistent with a previous publication from our group (6).
Statistical Analyses
Values are expressed as the mean ± SEM. Differences between the TmG and renal threshold for glucose at baseline versus 48 h and after 14 days after of empagliflozin treatment in the groups with and without diabetes were compared with repeated-measures ANOVA. The effect of empagliflozin on urine electrolyte excretion and GFR was similarly compared. Statistical significance was set at P < 0.05.
Study Approval
The study protocol was approved by the Institutional Review Board of The University of Texas Health Science Center in San Antonio, TX, and all subjects gave their written informed voluntary consent prior to participation.
Results
Effect of Empagliflozin on Plasma Glucose Concentration
The FPG concentration (mean of four determinations performed on days −5, −4, −2, and −1 prior to the start of empagliflozin) was 195 ± 9 and 103 ± 2 mg/dL in subjects with T2DM and without diabetes, respectively. At 24 and 48 h after starting empagliflozin, the FPG declined to 169 ± 11 and 165 ± 7 mg/dL, respectively, in the group with diabetes (P < 0.001) and remained unchanged (101 ± 2 and 100 ± 2 mg/dL) in the group without diabetes. On day 14 after starting empagliflozin, the FPG declined further in the group with diabetes (160 ± 7; P < 0.001) and remained unchanged in the group without diabetes (101 ± 2 mg/dL).
The mean plasma glucose concentrations during each of the nine steps of the hyperglycemic clamp performed at baseline in subjects with and without diabetes are displayed in Supplementary Fig. 2. During each hyperglycemic step, the plasma glucose concentration was maintained close to the goal with a coefficient of variation of <5%. The plasma glucose concentrations during the stepped hyperglycemic clamps performed 48 h and 14 days after empagliflozin were superimposable to those during the pre-empagliflozin hyperglycemic clamp.
Effect of Empagliflozin on UGE
In the group with diabetes, UGE (mean of four 24-h determinations prior to empagliflozin) was 19 ± 5 g/day, rose to 104 ± 12 and 101 ± 10 g/day (P < 0.0001 vs. baseline) on days 0 (start of empagliflozin) and 1 postempagliflozin, respectively, and remained elevated on days 12 and 13 postempagliflozin (117 ± 12 and 105 ± 12 g/day, respectively). In the group without diabetes, UGE before empagliflozin was 0.2 ± 0.1 g/day, rose to 47 ± 3 and 52 ± 3 g/day (mean of four 24-h determinations prior to empagliflozin) (P < 0.0001 vs. baseline) on days 0 and 1 postempagliflozin, respectively, and remained elevated on days 12 and 13 after empagliflozin (43 ± 4 and 42 ± 5 g/day).
