Abstract
Patellar instability remains a ubiquitous and troublesome problem in orthopaedics and represents a challenge in the pediatric population. Reconstruction of the medial patellofemoral ligament (MPFL) has become a mainstay of patellar instability management in recent years. As with any procedure at or around the physes, there is concern among surgeons regarding safe placement of hardware and drilled tunnels. The authors describe a technique for anatomic MPFL reconstruction with the aid of fluoroscopic guidance to maintain a “safe zone” without violating the distal femoral physis. This technique allows for reliable MPFL reconstruction in the skeletally immature population with concomitant lateral lengthening, while requiring only minor deviations from the procedure in a skeletally mature patient.
Patellar instability (PI) is a common problem in children and adolescents that can be associated with irreversible cartilage damage, eventual osteoarthritis, continued pain, and limitation in activity.1,2 PI chiefly results from underlying pathoanatomy that predisposes the patella to dislocate. Common pathoanatomic risk factors include trochlear dysplasia, patella alta, femoral anteversion, genu valgum, and ligamentous laxity.3,4 The medial patellofemoral ligament (MPFL) is the chief restraint to lateral translation of the patella, particularly in early knee flexion, and is the primary soft tissue structure that is injured when the patella dislocates.5,6 In cases of recurrent patellar instability, the MPFL becomes elongated and incompetent, which can cause the patella to tilt and translate laterally. The shift in patellar position can cause the lateral retinacular structures to correspondingly shorten (Figure 1).
Fig 1.
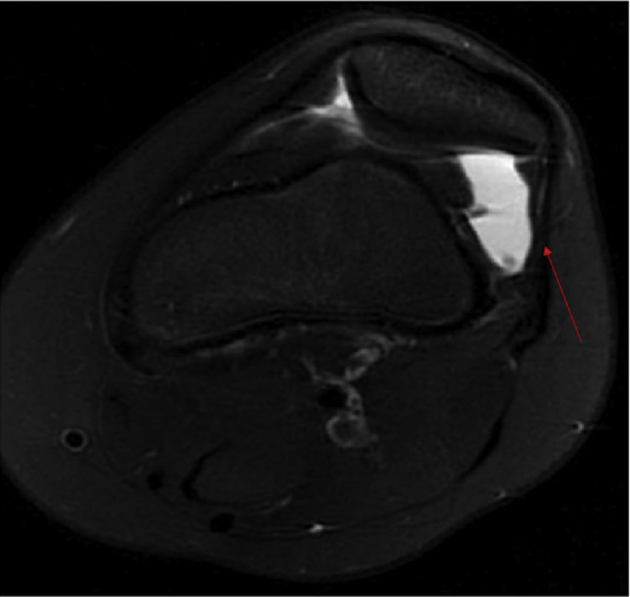
Representative image of an axial series of T2-weighted MRIs, showing correspondent shortening of lateral retinacular structures (arrow) in the setting of lateral patellar instability. This is characteristic of many patients with moderate to severe patellar instability. MRI, magnetic resonance imaging.
Many surgical procedures have been proposed to best treat recalcitrant cases of instability. Based on the underlying pathoanatomy, the options include MPFL reconstruction with or without a lateral lengthening or release, tibial tubercle transfers, trochleoplasty, and femoral osteotomies.1 In cases in which the underlying pathoanatomy is not severe enough to require correction, an MPFL reconstruction can be performed to augment and strengthen the native MPFL and thus compensate for the underlying cause of the instability. A well-positioned MPFL graft is paramount to restoring patellar stability.7 Conversely, a malpositioned MPFL graft can lead to poor surgical outcomes.5,6,8
Some surgeons hesitate to perform an MPFL reconstruction in the skeletally immature population because of appropriate concerns about physeal violation and subsequent growth disturbance. Although the exact location of the femoral insertion of the MPFL can be variable in pediatric patients, there is general consensus that a reconstruction tunnel or socket should be placed distal to the distal femoral physis.9, 10, 11, 12 A recent study showed a technique for safe drilling paths across the distal femoral epiphysis such that the distal femoral physis, intercondylar notch, and trochlear articular cartilage can be avoided.13 With this information in mind, development of a reliable and reproducible technique that is able to provide precise anatomic placement of MPFL grafts in skeletally immature patients is achievable.
The following technique is the senior author's method for achieving an anatomy-based MPFL reconstruction and combined lateral lengthening procedure while respecting the physeal margins in the skeletally immature patient.
Surgical Technique
A complete demonstration of the surgical technique described in this section is presented in the Video.
After induction of general anesthesia and administration of a regional block (typically an adductor canal block), with the patient in the supine position, a thigh-high tourniquet is placed. The affected extremity is prepped and draped in the usual sterile fashion. Diagnostic arthroscopy is not routinely performed unless there is a known loose body or the health of the patellofemoral cartilage is in question. If a surgical chondral injury is present, it can typically be addressed in the same surgical setting.
The leg is exsanguinated with an Esmarch, and the tourniquet is inflated. The leg is placed over a tibial triangle to keep the knee flexed ∼45°. A lateral lengthening procedure is first performed as outlined below. A 5- to 6-cm incision is made on the lateral border of the patella (Figure 2), and dissection is carried down to the lateral retinaculum. The retinaculum is opened in a Z fashion with the superficial layer incised off the patella. The dissection is carried approximately 2 to 3 cm posterior to this incision, and a deeper incision is made that will provide the deep flap of the Z lengthening. Typically, both transverse and longitudinal fibers of the lateral retinacular structures will be visible in this dissection. This will be sutured closed after completion of the medial reconstruction (Figures 3 to Fig 4, Fig 5).
Fig 2.
