Abstract
Background
Early diagnosis and treatment of juvenile idiopathic arthritis (JIA) with conventional and biologic disease modifying anti-rheumatic drugs (DMARDs) have vastly improved outcomes for children with these diseases. Currently, a large proportion of children with JIA are able to achieve clinical inactive disease and remission. With this success have come important questions about when medications can be stopped and how to balance the risks and benefits of continuing medications versus the potential for flare after stopping.
Aim
To conduct a systematic review of the available literature to summarize current evidence about medication withdrawal for JIA in remission.
Methods
We conducted a systematic literature search in PubMed and Embase from 1990 to 2019. References were first screened by title and then independently screened by title and abstract by two authors. 77 original papers were selected for full-text review. Data were extracted from 30 papers on JIA and JIA-associated uveitis, and quality of evidence was evaluated using NIH/NHLBI tools. Studies on biochemical and radiologic biomarkers were also reviewed and summarized.
Results
Most studies investigating treatment withdrawal in JIA have been observational and of poor or fair quality; interpretations of these studies have been limited by differences in study populations, disease and remission durations, medications withdrawn, approaches to withdrawal, and definitions of disease outcomes. Overall the data suggest that flares are common after stopping JIA medications, particularly biologic medications. Clinical characteristics associated with increased risks of flare have not been consistently identified. Biochemical biomarkers and ultrasound findings have been shown to predict outcomes after stopping medications, but to date, no such predictor has been consistently validated across JIA populations. Studies have also not identified optimal strategies for withdrawing medication for well-controlled JIA. Promising withdrawal strategies include discontinuing methotrexate before biologic medications in children receiving combination therapy, dose reduction for children on biologics, and treat-to-target approaches to withdrawal. These and other strategies require further investigation in larger, high-quality studies.
Conclusions
The published literature on treatment withdrawal in JIA has varied in design and quality, yielding little conclusive evidence thus far on the management of JIA in remission. Given the importance of this question, international collaborative efforts are underway to study clinical and biologic predictors of successful medication withdrawal in JIA. These efforts may ultimately support the development of personalized approaches to withdrawing medication in children with JIA in remission.
1. Introduction
The use of conventional and biologic disease-modifying antirheumatic drugs (DMARDs) have led to improvements in both short-term and long-term outcomes in children with juvenile idiopathic arthritis (JIA). While the benefit of DMARDs for ameliorating symptoms and preventing damage in children with JIA is clear, there is no consensus on whether or how withdrawal of medication(s) should be attempted in those patients who achieve remission. Given cost and safety considerations with DMARDs, as well as the challenges of administering these medications which are often given parenterally, it might be reasonable to attempt withdrawal in selected patients. However, there is substantial variability in how clinicians withdraw DMARDs for children with JIA. In this systematic review, we summarize the published evidence around treatment withdrawal in JIA patients who have achieved remission.
1.1. Heterogeneity of Disease
JIA is the most common rheumatic disease of childhood, with an estimated prevalence of 3.8–400 per 100,000 children, reflecting both geographic variation and evolving disease definitions over time [1]. Notably, JIA is not a single disease but several clinically and biologically distinct entities sharing the common feature of chronic joint inflammation with onset in childhood. Extra-articular manifestations, including eye inflammation (uveitis), may occur. The current classification system of JIA encompasses seven major categories based on clinical characteristics, as derived by consensus among an international panel of experts [2], [3]. These criteria have not been without criticism [4], [5], [6], [7]. Others have proposed alternative classification schemes, e.g., a group of 5 discrete entities (not including systemic JIA (sJIA)) based on demographics, clinical features, and cytokine/chemokine profiles [8]; a group of 4 primary disorders spanning childhood and adulthood based on demographic, clinical, and genetic features [9]; and a group of 6 clinically-defined, childhood-onset inflammatory disorders [10]. While the optimal classification of JIA has not yet been definitively established, its genetic heterogeneity and corresponding variability in the definitions and composition of JIA populations represent major challenges in drawing firm conclusions regarding treatment withdrawal.
1.2. Heterogeneity of Disease Inactivity
Similar to the diagnosis of JIA itself, disease activity states—specifically clinical inactive disease (CID) and remission—have been defined in primarily clinical (rather than biologic) terms and applied variably across the literature [11], [12], [13]. CID generally refers to a state of inactivity at a point in time. Various definitions of CID have been developed, usually including measures of inactive arthritis and a low, physician-assessed global score of disease activity. CID definitions have been more variable with regard to inclusion of other clinical characteristics, including inflammatory markers, duration of joint stiffness, and patient- and parent-reported global scores [14]. Distinct CID definitions may correspond differently to other aspects of disease control that are meaningful to patients and families [15]. Alternative activity states, including minimal disease activity, have also been proposed [13]. While these various definitions have been validated [12], [16] they lack in agreement for a given patient population [14], [15]. The key inclusion of a physician global score across definitions raises further questions, given its poor inter-rater reliability [17].
Remission has been defined as sustained CID over a period of time—6 months while on medication or 12 months after stopping all medication [11]. However, this concept of remission as a state of sustained inactivity is not universal [13] The various definitions of CID and remission also contrast with definitions used in adults with rheumatoid arthritis (RA), which are less strictly defined and allow some degree of joint inflammation [18]. Adding to the challenges of these definitions, clinically defined states of CID and remission do not always agree with biologic assessments of inflammation and immune activation [19], [20].
For patients with JIA-associated uveitis, articular and ocular disease activity may not parallel one another in timing or severity. One disease manifestation, arthritis or uveitis, is often more severe than the other and may drive decisions about starting or stopping systemic treatment [21],[22].
1.3. Heterogeneity of Disease Management
As a group of inflammatory disorders, JIA is treated with medications that suppress inflammation. Different categories of medications are used, depending on disease severity, presence of extra-articular manifestations, and therapeutic response [23]. Table 1 summarizes the 5 major categories of JIA medications.
Table 1.
Major Classes of Medications Used in the Treatment of JIA
| Class of Medication | Description | Examples |
|---|---|---|
| Glucocorticoids [80] | -Potent anti-inflammatories-Administered by
PO, IV, or IA routes -Used short-term/as bridging therapy -IA can be definitive treatment of oligo-JIA |
-Prednisone-Prednisolone -Methylprednisolone -Triamcinolone (hex)acetonide |
| Non-Steroidal Anti-Inflammatory Drugs [23] | -Oral medications used as monotherapy in mild
disease -Often taken in conjunction with other medications |
-Naproxen -Meloxicam -Indomethacin -Celecoxib |
| Conventional Synthetic DMARDs [23] [81] | -Widely used class of JIA
medications -Slow-acting -Toxicity often associated with intolerance |
-Methotrexate -Sulfasalazine -Leflunomide |
| Biologic DMARDs [82] | -Targeted parenteral drugs used to treat patients with more severe or refractory JIA | -Etanercept -Adalimumab -Tocilizumab -Abatacept |
| Targeted Synthetic DMARDs [83] [84] [85] | -Newer class of targeted, oral
drugs -Little known about efficacy and safety in JIA to date |
-Tofacitinib -Baricitinib |
DMARD disease-modifying anti-rheumatic drug, IA intraarticular, IV intravenous, JIA juvenile idiopathic arthritis, oligo-JIA oligoarticular juvenile idiopathic arthritis, PO oral
Multiple options exist for withdrawing JIA treatment. Clinicians in the US and Canada most commonly advise tapers of variable duration with the goal of discontinuing therapy [24]. Tapers may involve decreases in drug dosage, increases in the interval between doses, or a combination thereof. Other clinicians may stop medications abruptly, without taper. Clinicians may also reduce dosage and/or frequency with the goal of identifying lower-intensity regimens that suppress inflammation and flares. Some clinicians may maintain the same regimen in growing children, which amounts to a gradual taper as children outgrow their medication. As detailed within this review, we currently lack high-quality evidence guiding practices for treatment withdrawal, even for commonly used antirheumatic drugs. As a result, clinical approaches to withdrawing medications vary widely across clinicians and centers [24, 25]. This additional source of variability further complicates our ability to draw firm conclusions from studies on treatment withdrawal.
Notably, variable treatment decisions early in patients’ disease course may also affect outcomes after treatment withdrawal. For example, one group of physicians may start conventional synthetic DMARDs (csDMARDs) first on all patients with more severe JIA, adding biologic DMARDs (bDMARDs) only for those who do not adequately respond after many months of treatment. Another group of physicians may start bDMARDs shortly after or at the same time as csDMARDs for patients with similar disease severity; some of these patients may in fact have responded to a csDMARD alone. Thus, biologic users in the first group may be expected to have more refractory disease, on average, than biologic users in the second group and, as a result, may have an increased rate of flares after treatment withdrawal. The heterogeneity of early treatment practices and resultant differences in patient populations in remission on the same medicine (e.g., bDMARD) are usually not well recognized or described. Nonetheless, these group differences may also lead to selection bias that limits the interpretability and generalizability of observational studies of treatment withdrawal.