Effect of Empagliflozin on GFR and Urinary Electrolyte Excretion
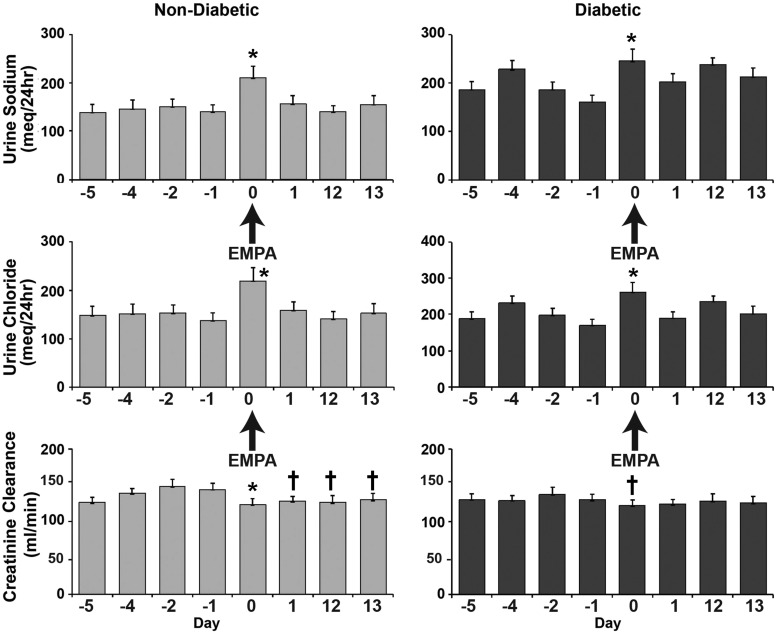
Prior to empagliflozin, GFR measured as creatinine clearance (mean of four 24-h determinations), was 132 ± 8 and 139 ± 7 mL/min in the groups with and without diabetes, respectively, and was significantly reduced by empagliflozin on day 1 (125 ± 11 [group with diabetes] and 128 ± 7 [group without diabetes] mL/min; P < 0.05 vs. baseline) and day 13 (129 ± 10 [group with diabetes] and 126 ± 10 [group without diabetes] mL/min; P < 0.05 vs. baseline). Urinary sodium excretion prior to empagliflozin was 190 ± 7 and 144 ± 1 meq/day (mean of four 24-h urine collections) in groups with and without diabetes, respectively, rose significantly (P < 0.01) in both groups on the first day of empagliflozin administration, returned to baseline values on the second day after empagliflozin in both groups, and remained at the baseline value on days 12 and 13 in the groups without diabetes (Fig. 1). In the group with diabetes, the 24-h sodium excretion on day 13 had returned to a value that was not significantly different from baseline. There were two outliers that were responsible for the increased 24-h sodium excretion on day 12. Urinary chloride excretion paralleled that of sodium excretion. Empagliflozin had no effect on urinary potassium excretion (data not shown).
Figure 1.
GFR (creatinine clearance) and urinary excretion of sodium and chloride in subjects without diabetes and with T2DM at baseline, on the day that empagliflozin (EMPA) was started (day 0), and during the 24–48-h period (day 1) after the start of empagliflozin. *P < 0.01 vs. baseline; †P < 0.05 vs. baseline.
Effect of Empagliflozin on UGE and Fractional Excretion of Glucose During Hyperglycemic Clamp
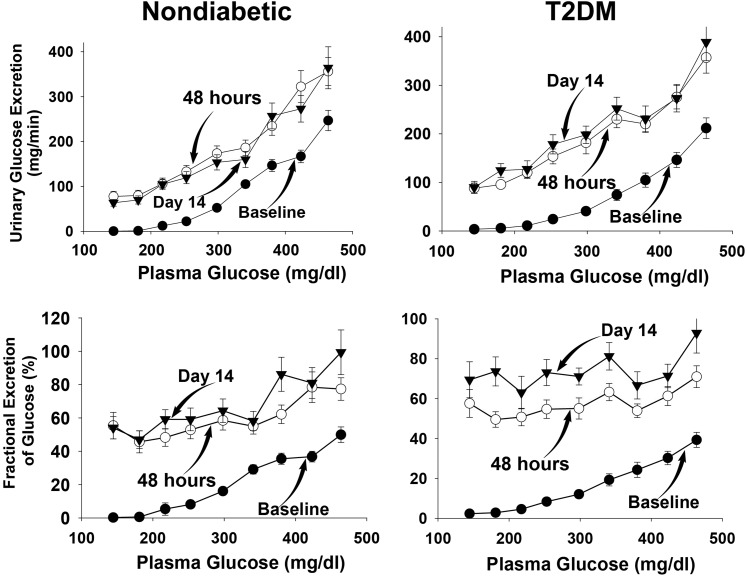
The rate of UGE in subjects with and without diabetes during each step of the three hyperglycemic clamp studies is shown in Fig. 2. The rise above baseline in UGE during the hyperglycemic clamp performed before the start of empagliflozin began during the third clamp step (plasma glucose concentration of 220 mg/dL). With each subsequent hyperglycemic step, there was a progressive rise in UGE, which was comparable in both groups with and without diabetes.
Figure 2.