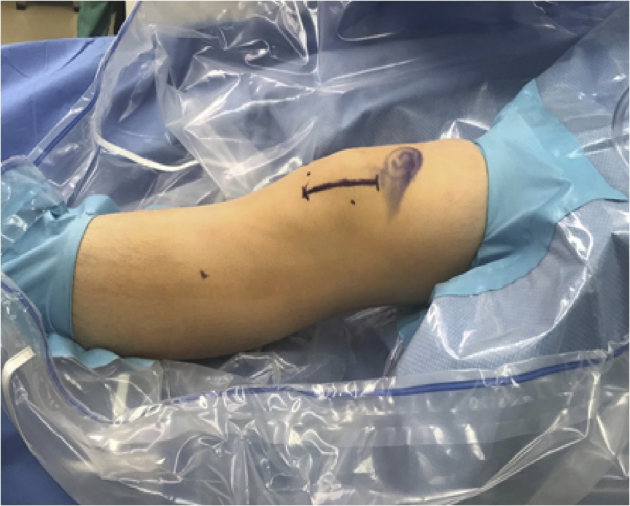
The patient is positioned supine on the operating room table, with knee in full extension. A ∼5- to 6-cm incision is marked out along the lateral aspect of the patella for the approach to the lateral lengthening portion of the procedure, in this case, of the left knee.
Fig 3.
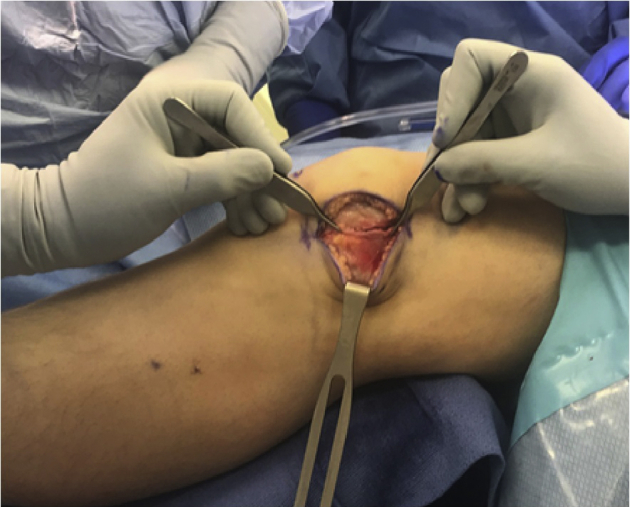
In the first portion of the procedure, lengthening of the lateral retinaculum is performed in an open and controlled fashion that can be directly visualized and quantified. Measurement of the lengthening can be assessed using a ruler to measure the anterior-to-posterior extent of the correction (arrow and brackets in Figure 4).
Fig 4.
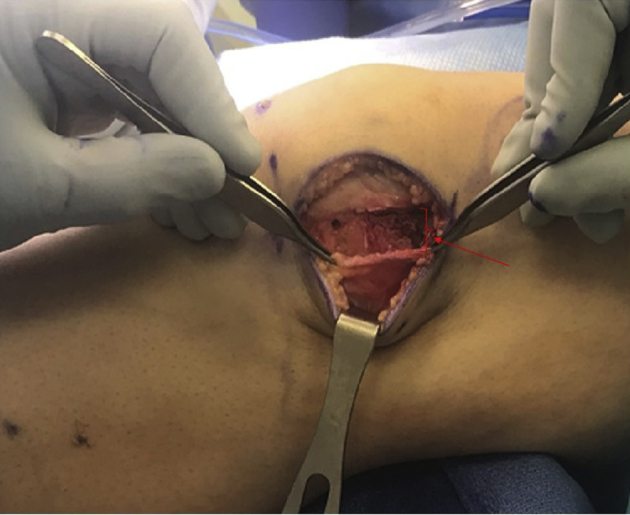
In the first portion of the procedure, lengthening of the lateral retinaculum is performed in an open and controlled fashion that can be directly visualized and quantified. Measurement of the lengthening can be assessed using a ruler to measure the anterior-to-posterior extent of the correction (arrow and brackets in Figure 4).
Fig 5.
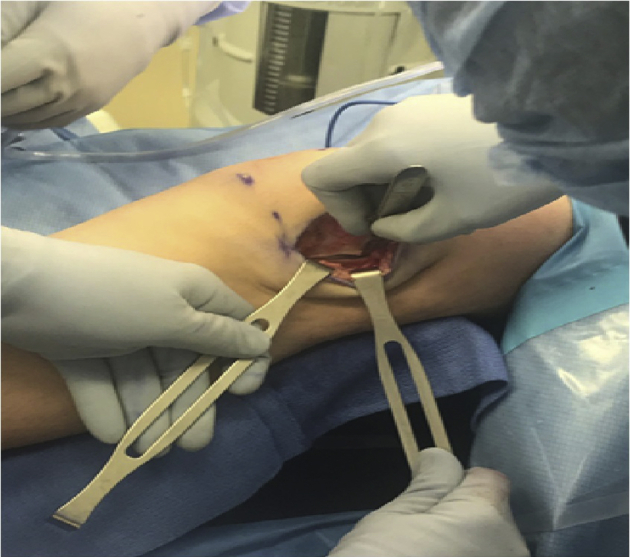
In the first portion of the procedure, lengthening of the lateral retinaculum is performed in an open and controlled fashion that can be directly visualized and quantified. Measurement of the lengthening can be assessed using a ruler to measure the anterior-to-posterior extent of the correction (arrow and brackets in Figure 4).
MPFL reconstruction is commenced by dissecting through the lateral incision over the anterior surface to the medial border of the patella. Guide pins are placed in the upper half of the patella in a parallel fashion: 1 at approximately the midpoint between the superior and inferior pole, and 1 at the midpoint between the superior pole and the distal wire. Fluoroscopy is used to confirm appropriate pin placement in the patella. Attention is paid to appropriate placement in the anterior-to-posterior dimension to avoid violating the articular cartilage or anterior patellar cortical bone (Figures 6 and 7). Femoral tunnel placement is performed under fluoroscopic guidance to determine Schottle's point,14 as described by Nelitz et al.15 through a 2-cm incision on the medial femur. Initial placement is determined on a lateral view, which typically gives the appearance that the guide pin will violate the physis. On the corresponding anteroposterior (AP) view, the pin is shown to be safely distal to the medial margin of the distal femoral physis (Figures 8 and 9). Under fluoroscopic guidance, the femoral Beath pin is aimed slightly distal and slightly anterior. Careful attention is paid to remain above Blumensaat's line, distal to the distal femoral physis and posterior to the trochlear groove, throughout the duration of pin passage. This radiographic triangle is referred to as the “kid's zone” (Figure 10). Imaging is toggled back and forth between the AP and lateral image. This allows for safe drilling of the tunnel without disruption of the physis, intercondylar notch, or trochlear cartilage (Figures 11 and 12). The Beath pin is advanced through the lateral femoral cortex and out the skin of the lateral thigh.