1.4. Benefits and Risks of Treatment Withdrawal
Maintenance of JIA remission on medication has numerous potential costs for patients, families, and society. All medicines cause side effects and toxicities, and many patients treated for JIA experience adverse effects, such as nausea, abdominal pain, or injection site reactions. Some toxicities may be severe, including teratogenicity, malignancy, or infection requiring hospitalization, although parenthetically the role of antirheumatic drugs (e.g., tumor necrosis factor inhibitors [TNFi]) in causing certain serious outcomes in children with JIA remains unclear [26, 27]. Many JIA medications, particularly bDMARDs, are expensive and financially costly to families as well as to society [28]. JIA medications also have additional, hidden costs, including the inconvenience of administering shots or receiving infusions; the opportunity costs of missed school, work, and activities; and the psychologic burdens of taking/giving medicine and experiencing or fearing treatment toxicities [24, 28]. Given the many risks and costs of JIA medications, treatment withdrawal can give patients and families welcome relief and may improve their quality of life.
Nonetheless, treatment withdrawal itself is not without costs. Some patients who stop JIA medication will flare, leading to new pain, disability, and disruption of activities. Some flares may not respond to prior treatment regimens, requiring use of other, potentially toxic or expensive medications. Irreversible damage to the joints, eyes, or other organs may result from flares after remission. In addition to the physical toll of flares, patients and families may worry about such flares occurring and not responding to treatments that previously maintained remission. Physicians’ inability to accurately predict such flares and future treatment response can add to these psychologic burdens.
Thus, withdrawing medicines from children with JIA in remission has important trade-offs in risks. These trade-offs and the limited evidence quantifying the risks (as described below) makes decisions about withdrawal challenging for patients, families, and clinicians. Consequently, these decisions often revolve around perceptions of the relative risks of continuing versus stopping treatment [24], [26], [29].
2. Methods
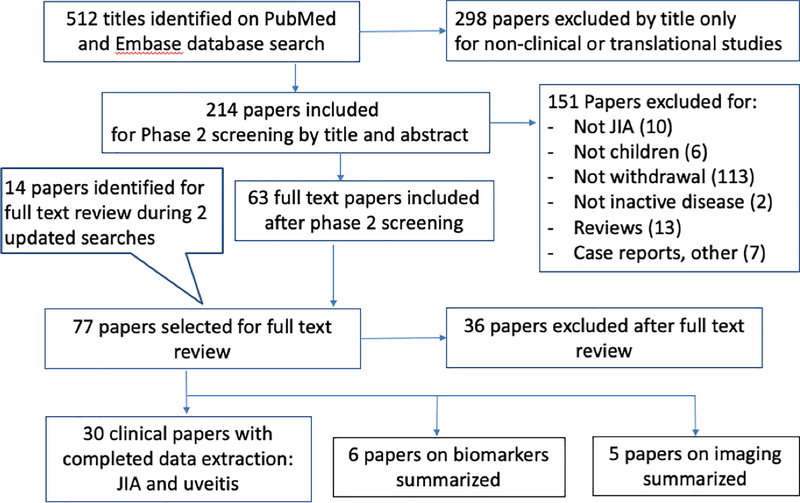
To identify literature about treatment withdrawal for well-controlled JIA, we performed a systematic search in PubMed and Embase of English-only publications from January 1, 1990, until May 17, 2018, with subsequent updated searches after completion of initial data extraction (see below). We used the following search strategy: (“arthritis, juvenile”[MeSH Terms] OR “juvenile idiopathic arthritis”[All Fields]) AND (Discontinu* OR Withdraw* OR Cessation OR Stopping OR Ceasing OR Taper* OR “down titration” OR “dose reduction” OR “dose de-escalation” OR “dose tapering” OR “spacing” OR “interval widening” OR “dose titration”). 512 papers were initially identified. After exclusion by title of 298 papers that were not considered JIA-related clinical or translational studies, 214 papers were screened for inclusion based on titles and abstract by pairs of authors (OH and DBH, JM and DR) (Figure 1). Disagreements were resolved by consensus. 77 original studies were marked for full-text review along with their references and additional references from three identified review papers on JIA treatment withdrawal. We performed an updated search on November 8, 2018, using the same search criteria, which yielded 35 additional papers, of which 11 were marked for full-text review. A final, more inclusive search was conducted on April 26, 2019, where the search terms for title/abstract keyword search also included “Drug Free Remission”, as detailed below: (‘juvenile rheumatoid arthritis’/exp/mj OR ‘juvenile idiopathic arthritis’:ti,ab) AND (‘treatment withdrawal’/exp/mj OR discontinu*:ti OR withdraw*:ti OR cessation OR stopping OR ceasing OR taper* OR ‘down titration’ OR ‘dose reduction’ OR ‘dose de-escalation’ OR ‘dose tapering’ OR spacing OR ‘interval widening’ OR ‘dose titration’ OR ‘Drug Free remission’) AND (‘article’/it OR ‘article in press’/it OR ‘review’/it) AND [english]/lim AND [1980–2018]/py. The search yielded 3 extra articles for review, confirmed by consensus among all authors for inclusion and exclusion criteria. Data were extracted by OH and independently reviewed by another author (DH, JM, DR, or SR).
Fig 1.
Selection of literature for inclusion
JIA, juvenile idiopathic arthritis
Quality of evidence assessments were independently completed for each clinical paper by DH and another author (DR, JM, or OH) using the NIH/NHLBI Study Quality assessment tool [30] (Tables S1–5). Differences in assessments were resolved via consensus discussion. Of note, we assessed the quality of included studies from the perspective of JIA medication withdrawal, which was not the primary focus of several studies. Thus, our assessments may not have reflected the overall quality of included studies.
3. Results
In total, 23 clinical papers on JIA and 7 clinical papers on uveitis met inclusion criteria and underwent data extraction using the pre-approved data extraction forms (Figure 1, Tables 2–4). An additional search on serum biomarkers yielded 6 papers and 5 papers on imaging, which were reviewed and summarized.
Table 2.