UGE (top) and fractional excretion of glucose (bottom) during the hyperglycemic clamp in subjects without diabetes (left) and with T2DM (right) at baseline and at 48 h and 14 days after the start of empagliflozin. The plasma glucose concentration during each of the nine steps of the hyperglycemic clamp is shown on the horizontal axis.
During the hyperglycemic clamp studies performed at 48 h and 14 days after the start of empagliflozin, significant glucosuria was present prior to the start of glucose infusion, and UGE rose progressively in both groups with and without diabetes, starting with the first (40 mg/dL) hyperglycemic clamp step (Fig. 2). Extrapolating the regression line relating UGE and plasma glucose concentration during the hyperglycemic clamp yields a plasma glucose concentration threshold for glucosuria of 32 and 34 mg/dL in subjects without diabetes and 41 and 35 mg/dL in subjects with diabetes on days 2 (i.e., 48 h after the start of empagliflozin) and 14, respectively.
The fraction (mean from 0–360 min) of filtered glucose excreted during the baseline hyperglycemic clamp was 16 ± 1 and 20 ± 2% in subjects with and without diabetes, respectively. However, this fraction varied markedly based upon the plasma glucose concentration during the stepped hyperglycemic clamp, rising progressively as the plasma glucose concentration increased >180 mg/dL (Fig. 2); at each hyperglycemic clamp step, the fractional glucose excretion was comparable in individuals with and without diabetes.
Empagliflozin caused a marked increase in the fraction of filtered glucose excreted in the urine to 62 ± 4 and 57 ± 3%, respectively (both P < 0.001 vs. baseline), during the hyperglycemic clamp performed 48 h after the start of empagliflozin, and to 69 ± 6 and 73 ± 3% during the hyperglycemic clamp performed on day 14 after the start of empagliflozin in subjects without and with diabetes, respectively. The increase in the fraction of filtered glucose excreted caused by empagliflozin was comparable in individuals with and without diabetes (Fig. 2).
Renal Glucose Reabsorption, TmG, and Threshold for Renal Glucose Excretion
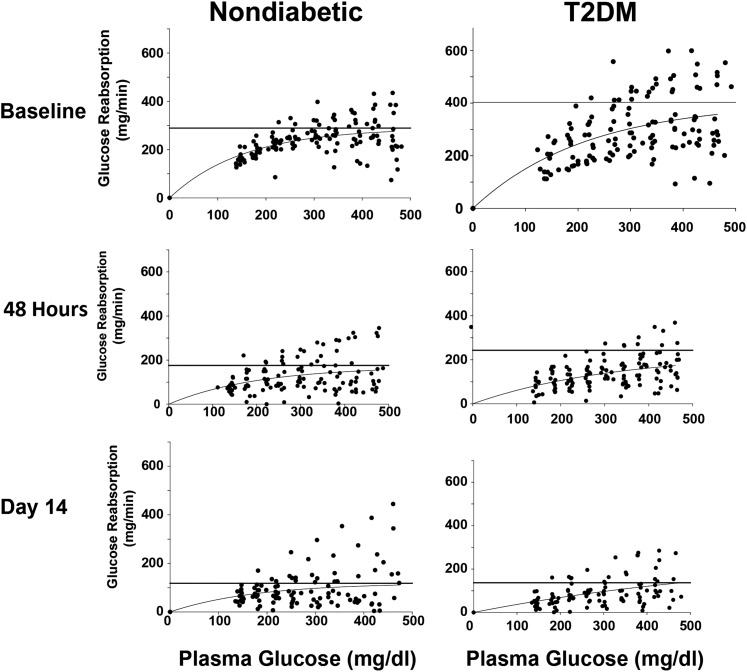
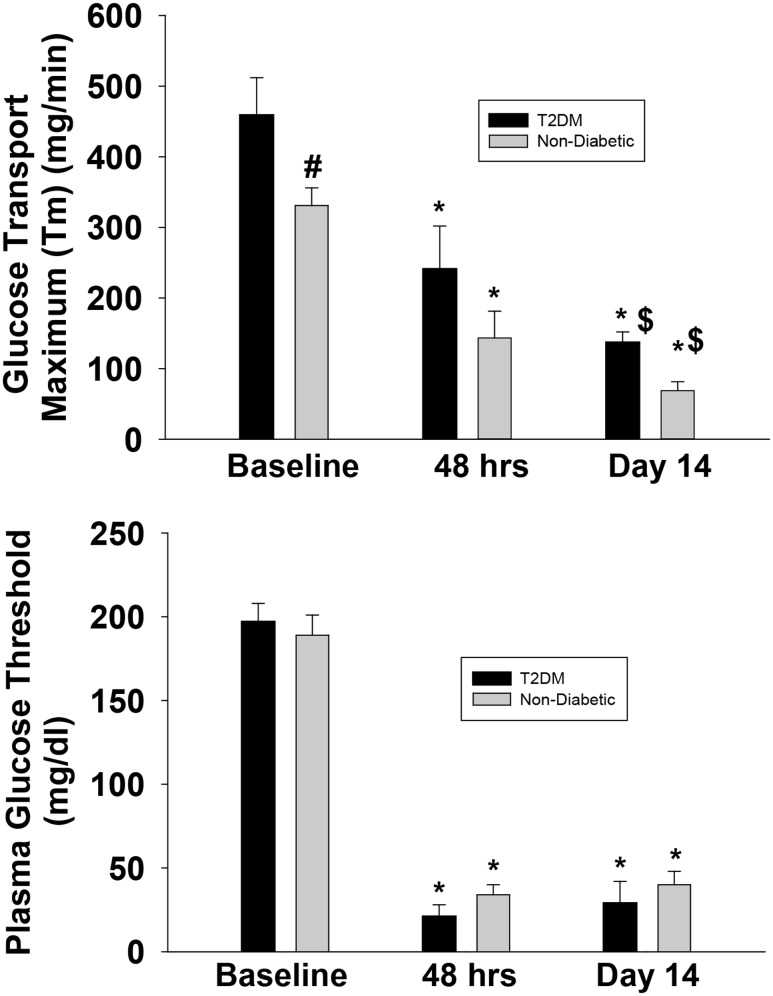