Fig 6.
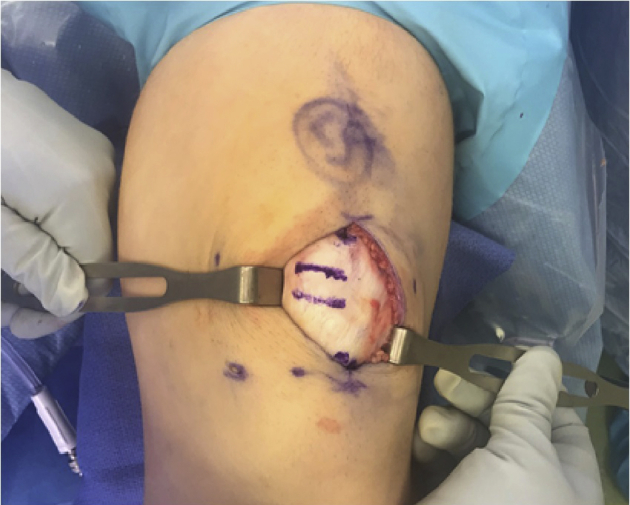
With the knee in slight flexion over a radiolucent triangle, a medial exposure is used to allow for preparation of patellar tunnels for the MPFL graft. Exposure of the patella and provisional positioning of tunnels are checked with guidewires before drilling tunnels (Figure 7, with arrows).
Fig 7.
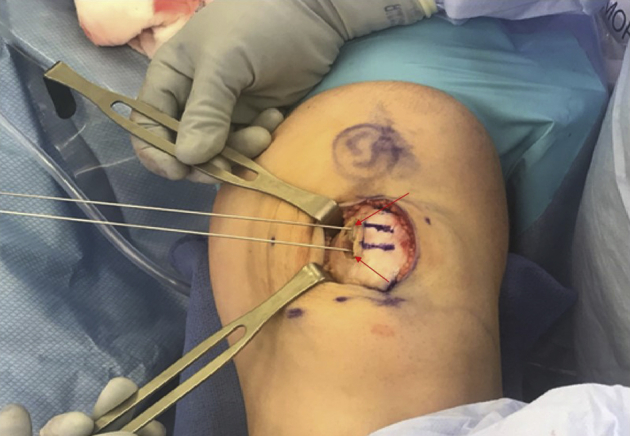
With the knee in slight flexion over a radiolucent triangle, a medial exposure is used to allow for preparation of patellar tunnels for the MPFL graft. Exposure of the patella and provisional positioning of tunnels are checked with guidewires before drilling tunnels (Figure 7, with arrows).
Fig 8.
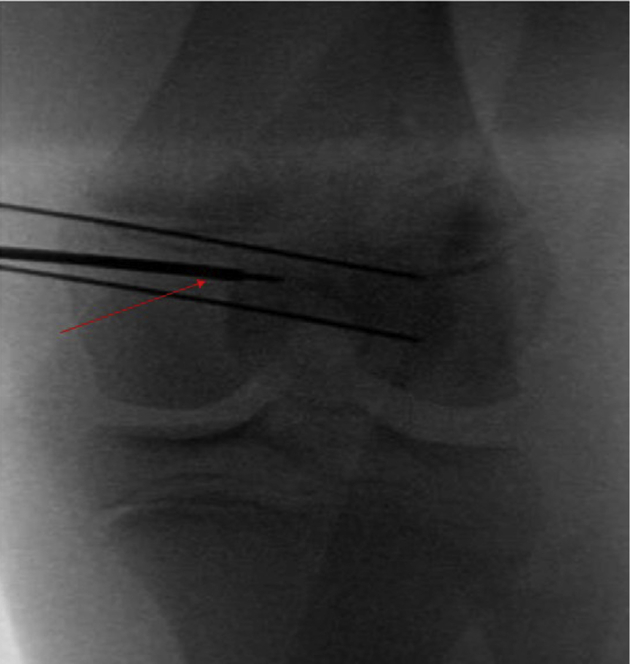
Intraoperative C-arm fluoroscopy is used to assess femoral tunnel starting point. Both anteroposterior (Figure 8 with arrow denoting femoral guide wire path) and lateral (Figure 9) imaging is used to confirm proper starting point of femoral tunnel positioning based on drill tip location.
Fig 9.
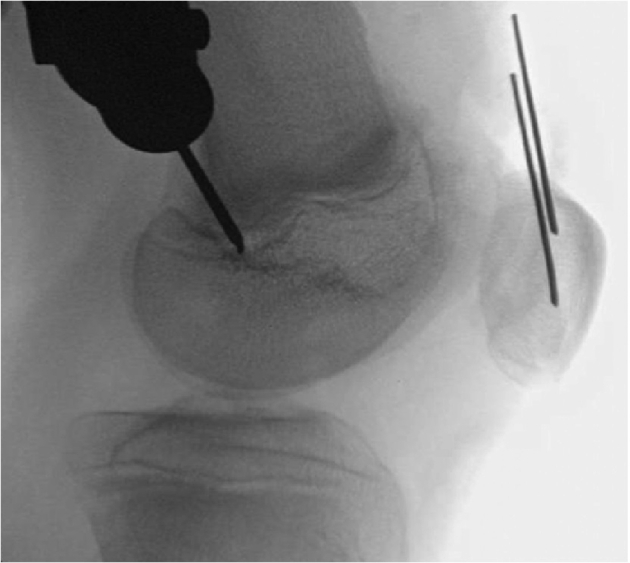
Intraoperative C-arm fluoroscopy is used to assess femoral tunnel starting point. Both anteroposterior (Figure 8 with arrow denoting femoral guide wire path) and lateral (Figure 9) imaging is used to confirm proper starting point of femoral tunnel positioning based on drill tip location.