Summary of withdrawal data in juvenile idiopathic arthritis
| Publication First Author, Year, Country | Study Design | N, Total | N, Wtd | CID Criteria | N, Centers | Years of Study | JIA Category | Med | Wtd Approach | Main Wtd Outcome | Flare Definition | Follow-up | Main Results | Uveitis | Recapture | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MTX | ||||||||||||||||
| Klotsche, 2018, Germany [39] | PCo/NCC | 1514 | 316 | cJADAS10 <=1 | NR1 | 2005-NR (after 2011) | All | MTX | Abrupt stop (62%), taper (38%) | Risk of flare | cJADAS10 >1.5 for oJIA or ≥2.5 for pJIA, or restart DMARD for any subject | Mean 3.6yr | 58% flared at mean 7.4mo; 46% flared within 12mo (78% of those who flared in follow-up); flare risk higher for CID <6mo (72%) than for CID >12mo (49%) | 6/7 patients with documented eye exam had active uveitis at time of flare | NR | Prospective observational study; 63% of those with flares received MTX or biologic |
| Foell, 2010, multiple countries [31] | RCT | 364 | 297 | Wallace2 | 61 | 2005–2008 | All | MTX | Abrupt stop 6mo or 12mo after trial entry | Flare within 2yr of study entry | Loss of CID | Mean 2.8yr | Equivalent risk of flare in each arm within 2yr of study entry (56–57%); equivalent risk of flare at 12mo after stopping (40%) | NR | NR | Those assigned to 12mo of MTX had higher risk of flares before stopping |
| Gottlieb, 1997, USA[86] | RCo/NCC | 101 | 25 | No active synovitis, normal labs | 2 | 1986–1996 | eoJIA, pJIA, sJIA | MTX | Taper by 2.5mg/mo (92%); abrupt stop (8%) | Time to flare | NR | Mean 1.1yr after stopping | 52% flare after mean 11mo | NR | 90% (9/10) with follow-up data reached CID on same dose of MTX after mean 7mo | Small, retrospective study; small N |
| Ravelli, 1995, Italy [40] | RCo | 30 | 17 | Morning stiffness ≤15 minutes, no fatigue, no active arthritis for ≥2 months, ESR<20 | 1 | 1986–1993 | eoJIA, pJIA, sJIA | MTX | NR | Risk of flare | Recurrent arthritis | Range 3–52mo | 59% flared: 30% within 3–9mo, 29% within 12–52mo | NR | 90% (9/10) flare restarted MTX; only 1/6 with available follow-up regained remission within median 15 months. | Small, retrospective study; single center; wtd at median 3 mo after clinical remission; small N; low MTX dose |
| Biologic | ||||||||||||||||
| Minden, 2019, Germany[87] | PCo | 566 | NR | PhGA and cJADAS10 remission off drugs | NR (multiple, per BiKER and JuMBO registries | 2005–2016 | All | Mostly ETN and ADA, few TCL, ANK | NR | PhGA and cJADAS10 remission off medications at 10 years ater JIA onset | NR | Mean observation 9.1yr | cJADAS10 remission off drugs at 10 yrs of disease duration significantly higher for early bDMARD starters (<2yrs)–15.7% vs 2–5 yrs (6%, OR 0.34), or >5yrs - 3.8% (OR 0.25). | NR | NR | Early start of bDMARDs (<2yrs) -also higher functional capability, lower requirements for joint and eye surgeries |
| Ter Haar, 2019, The Netherlands [49] | PCo | 42 | 25 | Modified Wallace2 (PhGA <1) | 1 | 2008–2017 | sJIA | ANK7 as first line monotherapy | After 3 mo CID, Tapered to alternate day for 1 mo | % in CID off medications at 1 yr | NR | Median 5.8yrs | At 1 yr- 52% off medications, at 5yrs-72% | NR | NR | |
| Aquilani, 2018, Italy [42] | RCo/NCC | 110 | 110 | Wallace2 | 1 | 2005–2016 | oJIA, pJIA | ETN | Abrupt stop (75%) or taper (25%), dose or frequency | Risk of flare | Recurrent arthritis or uveitis | 1yr | 60% flared within 12mo (median time to flare 4mo); 6/7 (86%) patients with CID >2yrs before wtd flared | 11% of arthritis flares also with concurrent uveitis (2 of 7 without prior uveitis) | NR | Retrospective study; did not include patients who flared while tapering; patients on ETN for ≥18mo, in CID on ETN for ≥6mo |
| Lovell, 2018, USA [33] | PCo/NCC3 | 137 | 106 | Modified Wallace2 (PhGA<0.5) | 16 | 2009–2014 | eoJIA, pJIA | TNFi | Abrupt stop | Risk of flare | ≥30% worsening in ≥3/6 JIA ACR core criteria + ≤1 improving by >30% | Median 8mo | 37% flared by 8mo | 75% (3/4) with prior uveitis flared with uveitis | NR | Withdrawal intervention at 6mo after study entry; 19% did not maintain CID while on TNFi within 6mo of study entry |
| Ruperto, 2018, multiple countries, [36] | RCT/PCo | 177 | 44 | Wallace2 or JADAS71 | 63 | 2009–2014 | sJIA | Canakinumab | Halved dose, then stopped | Risk of flare | Loss of CID | Median ∼3.5yr | 31% (44/144) in long-term extension study reduced drug to half dose; 59% (26/44) did not flare in median 25mo; 19% (5/26) stopped drug for ongoing remission | NR | 83% (15/18) who flared with taper regained control with full dose drug | Ongoing dedicated taper trial in responders ( NCT02296424) |
| Simonini, 2018, Italy[44] | RCo/NCC | 349 | 135 | Modified Wallace2,4 | 3 | 2000–2016 | eoJIA, ERA, oJIA, pJIA, PsJIA, sJIA | Biologics: ETN, ADA, IFX; ANK, rituximab, abatacept | NR | Time to flare | Loss of ≥ 2 Wallace criteria (not including stiffness) or Tx intensification | Median 6mo (3–109mo) | 75.6% flared; 31% had sustained remission 1yr after wtd; flares more common in those with CID <2yr (60%) than those with CID >2yr (12.5%) | NR | NR | Retrospective study; 68.1% were also on MTX |
| Su, 2017, Taiwan[45] | RCo/NCC | 30 | 10 | Wallace2 | 1 | 2003–2015 | eoJIA, pJIA, sJIA | ETN | Taper | Risk of flare | Loss of Wallace criteria for >1 visit | mean 26.4mo | 44% with CR off meds5, 17% with CR off meds for ≥2yrs | NR | NR | Small, retrospective study; analysis compared those in remission and those with flare, on or off treatment |
| Iglesias, 2014, Spain [88] | RCo/NCC | 18 | 18 | Wallace2 | 1 | 2000–2011 | ERA, oJIA, pJIA, uJIA, | ETN, ADA, IFX, +67% also on MTX | Abrupt, 6mo after MTX wtd | Risk of flare | Occurrence of new joint pain, new limited ROM, or new inflammatory signs on exam | Mean 5.1yr (SD 2.1) | 82% flared after wtd of all meds, mean time to flare 3mo | 1 patient with uncontrolled JIA and uveitis | NR | Small, retrospective study; did not include patients who flared while tapering; mean time to start of TNFi 18.6mo; 2/3 stopped TNFi 6mo after MTX wtd |
| Cai, 2013 China [32] | PCo/NCC3 | 31 | 31 | Wallace2 | 1 | 2008–2012 | eoJIA, ERA, pJIA | ETN | Dose decrease by 50%: 0.4 mg/kg per wk x12mo, then 0.4 mg/kg per mo | Risk of flare | Recurrent arthritis, systemic symptoms, or disease progression on MRI | Mean 5.1yr (SD 2.1) | 12.9% flared within 12mo, none during subsequent 12 mo; no disease progression on MRI in those who stayed in CR | NR | NR | Small, prospective observational study; single center; MRI performed at study entry, 1yr, or time of flare |
| Postepski, 2013, Poland [89] | RCo/NCC | 39 | 39 | Wallace | 2 | NR | ERA, oJIA, pJIA, PsJIA, sJIA | ETN | Abrupt | Duration of CID after ETN wtd | NR | Mean 25.4mo | 38.5% flared at 6mo; 30.8% remained in long-term CR off meds for mean 25.4 ± 12mo; mean duration of remission after ETN wtd 14.2mo | NR | 12/30 (40%) patients who started csDMARD for flare needed ETN, all of whom “responded satisfactorily” | Small, retrospective study; mean duration of remission on medication - 21.3mo (4–42mo) |
| Baszis, 2011, USA [90] | RCo/NCC | 171 | 136 | Wallace2 | 1 | 1998–2009 | All | ETN, ADA, IFX | Abrupt; those on csDMARD-TNFi combo stopped TNFi first | CR after stopping TNFi | NR | Mean 3.8yr after TNFi started | 33% with CR at 12mo; median CR duration 3.9mo; 40% of post-wtd flares while on MTX | Present in 16%, no other reported data | NR | Retrospective, single-center study; median duration TNFi Tx with CID 6mo (range 0–67.9mo) |
| Otten, 2011, Netherlands [37] | PCo/NCC | 262 | 39 | Modified Wallace2 (PhGA<1) | NR1,6 | 1999–2011 | All | ETN | NR | NR | NR | Median after ETN wtd 13.4mo (IQR 5.3–27.4mo) | 38% flared; compared to those with sustained CR off meds, those who flared had shorter prior ETN Tx (mean 29mo vs. 45mo) | NR | NR | Observational prospective study; performed within Dutch registry |