Figure 3 depicts the relationship between the rate of renal glucose reabsorption and plasma glucose concentration before and after empagliflozin treatment in subjects with and without diabetes during the three hyperglycemic clamps (baseline, 48 h after the start of empagliflozin, and on day 14 after the start of empagliflozin). The rate of renal glucose reabsorption was significantly higher in subjects with versus without diabetes and was markedly inhibited by empagliflozin. Prior to the start of empagliflozin, the Tm for glucose reabsorption was significantly higher, by 36%, in subjects with versus without diabetes (459 ± 53 vs. 337 ± 25 mg/min; P < 0.05) (Fig. 4, top panel). The renal threshold for glucose spillage into the urine occurred at a plasma glucose concentration of 197 ± 11 and 190 ± 2 mg/dL (P = NS) in groups with and without diabetes, respectively. There were two outliers (>5 SDs below the mean) in the group with diabetes, and they significantly lowered the mean threshold. If these two outliers are removed, the threshold is 211 ± 6 mg/dL (P = 0.003 vs. group without diabetes). At 48 h after the start of empagliflozin, the TmG for glucose was similarly decreased by 44 ± 7% in subjects with diabetes (to 241 ± 42 mg/min) and without diabetes by 53 ± 6% (to 150 ± 19 mg/min). The plasma glucose concentration threshold (pharmacodynamic model) for glucose spillage into the urine was 21 ± 7 mg/dL and 29 ± 5 mg/dL in groups with and without diabetes, respectively (Fig. 4, bottom panel), and these values agreed closely with the threshold determined by extrapolating the regression line relating UGE and plasma glucose concentration during the hyperglycemic clamp. On day 14 after the start of empagliflozin, the TmG was further reduced by 65 ± 5% in the group with diabetes (to 137 ± 15 mg/min; P < 0.0001 vs. day 3) and by 75 ± 3% in the group without diabetes (to 80 ± 9 mg/min; P < 0.05 vs. day 3). At day 14, the plasma glucose concentration threshold for glucose spillage into the urine remained unchanged compared with day 1 (i.e., 48 h after the start of empagliflozin) in both individuals with and without diabetes (29 ± 13 and 42 ± 8 mg/dL, respectively) (Fig. 4, bottom panel).