Fig 10.
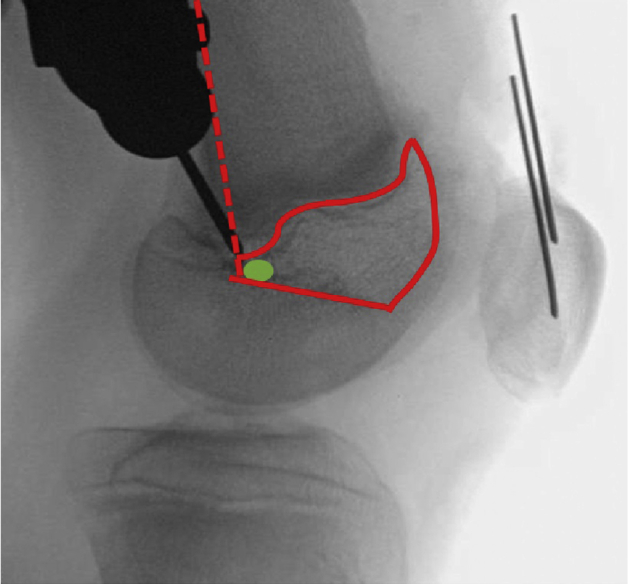
Illustration of “kid's zone,” outlined in red, with posterior margin of distal femur (dotted line), Blumensaat's line, and distal femoral physis outlined. The optimal starting point is marked with a green circle. This starting point for the femoral tunnel, based on the drill tip location, is optimal.
Fig 11.
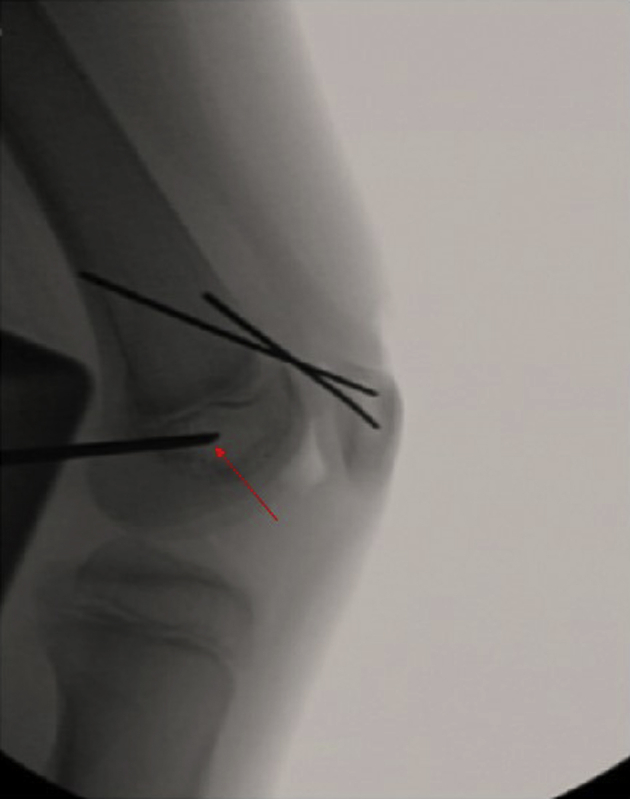
Frequent intraoperative fluoroscopy is used during creation of the femoral tunnel (both anteroposterior and lateral images). Attention is paid to keep the guide wire and subsequent reamer in the “kid's zone”: distal to the physis, anterior to Blumensaat's line, and posterior to the trochlear groove on both anteroposterior and lateral radiographs. In the included images, arrow denotes femoral guidewire path.
Fig 12.
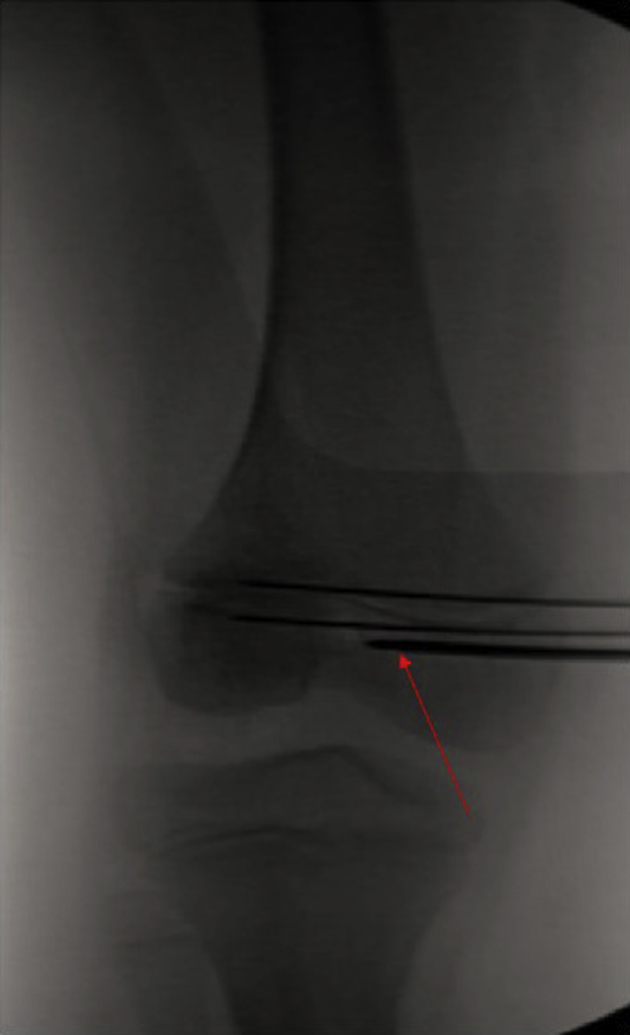
Frequent intraoperative fluoroscopy is used during creation of the femoral tunnel (both anteroposterior and lateral images). Attention is paid to keep the guide wire and subsequent reamer in the “kid's zone”: distal to the physis, anterior to Blumensaat's line, and posterior to the trochlear groove on both anteroposterior and lateral radiographs. In the included images, arrow denotes femoral guidewire path.