| Pratsidou-Gertsi, 2010, Greece [47] | RCo/NCC | 36 | 11 | Wallace2 | 1 | 2004–2008 | oJIA, pJIA | ETN | Abrupt stop (82%), taper by interval in ≤3mo (18%) | Risk of flare | NR | Median 3mo (1–15mo) | 100% flared (median time to flare 3mo); longer time to flare in those who also had stopped MTX prior to ETN | 1 uveitis flare | Milder disease activity with flare, controlled with MTX/CSA in 10/11 and ADA+MTX in 1 (also had uveitis) | Small retrospective study; ETN withdrawn after ≥12mo of CID |
| Remesal, 2010, Spain [46] | RCo/NCC | 26 | 24 | Wallace2 | 1 | 2004–2009 | ERA, pJIA, PsJIA, oJIA, sJIA | ETN | Abrupt (54%), Gradual (46%) by dose or frequency | Risk of flare and response to re-treatment | Active arthritis on physical exam | Mean 17+/−13mo | 69% relapsed after mean 5.8mo (0.6–15.9); in all 12 patients with taper, flare happened after complete wtd. | NR | 18 restarted ETN for flare and “responded satisfactorily”; 6/18 received IA or systemic GCs | Small retrospective study; ETN was weaned after 1–36mo (mean 14.7mo) in CID |
| Prince, 2009, Netherlands [43] | RCo/NCC | 19 | 19 | Modified Wallace2 (PhGA <1) | NR1 | 1999–2008 | eoJIA, ERA, pJIA, sJIA | ETN | Abrupt (26%), taper (74%) | Risk of flare, time to flare | NR | Median 0.8yr (IQR 0.5–2.8yr) | 47% flared; higher rates of flare in those with shorter time on ETN (2.1 vs. 3.5yr, p=0.21), shorter time in CID (0 vs. 1.5 yr, p<0.01), and who abruptly stopped ETN (80% vs. 36%, p=?) | NR | 8/9 resumed ETN and “reacted promptly to treatment” | Small, retrospective study; did not include patients who flared while tapering; performed within Dutch registry |
| Combination treatment | ||||||||||||||||
| Hissink Muller, 2018, Netherlands [34] | RCT/PCo | 94 | 54 | Modified Wallace2 (PhGA<1) | 1 | 2009–2014 | oJIA, pJIA, PsJIA | Arm 1: MTX or SSZ Arm 2: MTX + Pred Arm 3: MTX + ETN | Taper in 1–2mo | Time to flare | Recurrence of arthritis | Mean 2yr | Median time to flare 3mo (3.0–6.8mo); after 2y, CID off meds in 31–45% across arms | Excluded | 26% (14/54) restarted treatment; 71% (10/14) regained CID within 3mo | Single-center treatment strategy RCT; taper for ≥3mo CID for oJIA and 6 mo for pJIA |
| Guzman, 2016, Canada [38] | PCo/NCC | 1497 | 1146 | Modified Wallace2 (0 enthesitis, PhGA<1) | 16 | 2005–2012 | All | MTX ± biologics (TNFi and ANK for sJIA) | NR | Risk of flare | Recurrent disease activity or PhGA≥1 | Mean 2yr | 32% flared within 12mo of Tx wtd; 25% required treatment escalation | NR | NR | Prospective, observational study within national inception cohort; few RF+ pJIA and eoJIA stopped treatment |
| Chang, 2015 USA [41] | RCo/NCC | 455 | 335 | Wallace2 | 1 | 2000–2011 | ERA, pJIA | MTX and/or TNFi | MTX+ TNFi: abrupt stop (64%) or taper (36%) of first drug; NR for MTX or TNFi monotherapy | Risk of flare, time to flare | Loss of CID | Mean 3.8yr | 63% flared within 12mo; among those on TNFi+MTX, those stopping TNFi first had higher risk of flare within 12mo (78%) than those stopping MTX first (19%) (higher risk of flare in those with ERA); among those on TNFi±MTX, 83% flared within 12mo of stopping all medicines; among those on MTX monotherapy, 50% flared within 12 months | NR | 49% regained CID within 12mo | Retrospective, single-center study |
| Wallace, 2014, USA[35] | RCT/PCo | 48 | NR | Wallace2 | 12 | 2010–2012 | pJIA | MTX ± ETN and Pred | NR | Duration of CID | Loss of CID | Mean 1.8yr | 15% achieved CID without meds, 2 of 7 for ≥12mo | NR | NR | Performed with RCT extension study; subjects with highly active disease at trial entry; 65% of subjects with CID did not remain in CID (75% because of tapering) |
ADA adalimumab, ANK anakinra, CID clinical inactive disease, cJADAS10 clinical juvenile arthritis disease activity score 10 joints, CR clinical remission, csDMARD conventional synthetic disease-modifying antirheumatic drug, Dx diagnosis, eoJIA extended oligoarticular JIA, ERA enthesitis-related arthritis, ESR erythrocyte sedimentation rate, ETN etanercept, GC glucocorticoid, IA intra-articular, IFX infliximab, IQR interquartile range, JIA juvenile idiopathic arthritis, JADAS71 juvenile arthritis disease activity score 71 joints, Med medication, mo month, MTX methotrexate, NCC nested case-control, NR not reported, oJIA oligoarticular JIA, PaGA patient/parent global assessment, PCo prospective cohort PhGA physician global assessment, pJIA polyarticular JIA, Pred Prednisone, PsJIA juvenile psoriatic arthritis, RCT randomized controlled trial, RCo retrospective cohort, sJIA systemic JIA, SSZ sulfasalazine, TNFi tumor necrosis factor inhibitor, TCL tocilizumab, Tx treatment, uJIA undifferentiated JIA, wk week, wtd withdrawal, yr year
Multiple participating centers within the country
Wallace criteria: no joints with active arthritis; no fever, rash, serositis, splenomegaly, or generalized lymphadenopathy attributable to JIA; No active uveitis; best possible physician’s global assessment of disease activity score (or as modified); normal ESR and/or CRP; if elevated, not attributable to JIA; duration of morning stiffness ≤15 minutes [12]
Protocolized, single-arm withdrawal interventional study
Wallace criteria as defined above except including no enthesitis and not including stiffness criterion
Clinical remission defined as CID on medication for ≥6 months or CID off medication for ≥12 months
All Dutch patients with JIA who used ETN since 1999
Table 4.
Summary of withdrawal data in JIA-associated uveitis.
| First Author, Year, Country | Design | N, Initial Cohort | N, Wtd | Inactive Disease Criteria | N, Centers | Years of Study | JIA Cat | Med | Wtd Approaches | Definition of Flare [92] | Follow-up | Wtd Outcome Measure | Main Results | Associated Factors | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acharya, 2018 USA [51] | RCo/NCC | 51 | 14 | ≤0.5+ AC cells, ≤0.5+ VH, oral pred dose ≤10 mg/d, topical GCs ≤3 times/day | 2 | 1988–2011 | oJIA, pJIA, other | MTX, MMF, CSA, IFX, ADA, ETN | Taper or abrupt | ≥1+ AC cells or ≥1+ VH | Median 2.3yrs | Time to flare from wtd or (where applicable) start of taper | 57% (8/14) flared after Tx stopped for inactive uveitis; 5/13 (38%) flared during taper, before complete wtd | No significant differences in age at wtd, sex, time on TNFi, duration of GC-sparing control, and time from uveitis Dx to start of TNFi | Small, retrospective study; high flare rates - 82% for those who stopped TNFi, but unclear if all stopped for remission |
| Breitbach, 2017 Germany [93] | RCo | 59 | 3 | <0.5 AC cells | 1 | 2006–2013 | eoJIA, poJIA, pJIA | ADA + cDMARDs (mostly MTX, some AZA, CSA, others) | NR | ≥0.5+ AC cells | NR | None specific for wtd | Only 3/59 stopped due to inactive disease (uveitis and arthritis) for ≥2yr | NR | Small, retrospective, single-center study; outcomes not provided specifically for the 3 patients who stopped for inactive uveitis; some patients continued csDMARDs after ADA wtd |
| Simonini, 2017, Italy [54] | RCo/NCC | 941 | 941 | Rare cells or <1 cell per field | 4 | 2013–2015 | ERA, oJIA, pJIA, PsJIA1 | MTX, ADA ± MTX, IFX + MTX | NR | ≥1 inflammatory activity grade | Median 48mo for JIA uveitis, 50mo for idiopathic uveitis | Time to flare after wtd | ∼40% flared within 1yr and ∼80% flared within 2yr; 17.4% of patients with JIA remained in remission at 4yr | ANA+ and slower response to initial Tx (i.e., achieving inactive uveitis in >6 mo) - higher risk of uveitis flare; among those with inactive uveitis within 6mo of starting Tx, TNFi Tx a/w lower flare risk than MTX Tx; longer remission on Tx not associated with time to flare after wtd | Retrospective study; subjects were only included if they discontinued and remained off all Tx for ≥6 months, thus excluding those who could not wtd Tx and those with early flares after wtd |