Figure 3.
Renal glucose reabsorption is plotted as a function of the plasma glucose concentration during the hyperglycemic clamp in subjects without diabetes (left) and with T2DM (right) at baseline and at 48 h and 14 days after the start of empagliflozin.
Figure 4.
Maximum Tm (top) and renal threshold (bottom) for glucose spillage into the urine in subjects without diabetes and with T2DM at baseline and at 48 h and 14 days after the start of empagliflozin. #P < 0.05 vs. subjects without diabetes; *P < 0.0001 vs. baseline; $P < 0.05 vs. 48 h.
Safety Results
Increased frequency of urination was reported in 22 participants and primarily occurred during the initial 48 h after the start of empagliflozin. In order of frequency, the following number of participants experienced headache (n = 7), dry mouth (n = 5), nausea (n = 4), back pain (n = 4), light-headedness (n = 3), weakness (n = 3), emesis (n = 3), abdominal pain (n = 2), palpitation (n = 2), and rash (n = 1) on at least one occasion during the 14-day treatment period. Two males experienced a penile fungal infection; no vaginal mycotic infections occurred in any female. No participants experienced a bacterial urinary tract infection.
Discussion
The current study assessed the acute (after 48 h) and more chronic (14 days) effect of SGLT2 inhibition on renal glucose handling in healthy individuals without diabetes or with T2DM and provides two novel findings. First, it delineates the time course of effect of SGLT2 inhibition on the TmG and renal threshold for glucose spillage into the urine. Although empagliflozin similarly lowered the TmG in both individuals with and without diabetes, inhibition of SGLT2 and subsequent reduction in TmG in both groups was not complete during the hyperglycemic clamp performed 48 h after the start of empagliflozin, and further reduction in TmG was observed at day 14 compared with day 3 (Fig. 4). We believe that this is explained by the pharmacokinetic properties of empagliflozin, which have shown that the time required to achieve steady-state plasma concentration is 4 to 5 days (12).
The second novel finding of the current study is the documentation, for the first time in man, that SGLT1 has a large capacity to reabsorb glucose, removing approximately one-third of the filtered glucose load following inhibition of the SGLT2 transporter. Although SGLT2 is responsible for the absorption of >80–90% of filtered glucose in subjects without diabetes under normal physiologic conditions (1), SGLT2 inhibition with empagliflozin inhibited the absorption of only ∼40 and ∼50% of filtered glucose during the hyperglycemic clamp in individuals with and without diabetes, respectively. This apparent paradox can be explained by the relationship between the amount of glucose filtered and the fraction of filtered glucose reabsorbed by each sodium glucose cotransporter in the presence and absence of the SGLT2i (13). Because SGLT2 is located in the proximal part of the proximal tubule (S1 segment), whereas SGLT1 is located in a more distal part (S2/S3) of the proximal tubule, the glomerular filtrate must first pass through SGLT2, where the majority of filtered glucose (∼80–90%) is taken up. Thus, by the time glomerular filtrate reaches the S3 segment, only a small amount of the filtered glucose (10–20%) remains to be “cleaned up” by the SGLT1 transporter. However, under conditions of complete SGLT2 inhibition, SGLT1 remains the sole mechanism of renal glucose reabsorption and, contrary to common belief, has the ability to reabsorb significant amounts of glucose. Following inhibition of SGLT2, the great majority of filtered glucose reaches the S2/S3 segment, and SGLT1 is forced to reabsorb glucose at its maximal capacity. Thus, the amount of glucose excreted in the urine following maximal SGLT2 inhibition will highly depend upon the maximal SGLT1 glucose transport capacity and equals the amount of glucose that is filtered minus the SGLT1 maximal glucose transport capacity. The renal Tm for glucose reabsorption in the absence of SGLT2 inhibition reflects the sum of the maximal transport capacity (TmG) of SGLT1 plus SGLT2, whereas the renal TmG in the presence of maximal inhibition of SGLT2 reflects the maximal transport capacity (TmG) of SGLT1.