Patellar sockets are drilled with a 3.5-mm drill to accommodate 3.5-mm Swivelock suture anchors (Arthrex, Naples, FL). A gracilis allograft is folded in half, reinforced on each free end with 2-0 Fiberwire (Arthrex), and loaded with a Tightrope RT (Arthrex) (Figure 13). The ends of the graft are first placed in the patella docking sites and secured with the Swivelock anchors. It is recommended to cut down the size of the graft to allow the doubled-over portion to easily fit through a 4.5-mm tunnel. The interval between layers 2 and 3 of the medial retinacular structures and the capsule is dissected from the patella to the femoral tunnel site and incision. A shuttle suture is passed through this incision up to the patella. The Tightrope-graft construct is then shuttled down to the femur. To confirm appropriate femoral tunnel placement, the graft is wrapped around the femoral Beath pin. The knee is flexed, and any change in graft tension is observed. Ideally, the graft loosens slightly in flexion or is isometric. If the graft tightens in flexion, the femoral Beath pin needs to be repositioned. Typically, in this situation, it would need to be repositioned posteriorly. Once appropriate femoral tunnel position is confirmed, the femoral tunnel is drilled with a 4.5-mm reamer. This small reamer further minimizes the chance of violation of the distal femoral physis. The femoral tunnel is typically drilled all the way across the epiphysis to allow easy passage of the graft as well as the button. The suture tails of the Tightrope RT are loaded into the eyelet of the Beath pin and passed through the femoral epiphysis from medial to lateral (Figures 14 to Fig 15, Fig 16). At this point, the Tightrope button is passed to the lateral cortex, and confirmation that it is flipped onto the lateral cortex without underlying soft tissue is made under direct visualization.
Fig 13.
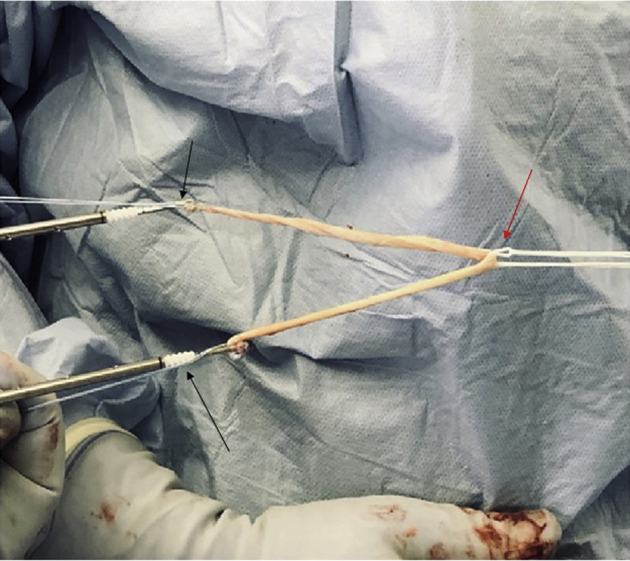
Demonstration of MPFL graft preparation, with folded gracilis allograft, suture anchors on either free end (black arrows), and Tight Rope RT at the midportion of the graft (red arrow). The graft can be prepared on the back table by a surgical assistant during tunnel preparation.
Fig 14.
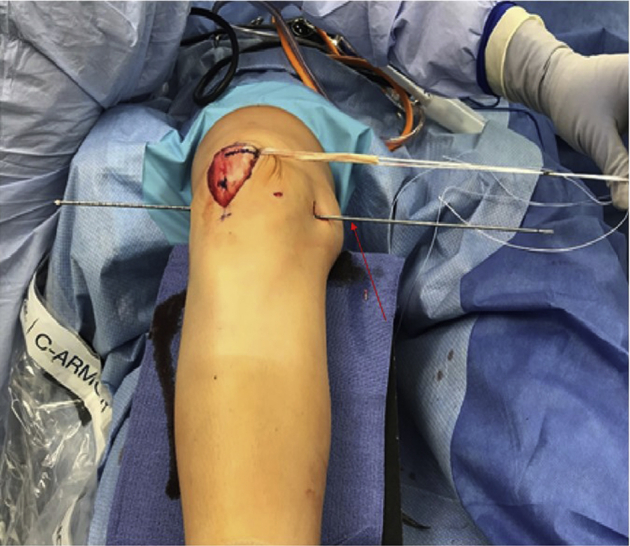
Once the allograft is anchored in the patella tunnels, passage of the graft first through the medial interval (Figure 15), then through the previously drilled femoral tunnel (Figure 16) can be performed. Passage through the femoral tunnel is aided by a Beath pin (arrow in Figure 14). The patient's knee remains in slight flexion over the radiolucent triangle.
Fig 15.
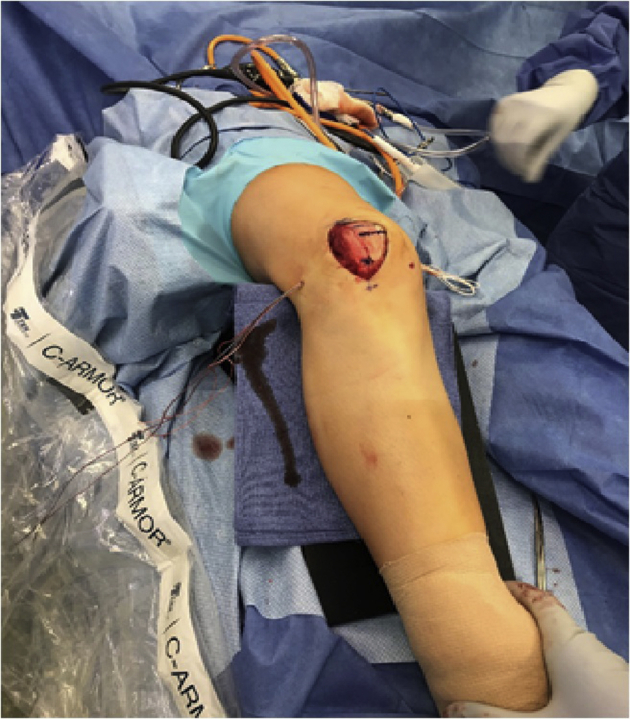
Once the allograft is anchored in the patella tunnels, passage of the graft first through the medial interval (Figure 15), then through the previously drilled femoral tunnel (Figure 16) can be performed. Passage through the femoral tunnel is aided by a Beath pin (arrow in Figure 14). The patient's knee remains in slight flexion over the radiolucent triangle.