| Lerman, 2015, USA [52] | RCo/NCC | 50 | 19 | “Slightly active” or inactive uveitis while on ≤2 drops/d topical GCs and no oral GCs for ≥2 visits spanning ≥28d | 1 | 2000–2012 | Non-infectious uveitis, 43.6% with JIA | IFX or ADA ± other (GCs, MTX, MMF) | NR | Active uveitis | NR | Time to flare after TNFi wtd | 63.8% flare after wtd, higher in ADA vs IFX group (HR 13.4) | Older age at onset of uveitis a/w flare rate (HR1.3); factors not a/w flare: duration of remission on meds, sex, race, systemic Dx, time to drug initiation, disease severity at drug initiation (for all types of uveitis) | Small, retrospective, single-center study |
| Shakoor, 2014, USA [55] | RCo/NCC | 65 | 18 | ≤0.5+ AC cells, ≤0.5+ VH, no active vasculitis, retinitis, or choroiditis | 1 | 1998–2010 | NR, patients with various types of inflammatory uveitis | IFX ± MTX or MMF | NR | Active uveitis by SUN criteria | NR | Time to flare after IFX wtd | 4/18 patients had JIA, all 4/4 flared at median 76days | Higher rate of flare if GC-sparing control (≤10mg pred/day) took >6mo; factors not a/w flare: age, gender, duration of IFX Tx, duration of inactive uveitis before wtd, duration to IFX initiation | Small, retrospective, single-center study; patients with JIA flared sooner (median 76d) than those without JIA (median 1169d) |
| Saboo, 2013, USA[50] | RCo/NCC | 30 | 30 | <1+ AC cells and <1+ VH | 1 | 1990–2011 | mostly oJIA | MTX, AZA, MMF, CSA, IFX, chlorambucil | Stopped after 2yr in remission w/o GCs | ≥1+ AC cells or ≥1+ VH | Median 20mo for flare group, 56mo for remission group | Risk factors a/w flare after remission ≥1 yr | 43% flared | Longer time to DMARD and older age at DMARD initiation a/w flare; no other factors a/w flare (e.g., sex, JIA cat, ANA, duration of uveitis or inactivity before wtd) | Small, retrospective, single-center study; did not include patients who flared within 1yr of discontinuation |
| Kalinina Ayuso, 2011, Netherlands [53] | RCo/NCC | 22 | 13 | ≤0.5+AC cells | 1 | 1989–2009 | oJIA, pJIA, PsJIA | MTX | Abrupt | ≥1+ AC cells or any increase from time of wtd | Median 1.7yr | Time to flare after MTX wtd | 69% flared after mean 7.5mo | Flare a/w MTX Tx <3yr, inactive uveitis on MTX <2yr before wtd, age <8yrs at wtd | Small, retrospective, single-center study |
AC anterior chamber, ADA adalimumab, ANA antinuclear antibody, a/w associated with, AZA azathioprine, cat category, csDMARD conventional synthetic disease-modifying anti-rheumatic drug, CSA cyclosporine A, d day, DMARD disease-modifying anti-rheumatic drug, Dx diagnosis eoJIA extended oligoarticular JIA, ETN etanercept, GC glucocorticoid, IFX infliximab, JIA juvenile idiopathic arthritis, MMF mycophenolate mofetil, mo month, MTX methotrexate, NCC nested case-control, NR not reported, oJIA oligoarticular JIA, pJIA polyarticular JIA, poJIA persistent oligoarticular JIA, pred prednisone, PsJIA juvenile psoriatic arthritis, RCo retrospective cohort, SUN Standardization of Uveitis Nomenclature Working Group, TNFi tumor necrosis factor inhibitor, Tx treatment, VH vitreous haze, wtd withdrawal, yr year
Only 67/94 (71%) of subjects had JIA; the remainder had idiopathic uveitis
3.1. Treatment Withdrawal in JIA
Studies on JIA treatment withdrawal differ considerably in design, including population definitions, sample sizes, medications studied (e.g. MTX, bDMARDs, or combination MTX/bDMARD), withdrawal approaches, and outcomes assessed (Table 2). Most studies have been observational, many retrospective and/or involving a single academic center (Table 2). One published randomized controlled trial (RCT) focused primarily on JIA treatment withdrawal [31]. Two studies examined outcomes after a single-arm protocolized withdrawal intervention [32, 33] while three other RCTs on early treatment strategies secondarily examined outcomes after withdrawal [34–36]. Four additional studies reported results on treatment withdrawal from large prospective, multicenter cohorts from Europe or North America [37–39, 87].
Flares are common after JIA treatment withdrawal, ranging 30–100% across studies (Table 2). Reported outcomes appear to be better overall for children withdrawing MTX, with risks of flare within 12 months ranging 30–50% [31, 39–41]. Among children withdrawing bDMARDs, reported risks of flare are 37% at 8 months [33] and 60–83% at 12 months [41, 42]; however, lower apparent flare rates have been reported in other studies on bDMARD withdrawal with variable follow-up [37, 43] (Table 2). Large cohorts with patients withdrawing MTX or bDMARDs have suggested that prior bDMARD use could be a potential risk factor for flare [38, 41]. Higher flare rates among bDMARD users may reflect their greater disease severity compared to children using csDMARDs.
Few other clinical factors have consistently been associated with flares post-withdrawal (Table 3). Children with sJIA may, on average, have lower risks of flares after stopping treatment [38, 39], with higher risks among those with rheumatoid factor-positive (RF+) polyarticular JIA (pJIA) [38, 39, 43]. CID duration on treatment has also been associated with outcomes across studies, although the direction of this association has been inconsistent: longer CID duration has been linked to both lower rates of flare [39, 43, 44] and higher rates of flare [33, 45]. The purported protective effect of prolonged CID may reflect so-called “depletion of susceptibles”: individuals at higher risk for flare may flare sooner, even in preparation for withdrawal [31, 33] or while tapering. Studies limited to children who stopped treatment may be biased if, for example, those with longer CID durations were more likely to flare with tapering and excluded from analyses. Of note, the only RCT on JIA treatment withdrawal, which compared shorter and longer times to MTX discontinuation, found no difference after accounting for pre-withdrawal flares [31]. Other factors, such as age at diagnosis, sex, antinuclear antigen (ANA) positivity, and measures of early disease activity, have been inconsistently identified as predictors of flare (Table 3).
Table 3.
Associations between clinical factors and risk of flare after Juvenile Idiopathic Arthritis treatment withdrawal1
| Article: First Author, Year | Older Age at Dx | Female Sex | JIA Category | ANA Positive | Higher Early Dz Activity2 | Higher Inflam. Markers | Time from Dx to Tx | Time to CID | Time in CID Before Wtd | Total Duration of Tx | Wtd: Abrupt vs. Taper | Prior Use of GCs | Prior Use of Biologic | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methotrexate | ||||||||||||||
| Klotsche, 2018 [39] | 0 | (↑) poJIA ↑↑ RF+ pJIA ↓↓ sJIA | (↑) | 0 | 0 | ↓ | 0 | |||||||
| Gottlieb, 1997 [86] | (↓) 3 | 0 | 0 | 0 | 0 | 0 | 0 4 | |||||||
| Biologics | ||||||||||||||
| Minden, 2019[87] | ↑/0 5 | |||||||||||||
| Aquilani, 20186 [91] | (↓) | 0 | (↑) | 0 | (↑) | 0 | 0 | 0 | 0 | 0 | 07 | |||
| Lovell, 2018 [33] | ↓ | 0 | 0 | 0 | (↑) | (↑) | ↓8 | |||||||
| Simonini, 2018 [44] | (↓) ERA (↓) sJIA | 0 | (↓)9 | 0 | 010 | |||||||||
| Su, 20176, 11 [45] | 0 | 0 | 0 | 0 | 0 | (↑) | (↑) | |||||||
| Iglesias, 201411[88] | 0 | 0 | ||||||||||||
| Cai, 20136 [32] | 0 | 0 | 0 | 0 | 0 | 012 | ||||||||
| Postepski, 20136 [89] | 0 | 0 | 0 | 0 | 0 | |||||||||
| Baszis, 201112 [90] | 0 | 0 | 0 | 0 | 0 | |||||||||
| Pratsidou-Gertsi, 20106 [47] | 0 | 0 | ||||||||||||
| Remesal, 20106 [46] | 0 | 0 | ||||||||||||
| Prince, 20096 [43] | ↑ RF+ pJIA | (↑) | ||||||||||||
| Combination treatment | ||||||||||||||
| Guzman, 201614 [38] | (↓)3 | 0 | ↓sJIA, (↑)RF+ pJIA15 | (↑) | ↑ | 0 | (↑) | 0 | (↑)16 | ↑17 | ||||
| Chang, 2015 [41] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | (↑) |
ANA antinuclear antibody, CID clinical inactive disease, Dx diagnosis, Dz disease, ERA enthesitis related arthritis, GCs glucocorticoids (intra-articular or systemic), Inflam inflammatory, JIA juvenile idiopathic arthritis, poJIA persistent oligoarticular JIA, RF+ pJIA rheumatoid factor positive polyarticular JIA, sJIA systemic JIA, Tx treatment, Wtd withdrawal.