Because, during SGLT2 inhibition, a fixed amount of filtered glucose is taken back by SGLT1, the fraction of filtered glucose excreted will rise progressively as the plasma glucose concentration and subsequent increase in filtered glucose load increases (Fig. 2). Therefore, the efficacy of SGLT2i in lowering plasma glucose concentration, and consequently the HbA1c, will increase progressively with the increase in baseline HbA1c (which reflects the mean daylong plasma glucose concentration) because, as HbA1c increases, more glucose is removed from the body by the SGLT2i. Indeed, clinical studies (14,15) have demonstrated that, at high HbA1c levels (>8.5–9%), both empagliflozin and dapagliflozin have a significant advantage over dipeptidyl peptidase 4 inhibitors in lowering the plasma glucose concentration in patients with T2DM (12,16).
The renal Tm for glucose in subjects without diabetes in this study was comparable to that previously reported (6,16). The renal TmG of patients with T2DM at baseline was 39% higher than in subjects without diabetes, consistent with previous observations (6), which have shown a significant increase in TmG in subjects with compared with subjects without diabetes. However, the magnitude of increase in TmG in subjects with diabetes in the current study was greater than reported in previous studies. Farber et al. (16) and DeFronzo et al. (6) reported a 15 and 32% increase, respectively, in TmG in individuals with versus without diabetes. Because the plasma glucose concentration is the principal regulator of the renal TmG, the poorer glycemic control of subjects with T2DM in the current study compared with that of DeFronzo et al. (6) (HbA1c 7.9 vs. 6.5%) could explain the greater increase in TmG. Surprisingly, the magnitude of TmG correlated weakly with HbA1c (r = 0.31; P = NS). This could be explained by the possibility that worsening glucose control requires >3 months to exert its effect to increase renal TmG.
Because the TmG on day 14 reflects the TmG of SGLT1, whereas the TmG at baseline reflects the TmG for both SGLT1 plus SGLT2, the difference between the two reflects the TmG for SGLT2. The present results demonstrate that SGLT1 transport capacity accounts for ∼30% of the total renal glucose reabsorption capacity (assuming that the results at day 14 can be extrapolated to baseline (i.e., before empagliflozin), whereas SGLT2 accounts for the remaining ∼70% of renal glucose reabsorption capacity. In absolute terms, the TmG for SGLT2 was 322 and 239 mg/min in individuals with and without diabetes, respectively. Thus, approximately two-thirds of the increase in renal Tm in patients with T2DM (128 mg/min) is because of the increase in SGLT2 transport.
Under fasting conditions, the filtered glucose load following 48 h of empagliflozin treatment was lower than the renal TmG for glucose transport in the vast majority of patients (mean 154 ± 9 vs. 241 ± 42 mg/min in patients with diabetes and 98 ± 4 vs. 184 ± 38 mg/min in patients without diabetes). Nonetheless, large amounts of glucose are excreted in the urine during the fasting state. This is explained by the marked decrease in renal threshold for glucose excretion, which was reduced by empagliflozin to ∼30 and ∼40 mg/dL in subjects with and without diabetes, respectively. The renal threshold for glucose excretion in the current study, which was measured with two different methods (pharmacodynamic model and extrapolation of the regression line relating UGE and plasma glucose concentration during the hyperglycemic clamp), was comparable to that reported with dapagliflozin treatment but significantly lower than that reported by Sha et al. (7) and Polidori et al. (8) with canagliflozin. Although these differences could be explained by intrinsic differences between empagliflozin and dapagliflozin versus canagliflozin, we believe that this is unlikely. The method used by Sha et al. (7) has a major inherent flaw in that it assumes, like renal phosphate handling, that all of the increase in plasma glucose concentration above the fasting level will be excreted in the urine. This clearly is not the case in subjects without diabetes, and, to the extent that in subjects with diabetes the FPG concentration is below the threshold, this also will not be correct. The net result is that the true renal glucose threshold following SGLT2 inhibition will be markedly overestimated. In a subsequent publication using the stepped hyperglycemic clamp, the same authors (8) reported a much lower threshold for glucose excretion (49 mg/dL), which is much closer to that reported with empagliflozin in the current study and with dapagliflozin in a previous study (6). However, in this later study (8), the renal glucose threshold could not be measured in 2 of 14 subjects, and this likely would bring their mean value for the glucose threshold even closer to that observed in the current study.