Fig 16.
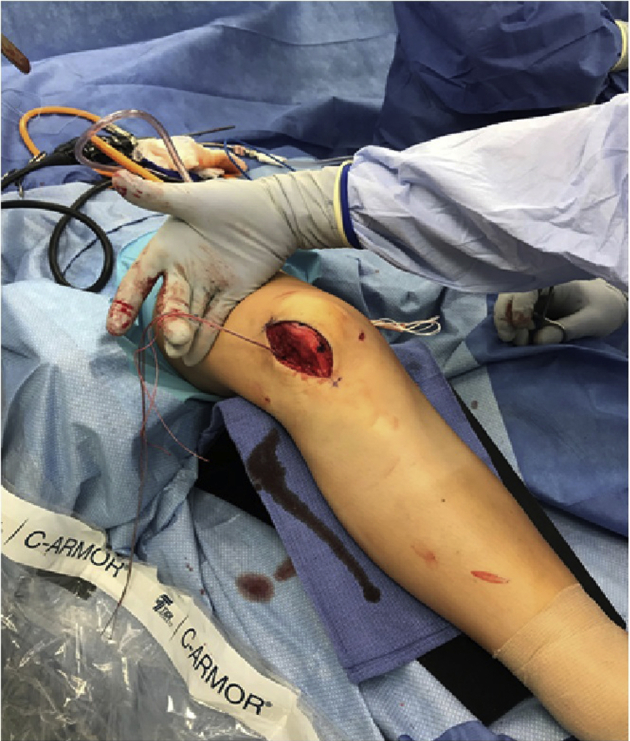
Once the allograft is anchored in the patella tunnels, passage of the graft first through the medial interval (Figure 15), then through the previously drilled femoral tunnel (Figure 16) can be performed. Passage through the femoral tunnel is aided by a Beath pin (arrow in Figure 14). The patient's knee remains in slight flexion over the radiolucent triangle.
With the knee maintained in 45° of flexion (typically by using a radiolucent tibial triangle), the adjustable loop of the Tightrope is shortened to pull the graft into the femoral tunnel. A curved clamp is placed under the 2 limbs of the graft immediately adjacent to the medial aspect of the patella, and the patella is held in neutral tilt in the trochlear groove at 45° of flexion. Provisional graft tension is achieved by watching the graft tension over the curved clamp. The surgeon then removes the tibial triangle, places the knee in full extension, and provides an anterior directed stress on the patella by placing their fingers on the articular surface of the patella and manually pulling the patella upward to remove any redundancy of the MPFL graft-Tightrope construct in the femoral tunnel. Further gentle tensioning is then performed, if needed, to achieve 1 quadrant of lateral translation with neutral tilt. The knee is ranged to ensure that it can be fully flexed. The wound and joint are washed with saline, and the knee is flexed to 70° for lateral retinacular closure. The lateral retinaculum is closed by suturing the superficial layer of the retinaculum to the deep layer at whatever point the superficial layer comfortably lies. This completes the lateral retinacular lengthening. The senior author has observed that 1 to 2 cm of length is typically achieved. At this point, all wounds are closed in a layered fashion, and dressing is applied.
Postoperative Management
The patient is discharged home as an outpatient. The patient is encouraged to bear weight as tolerated and range the knee as tolerated immediately postoperatively. No brace is used, and crutches are used for comfort and stability measures only. Physical therapy is commenced within 1 to 2 weeks of surgery to re-establish motion, work on early muscle activation, and aid with swelling reduction. If rehabilitation proceeds appropriately, the patient may begin a return to impact activities as early as 6 to 8 weeks after surgery. Full clearance for sports can often occur within 3 months after surgery based on the patient's efforts with rehabilitation.
Discussion
There is significant concern in the orthopaedic community regarding the potential for growth disturbance via iatrogenic physeal injury in patients undergoing MPFL reconstruction, but fortunately there has been little reported of this occurring.16,17 Several methods of MPFL reconstruction have been described that have successfully avoid disruption of the distal femoral physis with good clinical results, including use of a suture anchor, rather than a longer tunnel, for interference-fit femoral fixation.12,15 Alternative methods of patellar stabilization, such as turndown of the quadriceps tendon, have shown some success, but this technique incorporates a nonanatomic recreation of the MPFL femoral attachment, in an effort to avoid the distal femoral physis.18 A recent systematic review by Shamrock et al.19 echoes the above point, noting the diversity of techniques, particularly in femoral fixation. However, they go on to conclude that there was not a single reported case of physeal growth disruption, regardless of technique, among any of the trials they reviewed.19
The described technique provides an attractive option for 2-sided soft tissue stabilization via MPFL reconstruction and open lateral retinacular lengthening in the skeletally immature population. Advantages and potential disadvantages of this technique are described in Table 1. The only modification from what would be done for a skeletally mature patient is the trajectory of the femoral tunnel. Thus, an anatomic reconstruction with suspensory cortical fixation is performed, which has been shown to have superior biomechanical strength and can allow for early and aggressive rehabilitation.20 It is our observation that if attention is paid to anatomic and radiographic landmarks during the case, a safe femoral tunnel can be drilled completely across the epiphysis that does not violate the physis, intercondylar notch, or articular cartilage and provides an anatomic placement of the graft.
Table 1.