0 no significant association, (↑) increased risk of flare in unadjusted analysis only, ↑ significantly increased risk of flare with adjusted OR/HR 1.01–2, ↑↑ significantly increased risk of flare with adjusted OR/HR>2; (↓) decreased risk of flare in unadjusted analysis only, ↓ significantly decreased risk of flare with adjusted OR/HR 0.5–0.99, ↓↓ significantly decreased risk of flare with adjusted OR/HR <0.5. Light grey fields: factor not reported in the study.
Disease activity variably assessed as joint counts or Juvenile Arthritis Disease Activity Score
Age <4.5 (Gottlieb) or <6 (Guzman) at diagnosis associated with higher risk of relapse after stopping treatment in unadjusted analysis
No association with cumulative dose of methotrexate
Study included multiple outcomes, some of which were associated with time to biologic initiation
Study of etanercept only
No association with concomitant use of methotrexate
Use of methotrexate in those on adalimumab associated with decreased risk of flare
Longer CID while on medicine (>2yrs) was associated with higher probability of sustained remission off medicine; those with clinical remission off medicine had mean CID duration 7 months longer than those who flared within 1 year (18.6+/−3.3 months vs 11.5+/−2.7 months, p<0.009)
No association with type of biologic or concomitant use of methotrexate
Study evaluated factors associated with flare versus remission, on or off medication
Study of various TNF inhibitors
No association with HLA-B27 positivity or arthritis severity scores by magnetic resonance imaging
Primarily studied risk of flare after attaining inactive disease and secondarily, flare after stopping treatment
Association with any flare after CID, not after stopping treatment
Increased risk of flare after achieving inactive disease but not after stopping treatment
High prior physician global score associated with subsequent risk of flare
Even fewer studies have provided actionable evidence on preferred strategies for treatment withdrawal. A large (N=335), single-center retrospective cohort study of children on combination MTX-TNFi therapy showed markedly lower risks of flare in children who stopped MTX first compared to children who stopped TNFi first (19% vs. 78% within 12 months) [41]. This treatment strategy is used by a majority of pediatric rheumatologists in the US and Canada [24], although this finding has not been replicated. Another study- a small (N=31), single-center, single-arm, unblinded interventional study - showed an impressively low rate of flares (12.9% within 2 years) with a very slow, stepwise taper of etanercept [32]. This intriguing approach, echoing effective approaches used for adults with RA (see below), has also not been replicated to date in larger JIA populations. Several other studies, most focused on bDMARDs, have shown no apparent differences in outcomes between tapering vs. abrupt drug discontinuation [41, 42, 46, 47]. Recent single-center studies of treat-to-target approaches have suggested that some patients can remain in CID following treatment withdrawal after relatively short times of CID (3–6 months) [48], particularly patients with sJIA [49]. These strategies warrant further examination and validation in other populations.
For those who do flare after treatment withdrawal, the timing and preferred approach to controlling the disease is even less clear. Published rates of “recapture” of well-controlled JIA in the biologic era have ranged 49–89% (Table 2).
The quality of evidence for most studies investigating JIA treatment withdrawal has ranged from fair to poor (Tables S1–4). Common deficiencies of reviewed articles included lack of blinding (87%), lack of sufficient adjustment for key confounders (61%), and lack of clearly defined and valid outcome measures (39%). Small sample sizes and exclusion of subjects who tapered but did not stop treatments were also commonly noted limitations.
3.2. Treatment Withdrawal in JIA-associated Uveitis
All 7 included publications on treatment withdrawal for uveitis have been retrospective, 5 of them single-center. Reported rates of flare after withdrawal have ranged 43–100% [50–55], with most flares occurring within 1 year after withdrawal [51, 53, 55]. The largest of these studies (N=94, including children with idiopathic uveitis) showed that children with shorter time to CID (≤6 months) were less likely to flare after withdrawal (56% vs. 87%); rapid TNFi-responders had more favorable outcomes than rapid MTX-responders [54]. ANA positivity was associated with higher risk of flares, echoing findings in some studies on JIA arthritis flares [38, 42]. As with studies on arthritis, findings with respect to CID duration have been discrepant: one small study (N=13) showed lower risks of flare in children with longer CID duration [53] while other larger studies have not replicated this finding [50, 52, 54]. Nonetheless, based on this overall low-quality evidence, some experts have recommended waiting for at least 2 years of quiescent uveitis before attempting withdrawal [56].
The quality of most studies on treatment withdrawal for JIA-associated uveitis have ranged from fair to poor (Table S5). Common deficiencies of reviewed articles included lack of blinding (100%), lack of sufficient adjustment for key confounders (71%), selection of subjects from different populations (57%), and small sample sizes (57%).
3.3. Use of Biomarkers
Multiple papers have examined potential biomarkers of JIA flare after treatment withdrawal, but no such biomarker has been consistently validated across populations. Several publications have reported an association between higher levels of S100 proteins (toll-like receptor-4 ligands produced by inflammatory neutrophils and monocytes, e.g., S100A8/A9 and S100A12) in medicated remission and risk of subsequent flare after MTX or bDMARD withdrawal [31], [57], [58] [59] These studies came from 2 multicenter, predominantly European populations—a clinical trial on MTX withdrawal [31] and prospective, observational biologic registries [58]. A separate, smaller Japanese study suggested similar findings but did not directly test flare risk after withdrawal [60]. A recent large US-based single-arm bDMARD withdrawal trial did not find any significant relation between S100 levels and risk of flare, although elevated S100A12 levels appeared possibly predictive of early flare [61]. In a separate study including participants from the same cohort, higher levels of anti-DEK antibodies (which target DEK nuclear phosphoprotein, participating in multiple intracellular pathways) were seen in those with higher joint counts and those who flared after TNFi discontinuation [62]. However, antibody levels at the time of treatment discontinuation did not significantly differ between those who did and did not subsequently flare [62].
3.4. Use of Imaging
As with biomarkers, ultrasound and magnetic resonance imaging (MRI) have been studied as means to detect subclinical disease not captured by clinical definitions of inactivity and to predict future JIA flares, with mixed results. Two small studies using ultrasound in children with JIA in CID, one from Italy (N=39) and another from the US (N=40), showed no apparent association between sonographic abnormalities and risk of JIA flare [63, 64]. In contrast, two other studies of patients in CID both on and off treatment showed significantly more flares associated with joint abnormalities on ultrasound, with better predictive ability when combining grey-scale and power Doppler findings [65, 66]. In one sample (N=35, Brazil), flares rates were several-fold higher among participants with subclinical synovitis and positive power Doppler signal [65]. In another study (N=88, Italy), abnormal joints were more likely to flare within 4 years (75%) than normal joints (38%), and the probability of remission at 1 year was 94% for those with normal joints and 55% for those with abnormal joints [66]. MRI can detect subclinical synovitis in patients in CID,[67] but it remains unclear whether MRI-detected synovitis corresponds to increased risk of flare after treatment withdrawal.
4. Discussion
4.1. Treatment Withdrawal in Studies of Adults with Chronic Inflammatory Arthritis
Rheumatologists have considerably more evidence to inform the management of adults with well-controlled rheumatoid arthritis (RA). As reviewed elsewhere, multiple randomized controlled trials and observational studies have compared the efficacy or effectiveness of different strategies for bDMARD withdrawal for patients with early or established RA[68, 69]. Several lessons have emerged from these studies. First, adults with RA who discontinue bDMARDs have relatively high rates of flare, with approximately half of patients with early RA flaring within 1.5 years and up to 84% of patients with established RA flaring within 1year [69].
Second, disease activity-guided dose reduction, which entails stepwise dose-decreases or interval-spacing based on interim clinical evaluations to ensure sustained remission before further de-escalation, can help theoretically identify minimally effective doses for individual patients, sparing unnecessary cost and toxicity from higher intensity treatments while maintaining well-controlled disease. However, a recent Cochrane review suggested that, compared with continued treatment, both fixed dose reduction and disease activity-guided tapering might increase the risk of minimal radiographic progression [70]. Third, few clinical factors in adults with RA have been shown to reliably predict flares across studies; these factors include seropositivity (positive RF or anti-citrullinated peptide antibodies) (increased flare) and lower disease activity at time of treatment withdrawal (e.g., DAS28<2.2) (decreased flare) as reviewed by others [68, 69]. In contrast, there has been mixed evidence supporting the use of serum biomarkers to predict flare in these patients[71–75].
In addition to research on treatment withdrawal in RA, studies have examined the feasibility of bDMARD tapering and withdrawal in other forms of chronic arthritis in adults. In a 2018 review of tapering and discontinuation studies in psoriatic arthritis, the authors concluded that, while bDMARD discontinuation carries a substantial risk of the loss of remission, tapering the dose or frequency of bDMARDs in patients with low disease activity is feasible [76]. Similar conclusions were drawn in a review of studies looking at both dose reduction and interval spacing of TNF-inhibitors in axial spondyloarthritis [69]. However, based on low-quality evidence, recent treatment recommendations conditionally recommended against bDMARD tapering in adults with spondyloarthritis [77].