In summary, the present results demonstrate that: 1): empagliflozin reduces the threshold for glucose spillage into the urine to a level well below the FPG concentration in subjects without diabetes and explains why virtually all patients with diabetes with normal/near-normal GFR have a significant glucosuric effect and respond with a reduction in mean plasma glucose concentration; 2) there is a time-related effect that requires up to 2 weeks for empagliflozin to exert its maximum effect to inhibit renal glucose reabsorption; and 3) the glucosuric effect of empagliflozin and other SGLT2i is offset by ∼30% by SGLT1 reabsorption of glucose. These results provide credence for the use of combined SGLT2/SGLT1i therapy to maximize the effect of SGLT inhibition on glucosuria and reduction in HbA1c.
Supplementary Material
Article Information
Funding. This work was supported by Boehringer Ingelheim and Eli Lilly and Company. R.A.D.’s salary is supported, in part, by the South Texas Veterans Care Administration System.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. H.A.-J., G.D., E.C., C.T., R.M., L.N., R.A.D., and M.A.-G. performed the study and revised the original draft. R.A.D. and M.A.-G. wrote the original draft of the manuscript. R.A.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT01867307, clinicaltrials.gov.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-0100/-/DC1.
References
- 1.Abdul-Ghani MA, Norton L, Defronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev 2011;32:515–531 [DOI] [PubMed] [Google Scholar]
- 2.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011;91:733–794 [DOI] [PubMed] [Google Scholar]
- 3.Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merovci A, Mari A, Solis C, et al. Dapagliflozin lowers plasma glucose concentration and improves β-cell function. J Clin Endocrinol Metab 2015;100:1927–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeFronzo RA, Hompesch M, Kasichayanula S, et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 2013;36:3169–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sha S, Devineni D, Ghosh A, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab 2011;13:669–672 [DOI] [PubMed] [Google Scholar]
- 8.Polidori D, Sha S, Ghosh A, Plum-Mörschel L, Heise T, Rothenberg P. Validation of a novel method for determining the renal threshold for glucose excretion in untreated and canagliflozin-treated subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 2013;98:E867–E871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komoroski B, Vachharajani N, Boulton D, et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther 2009;85:520–526 [DOI] [PubMed] [Google Scholar]
- 10.Liang Y, Arakawa K, Ueta K, et al. Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS One 2012;7:e30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 12.Zhao X, Cui Y, Zhao S, et al. Pharmacokinetic and pharmacodynamic properties and tolerability of single- and multiple-dose once-daily empagliflozin, a sodium glucose cotransporter 2 inhibitor, in Chinese patients with type 2 diabetes mellitus. Clin Ther 2015;37:1493–1502 [DOI] [PubMed] [Google Scholar]
- 13.Abdul-Ghani MA, DeFronzo RA, Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30-50% of filtered glucose load in humans. Diabetes 2013;62:3324–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Lewin A, Patel S, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care 2015;38:384–393 [DOI] [PubMed] [Google Scholar]
- 15.Rosenstock J, Hansen L, Zee P, et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care 2015;38:376–383 [DOI] [PubMed] [Google Scholar]
- 16.Farber SJ, Berger EY, Earle DP. Effect of diabetes and insulin of the maximum capacity of the renal tubules to reabsorb glucose. J Clin Invest 1951;30:125–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.