Advantages and Disadvantages
| Advantages | Disadvantages |
|---|---|
| An anatomic reconstruction is performed that best approximates native anatomy | Intraoperative fluoroscopy is required |
| Does not violate distal femoral physis or articular surface | Creation of patellar bone tunnels carries a low risk of patellar fracture |
| Allograft choice allows for preservation of native tendon and avoidance of donor site morbidity | Lateral lengthening calls for additional dissection compared with MPFL reconstruction alone |
| Open lateral lengthening allows for measured improvement in lateral sided tension compared to arthroscopic alternatives | The adjustable loop button can be overtightened, which would require button removal and attaching a new adjustable loop cortical button |
| The laterally based parapatellar incision allows for easy visualization and manipulation of the cortical button | |
| Single-incision technique for lateral lengthening and MPFL reconstruction | |
| The cortical button does not run the risk of graft damage or screw loosening and migration |
It is the preference of the senior author to perform MPFL reconstruction with the use of gracilis allograft because of the success of the graft integrity, shorter operative time, and lack of donor-site morbidity. Numerous articles in the literature have supported the noninferiority of this graft choice. Hendawi et al.21 recently showed a lower graft rerupture rate in allograft MPFL reconstructions. This contributes to an overall lower cost of surgery and shorter surgical duration. The support for noninferiority in the usage of allograft tendon for MPFL reconstruction has also been made in review articles by Mcneilan et al.,22 Popkin et al.,12 and Hohn et al.23 Although there is a paucity of data to support its use in a pediatric population, there is likewise no evidence to discourage its use in this population. The senior author has not observed any adverse events related to allograft usage in this population.
Alhough the described technique has proven to be successful in the vast majority of cases, there are described risks and limitations associated with the procedure. There continues to be concern in the literature regarding the risk of patella fracture in the setting of MPFL reconstruction. Tanaka24 urges careful attention to orientation of patella drilling, specifically avoiding the anterior cortex to prevent fracture. Furthermore, although lateral lengthening and MPFL reconstruction is a solution for many patients with patellar instability, there is a role for more advanced procedures, including trochleoplasty in patients with more significant trochlear dysplasia in the setting of PI.25,26
Conclusion
This technique provides a safe, reproducible, and biomechanically superior option to reconstruct the MPFL and rebalance the lateral retinaculum of a skeletally immature patient with patellar instability. With attention to certain facets of the procedure (Table 2), this can be a predictable and very successful operation. This technique is attractive because it requires only a minor modification for what would be done for a skeletally mature patient, and the biomechanical strength of this construct allows for an aggressive rehabilitation protocol that can enable an early return to activity.
Table 2.
Pearls and Pitfalls
| Pearls | Pitfalls |
|---|---|
| Base the lateral parapatellar incision just medial to the lateral border of the patella. The patella is typically translated laterally, and once the patella has been stabilized, it will sit a bit more medial and the incision will then be just on the lateral border of the patella. Planning this incision here allows for a slightly smaller incision to mobilize over to the medial side of the patella for patellar tunnel drilling and graft placement. | The lateral lengthening should be carried posterior through the contributions of the iliotibial band, and the incision of the deep layer of the retinaculum should be 3 cm posterior to the patella. If this is not done, the deep layer may not be long enough to allow for appropriate closure at the end of the case. |
| Check fluoroscopy after the patellar guide pins are placed to ensure they stay in the cancellous portion of the patella. This will minimize the risk of patella fracture. | Avoid violating the anterior cortex of the patella with the patellar sockets to avoid the risk of patellar fracture. |
| Toggle between AP and lateral fluoroscopic imaging when advancing the femoral tunnel guide wire to ensure proper trajectory. | Always measure the gracilis allografts and cut the graft down as needed to be between 200 and 220 mm long. Sometimes a small pediatric knee will need a shorter graft. Grafts that are too long can bottom out in the femoral tunnel and not allow for adequate tensioning. |
| A 4.5-mm drill can be advanced through the lateral femoral cortex when preparing the femoral tunnel to allow for easier passage of the button. | To prevent creep, before final tightening of the graft, the patella should be manually stretched anteriorly to remove any inadvertent graft-adjustable loop foldover that may have occurred in the femoral tunnel. |
| Make sure the button is fully seated on the femoral cortex before shortening the adjustable loop. | |
| When performing final tensioning of the graft, put a curved clamp underneath the graft where it inserts into the patella and hold the patella in the trochlea with neutral tilt. Watch for the graft to tighten over the clamp. When this occurs, stop tensioning and check patellar translation in full extension. Retension as needed. This will minimize the chances of overtightening the graft. |
Footnotes
The authors report that they have no conflicts of interest in the authorship and publication of this article. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Demonstration of the open lateral lengthening and physeal-sparing MPFL reconstruction technique in a skeletally mature individual. The video begins with the patient positioned supine on the operating table with the knee in extension. The lateral lengthening is performed through a laterally based incision. A medially based incision is used for the MPFL reconstruction. The video details the operative technique as well as fluoroscopic images used for guidance during the technique.