While pediatric rheumatologists can learn from the high-quality research on treatment withdrawal in adults with RA, the evidence cannot be fully generalized to pediatric populations. For one, the majority of children with JIA have a disease that is genetically and biologically distinct from RA, with differences in treatment response and prognosis [9]. Compared to other JIA categories, children with RF+ pJIA (i.e., childhood-onset RA) have lower rates of clinical remission both on medication and off medication [78], [38]. Additionally, unlike the concept of CID in JIA, definitions of well-controlled disease (low disease activity or remission) used in many RA trials do not signify the clinical absence of all inflammation. Some definitions are based on having a low disease activity score 28-ESR or CRP (e.g., DAS28 <3.2 [low disease activity] or <2.6 [remission]), meaning that some joints may remain actively inflamed or inflammatory markers, modestly abnormal. Furthermore, many RA studies evaluating biologic tapering allow or require participants to continue conventional DMARDs (primarily MTX) in the background. While this approach may be more economical and acceptable to adults, this strategy is not favored by many pediatric rheumatologists or necessarily supported by evidence in populations with JIA [24],[41]. More research is needed to understand how well we can apply lessons from the management of arthritis in adults to children with well-controlled JIA.
4.2. Future Directions: What is the Need?
There is little evidence-based consensus on when and how to withdraw treatment in patients with well-controlled JIA. Most clinical trials for JIA have focused on when and how to start treatment. However, given the rising rates of remission in populations with JIA, combined with the physical, psychologic, and economic costs of ongoing treatment, better evidence is needed to understand the optimal strategies for safely withdrawing medications and identify the biochemical and radiographic biomarkers that can guide these strategies. The challenge is to ensure that the benefits of treatment withdrawal outweigh the risks - a difficult task at both the population-level and patient-level, given our current state of knowledge. With many unknowns and uncertainties around JIA treatment withdrawal, shared decision-making and consideration of patients’ and families’ priorities are paramount when deciding whether to withdraw JIA treatment. Patients and caregivers are also valuable partners in research on this topic and should be engaged from the planning stages and throughout knowledge translation and implementation.
4.3. Current and Future Studies Addressing Withdrawal
Other research on strategies and biomarkers for JIA treatment withdrawal are ongoing. The future of medicine, including pediatric rheumatology, is personalization of care for each patient, from medication initiation and monitoring to treatment withdrawal. International collaboration among pediatric rheumatologists is necessary to study large enough cohorts with JIA to support clinical and translational investigations of optimal and personalized withdrawal strategies, including identification and validation of predictive biomarkers. One example of such efforts is Understanding Childhood Arthritis Network-Canadian and Dutch (UCAN-CAN DU), an international investigation of personalized treatments for childhood arthritis, whose aims include prediction of which children will remain in remission after discontinuing bDMARD therapy [79].
5. Summary
The last two decades have seen marked changes in the general treatment approach to children with JIA. The acceptance of csDMARDs as appropriate first-line therapy and wider use of bDMARDs have made disease remission and damage prevention reasonable and increasingly expected targets. This progress has led to new pressing questions about when and how to withdraw treatment in children with JIA who have achieved prolonged remission. While the available data suggests that a large percentage of adults and children with arthritis flare upon withdrawal of treatment, some patients do remain quiescent. Important opportunities remain for future research to determine which patients will remain in remission after treatment withdrawal and which withdrawal strategies and biomarkers help achieve the optimal outcomes.
Supplementary Material
Key points.
Little conclusive evidence exists to help clinicians decide which patients with well-controlled JIA can safely stop treatment and how they should do so.
Time in medicated remission and other clinical factors have not consistently been associated with the likelihood of successful maintenance of remission off medications.
Promising strategies for treatment withdrawal from small or single-center studies (e.g., biologic tapering, treat-to-target withdrawal) bear confirmation in other populations.
Acknowledgments
The authors would like to thank Meaghan Muir for assisting with the original literature search and Chloe Rotman for assistance with the updated literature search and editing of references.
Compliance with Ethical Standards:
Funding: This paper was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health: L40-AR070497, K23-AR070286. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures: Dr. Horton has received grant funding on related topics from the Childhood Arthritis and Rheumatology Research Alliance and the Arthritis Foundation, as well as grant funding on unrelated matters from Bristol-Myers Squibb. Dr. Halyabar, Dr. Mehta, Dr. Ringold and Dr. Rumsey declare no conflicts of interest.
Glossary
- ACR
American College of Rheumatology
- bDMARD
biologic DMARD
- CRP
C-reactive protein
- CSA
cyclosporine A
- DMARD
disease-modifying anti-rheumatic drug
- MRI
magnetic resonance imaging
- RF+
rheumatoid factor-positive
- ROM
range of motion
- SD
standard deviation
References
- 1.Thierry S, Fautrel B, Lemelle I, Guillemin F. Prevalence and incidence of juvenile idiopathic arthritis: a systematic review. Joint Bone Spine. 2014. March;81(2):112–7. [DOI] [PubMed] [Google Scholar]
- 2.Petty RE, Southwood TR, Baum J, Bhettay E, Glass DN, Manners P, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol. 1998. October;25(10):1991–4. [PubMed] [Google Scholar]
- 3.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004. February;31(2):390–2. [PubMed] [Google Scholar]
- 4.Burgos-Vargas R, Rudwaleit M, Sieper J. The place of juvenile onset spondyloarthropathies in the Durban 1997 ILAR classification criteria of juvenile idiopathic arthritis. International League of Associations for Rheumatology. J Rheumatol. 2002. May;29(5):869–74. [PubMed] [Google Scholar]
- 5.Ravelli A, Varnier GC, Oliveira S, Castell E, Arguedas O, Magnani A, et al. Antinuclear antibody-positive patients should be grouped as a separate category in the classification of juvenile idiopathic arthritis. Arthritis and rheumatism. 2011. January;63(1):267–75. [DOI] [PubMed] [Google Scholar]
- 6.Stoll ML, Punaro M. Psoriatic juvenile idiopathic arthritis: a tale of two subgroups. Current opinion in rheumatology. 2011. September;23(5):437–43. [DOI] [PubMed] [Google Scholar]
- 7.Ravelli A, Consolaro A, Schiappapietra B, Martini A. The conundrum of juvenile psoriatic arthritis. Clin Exp Rheumatol. 2015. Sep-Oct;33(5 Suppl 93):S40–3. [PubMed] [Google Scholar]
- 8.Eng SW, Duong TT, Rosenberg AM, Morris Q, Yeung RS, Reacch OUT, et al. The biologic basis of clinical heterogeneity in juvenile idiopathic arthritis. Arthritis Rheumatol. 2014. December;66(12):3463–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nigrovic PA, Raychaudhuri S, Thompson SD. Review: Genetics and the Classification of Arthritis in Adults and Children. Arthritis Rheumatol. 2018. January;70(1):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martini A, Ravelli A, Avcin T, Beresford MW, Burgos-Vargas R, Cuttica R, et al. Toward New Classification Criteria for Juvenile Idiopathic Arthritis: First Steps, Pediatric Rheumatology International Trials Organization International Consensus. J Rheumatol. 2019. February;46(2):190–7. [DOI] [PubMed] [Google Scholar]
- 11.Wallace CA, Ravelli A, Huang B, Giannini EH. Preliminary validation of clinical remission criteria using the OMERACT filter for select categories of juvenile idiopathic arthritis. J Rheumatol. 2006. April;33(4):789–95. [PubMed] [Google Scholar]
- 12.Wallace CA, Giannini EH, Huang B, Itert L, Ruperto N. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis care & research. 2011. July;63(7):929–36. [DOI] [PubMed] [Google Scholar]