References
- 1.Weber A.E., Nathani A., Dines J.S. An Algorithmic approach to the management of recurrent lateral patellar dislocation. J Bone Joint Surg Am. 2016;98:417–427. doi: 10.2106/JBJS.O.00354. [DOI] [PubMed] [Google Scholar]
- 2.Sanders T.L., Pareek A., Johnson N.R., Stuart M.J., Dahm D.L., Krych A.J. Patellofemoral arthritis after lateral patellar dislocation: A Matched population-based analysis. Am J Sports Med. 2017;45:1012–1017. doi: 10.1177/0363546516680604. [DOI] [PubMed] [Google Scholar]
- 3.Parikh S.N., Lykissas M.G., Gkiatas I. Predicting risk of recurrent patellar dislocation. Curr Rev Musculoskelet Med. 2018;11:253–260. doi: 10.1007/s12178-018-9480-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franciozi C.E., Ambra L.F., Albertoni L.J. Increased femoral anteversion Influence over surgically treated recurrent patellar instability patients. Arthroscopy. 2017;33:633–640. doi: 10.1016/j.arthro.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Parikh S.N., Nathan S.T., Wall E.J., Eismann E.A. Complications of medial patellofemoral ligament reconstruction in young patients. Am J Sports Med. 2013;41:1030–1038. doi: 10.1177/0363546513482085. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy M., Ridley T.J., Bollier M., Wolf B., Albright J., Amendola A. Femoral tunnel placement in medial patellofemoral ligament reconstruction. Iowa Orthop J. 2013;33:58–63. [PMC free article] [PubMed] [Google Scholar]
- 7.Erickson B.J., Nguyen J., Gasik K., Gruber S., Brady J., Shubin Stein B.E. Isolated medial patellofemoral ligament reconstruction for patellar instability regardless of tibial tubercle-trochlear groove distance and patellar Height: Outcomes at 1 and 2 Years. Am J Sports Med. 2019;47:1331–1337. doi: 10.1177/0363546519835800. [DOI] [PubMed] [Google Scholar]
- 8.Bollier M., Fulkerson J., Cosgarea A., Tanaka M. Technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27:1153–1159. doi: 10.1016/j.arthro.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Shea K.G., Grimm N.L., Belzer J., Burks R.T., Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26:1083–1087. doi: 10.1016/j.arthro.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Nelitz M., Dornacher D., Dreyhaupt J., Reichel H., Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2011;19:2067–2071. doi: 10.1007/s00167-011-1548-3. [DOI] [PubMed] [Google Scholar]
- 11.Bishop M.E., Black S.R., Nguyen J., Mintz D., Stein B.S. A simple method of measuring the distance from the schottle point to the medial distal femoral physis with MRI. Orthop J Sports Med. 2019;7 doi: 10.1177/2325967119840713. 2325967119840713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popkin C.A., Bayomy A.F., Trupia E.P., Chan C.M., Redler L.H. Patellar instability in the skeletally immature. Curr Rev Musculoskelet Med. 2018;11:172–181. doi: 10.1007/s12178-018-9472-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen C.V., Farrow L.D., Liu R.W., Gilmore A. Safe drilling paths in the distal femoral epiphysis for pediatric medial patellofemoral ligament reconstruction. Am J Sports Med. 2017;45:1085–1089. doi: 10.1177/0363546516677795. [DOI] [PubMed] [Google Scholar]
- 14.Schöttle P.B., Schmeling A., Rosenstiel N., Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35:801–804. doi: 10.1177/0363546506296415. [DOI] [PubMed] [Google Scholar]
- 15.Nelitz M., Dreyhaupt J., Reichel H., Woelfle J., Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: Surgical technique and clinical outcome. Am J Sports Med. 2013;41:58–63. doi: 10.1177/0363546512463683. [DOI] [PubMed] [Google Scholar]
- 16.Vavken P., Wimmer M.D., Camathias C., Quidde J., Valderrabano V., Pagenstert G. Treating patella instability in skeletally immature patients. Arthroscopy. 2013;29:1410–1422. doi: 10.1016/j.arthro.2013.03.075. [DOI] [PubMed] [Google Scholar]
- 17.Uppstrom T.J., Price M., Black S., Gausden E., Haskel J., Green D.W. Medial patellofemoral ligament (MPFL) reconstruction technique using an epiphyseal femoral socket with fluoroscopic guidance helps avoid physeal injury in skeletally immature patients. Knee Surg Sports Traumatol Arthrosc. 2019;27:3536–3542. doi: 10.1007/s00167-019-05412-7. [DOI] [PubMed] [Google Scholar]
- 18.Noyes F.R., Albright J.C. Reconstruction of the medial patellofemoral ligament with autologous quadriceps tendon. Arthroscopy. 2006;22 doi: 10.1016/j.arthro.2005.12.058. 904.e1-e7. [DOI] [PubMed] [Google Scholar]
- 19.Shamrock A.G., Day M.A., Duchman K.R., Glass N., Westermann R.W. Medial patellofemoral ligament reconstruction in skeletally immature patients: A systematic review and meta-analysis. Orthop J Sports Med. 2019;7 doi: 10.1177/2325967119855023. 2325967119855023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joyner P.W., Bruce J., Roth T.S. Biomechanical tensile strength analysis for medial patellofemoral ligament reconstruction. Knee. 2017;24:965–976. doi: 10.1016/j.knee.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Hendawi T., Godshaw B., Flowers C., Stephens I., Haber L., Waldron S. Autograft vs allograft comparison in pediatric medial patellofemoral ligament reconstruction. Ochsner J. 2019;19:96–101. doi: 10.31486/toj.18.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNeilan R.J., Everhart J.S., Mescher P.K., Abouljoud M., Magnussen R.A., Flanigan D.C. Graft choice in isolated medial patellofemoral ligament reconstruction: A systematic review with meta-analysis of rates of recurrent instability and patient-reported outcomes for autograft, allograft, and synthetic options. Arthroscopy. 2018;34:1340–1354. doi: 10.1016/j.arthro.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 23.Hohn E., Pandya N.K. Does the utilization of allograft tissue in medial patellofemoral ligament reconstruction in pediatric and adolescent patients restore patellar stability? Clin Orthop Relat Res. 2017;475:1563–1569. doi: 10.1007/s11999-016-5060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka M.J. Socket or knock it? Considerations in patellar fixation during medial patellofemoral ligament reconstruction. Arthroscopy. 2019;35:1629–1630. doi: 10.1016/j.arthro.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Vogel L.A., Pace J.L. Trochleoplasty, medial patellofemoral ligament reconstruction, and open lateral lengthening for patellar instability in the setting of high-grade trochlear dysplasia. Arthrosc Tech. 2019;8:e961–e967. doi: 10.1016/j.eats.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rush J., Diduch D. When is trochleoplasty a rational addition? Sports Med Arthrosc Rev. 2019;27:161–168. doi: 10.1097/JSA.0000000000000254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demonstration of the open lateral lengthening and physeal-sparing MPFL reconstruction technique in a skeletally mature individual. The video begins with the patient positioned supine on the operating table with the knee in extension. The lateral lengthening is performed through a laterally based incision. A medially based incision is used for the MPFL reconstruction. The video details the operative technique as well as fluoroscopic images used for guidance during the technique.