- 13.Consolaro A, Bracciolini G, Ruperto N, Pistorio A, Magni-Manzoni S, Malattia C, et al. Remission, minimal disease activity, and acceptable symptom state in juvenile idiopathic arthritis: defining criteria based on the juvenile arthritis disease activity score. Arthritis Rheum. 2012;64(7):2366–74. [DOI] [PubMed] [Google Scholar]
- 14.Shoop-Worrall SJW, Verstappen SMM, Baildam E, Chieng A, Davidson J, Foster H, et al. How common is clinically inactive disease in a prospective cohort of patients with juvenile idiopathic arthritis? The importance of definition. Ann Rheum Dis. 2017. August;76(8):1381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoop-Worrall SJW, Verstappen SMM, McDonagh JE, Baildam E, Chieng A, Davidson J, et al. Long-Term Outcomes Following Achievement of Clinically Inactive Disease in Juvenile Idiopathic Arthritis: The Importance of Definition. Arthritis & rheumatology (Hoboken, NJ). 2018. September;70(9):1519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Consolaro A, Ruperto N, Bracciolini G, Frisina A, Gallo MC, Pistorio A, et al. Defining criteria for high disease activity in juvenile idiopathic arthritis based on the juvenile arthritis disease activity score. Ann Rheum Dis. 2014. July;73(7):1380–3. [DOI] [PubMed] [Google Scholar]
- 17.Taylor J, Giannini EH, Lovell DJ, Huang B, Morgan EM. Lack of Concordance in Interrater Scoring of the Provider’s Global Assessment of Children With Juvenile Idiopathic Arthritis With Low Disease Activity. Arthritis Care Res (Hoboken). 2018. January;70(1):162–6. [DOI] [PubMed] [Google Scholar]
- 18.Mack ME, Hsia E, Aletaha D. Comparative Assessment of the Different American College of Rheumatology/European League Against Rheumatism Remission Definitions for Rheumatoid Arthritis for Their Use as Clinical Trial End Points. Arthritis Rheumatol. 2017. March;69(3):518–28. [DOI] [PubMed] [Google Scholar]
- 19.Knowlton N, Jiang K, Frank MB, Aggarwal A, Wallace C, McKee R, et al. The meaning of clinical remission in polyarticular juvenile idiopathic arthritis: gene expression profiling in peripheral blood mononuclear cells identifies distinct disease states. Arthritis and rheumatism. 2009. March;60(3):892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang K, Wong L, Sawle AD, Frank MB, Chen Y, Wallace CA, et al. Whole blood expression profiling from the TREAT trial: insights for the pathogenesis of polyarticular juvenile idiopathic arthritis. Arthritis research & therapy. 2016. July 7;18(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anink J, Otten MH, Gorter SL, Prince FH, van Rossum MA, van den Berg JM, et al. Treatment choices of paediatric rheumatologists for juvenile idiopathic arthritis: etanercept or adalimumab? Rheumatology (Oxford). 2013. September;52(9):1674–9. [DOI] [PubMed] [Google Scholar]
- 22.Kearsley-Fleet L, Davies R, Baildam E, Beresford MW, Foster HE, Southwood TR, et al. Factors associated with choice of biologic among children with Juvenile Idiopathic Arthritis: results from two UK paediatric biologic registers. Rheumatology (Oxford). 2016. September;55(9):1556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Textbook of Pediatric Rheumatology, 7th Edition. : Saunders; 2015. [Google Scholar]
- 24.Horton DB, Onel KB, Beukelman T, Ringold S. Attitudes and Approaches for Withdrawing Drugs for Children with Clinically Inactive Nonsystemic JIA: A Survey of the Childhood Arthritis and Rheumatology Research Alliance. J Rheumatol. 2017. March;44(3):352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broughton T, Armon K. Defining juvenile idiopathic arthritis remission and optimum time for disease-modifying anti-rheumatic drug withdrawal: why we need a consensus. Paediatr Drugs. 2012. February 1;14(1):7–12. [DOI] [PubMed] [Google Scholar]
- 26.Beukelman T, Xie F, Chen L, Horton DB, Lewis JD, Mamtani R, et al. Risk of malignancy associated with paediatric use of tumour necrosis factor inhibitors. Ann Rheum Dis. 2018. July;77(7):1012–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aeschlimann FA, Chong SL, Lyons TW, Beinvogl BC, Goez-Mogollon LM, Tan S, et al. Risk of Serious Infections Associated with Biologic Agents in Juvenile Idiopathic Arthritis: A Systematic Review and Meta-Analyses. J Pediatr. 2019. January;204:162–71 e3. [DOI] [PubMed] [Google Scholar]
- 28.Gidman W, Meacock R, Symmons D. The humanistic and economic burden of juvenile idiopathic arthritis in the era of biologic medication. Curr Rheumatol Rep. 2015. May;17(5):31. [DOI] [PubMed] [Google Scholar]
- 29.Horton DB, Salas J, Wec A, et al. Making Decisions About Stopping Medicines for Well-Controlled Juvenile Idiopathic Arthritis: A Mixed-Methods Study of Patients and Caregivers [published online ahead of print, 2019 Dec 27]. Arthritis Care Res (Hoboken). 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.NHLBI. 2019. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools)
- 31.Foell D, Wulffraat N, Wedderburn LR, Wittkowski H, Frosch M, Gerss J, et al. Methotrexate withdrawal at 6 vs 12 months in juvenile idiopathic arthritis in remission: a randomized clinical trial. JAMA. 2010. April 7;303(13):1266–73. [DOI] [PubMed] [Google Scholar]
- 32.Cai Y, Liu X, Zhang W, Xu J, Cao L. Clinical trial of etanercept tapering in juvenile idiopathic arthritis during remission. Rheumatol Int. 2013. September;33(9):2277–82. [DOI] [PubMed] [Google Scholar]
- 33.Lovell DJ, Johnson AL, Huang B, Gottlieb BS, Morris PW, Kimura Y, et al. Risk, Timing, and Predictors of Disease Flare After Discontinuation of Anti-Tumor Necrosis Factor Therapy in Children With Polyarticular Forms of Juvenile Idiopathic Arthritis With Clinically Inactive Disease. Arthritis Rheumatol. 2018. September;70(9):1508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hissink Muller P, Brinkman D, Schonenberg-Meinema D, Van Den Bosch W, Koopman-Keemink Y, Brederije I, et al. Treatment strategy study in new onset DMARD naive juvenile idiopathic arthritis first results on 24 months clinical outcome. Annals of the Rheumatic Diseases. 2018;77:478. [DOI] [PubMed] [Google Scholar]
- 35.Wallace CA, Ringold S, Bohnsack J, Spalding SJ, Brunner HI, Milojevic D, et al. Extension study of participants from the trial of early aggressive therapy in juvenile idiopathic arthritis. Journal of Rheumatology. 2014;41(12):2459–65. [DOI] [PubMed] [Google Scholar]
- 36.Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat NM, Horneff G, et al. Canakinumab in patients with systemic juvenile idiopathic arthritis and active systemic features: results from the 5-year long-term extension of the phase III pivotal trials. Ann Rheum Dis. 2018. September 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otten MH, Prince FH, Armbrust W, ten Cate R, Hoppenreijs EP, Twilt M, et al. Factors associated with treatment response to etanercept in juvenile idiopathic arthritis. Jama. 2011. December 7;306(21):2340–7. [DOI] [PubMed] [Google Scholar]
- 38.Guzman J, Oen K, Huber AM, Watanabe Duffy K, Boire G, Shiff N, et al. The risk and nature of flares in juvenile idiopathic arthritis: results from the ReACCh-Out cohort. Ann Rheum Dis. 2016. June;75(6):1092–8. [DOI] [PubMed] [Google Scholar]
- 39.Klotsche J, Minden K, Niewerth M, Horneff G. Time spent in inactive disease before MTX withdrawal is relevant with regard to the flare risk in patients with JIA. Ann Rheum Dis. 2018. February 16. [DOI] [PubMed] [Google Scholar]
- 40.Ravelli A, Viola S, Ramenghi B, Aramini L, Ruperto N, Martini A. Frequency of relapse after discontinuation of methotrexate therapy for clinical remission in juvenile rheumatoid arthritis. J Rheumatol. 1995. August;22(8):1574–6. [PubMed] [Google Scholar]
- 41.Chang CY, Meyer RM, Reiff AO. Impact of medication withdrawal method on flare-free survival in patients with juvenile idiopathic arthritis on combination therapy. Arthritis care & research. 2015. May;67(5):658–66. [DOI] [PubMed] [Google Scholar]
- 42.Aquilani A, Pires Marafon D, Marasco E, Nicolai R, Messia V, Perfetti F, et al. Predictors of Flare Following Etanercept Withdrawal in Patients with Rheumatoid Factornegative Juvenile Idiopathic Arthritis Who Reached Remission while Taking Medication. J Rheumatol. 2018. May 1. [DOI] [PubMed] [Google Scholar]
- 43.Prince FH, Twilt M, Simon SC, van Rossum MA, Armbrust W, Hoppenreijs EP, et al. When and how to stop etanercept after successful treatment of patients with juvenile idiopathic arthritis. Ann Rheum Dis. 2009. July;68(7):1228–9. [DOI] [PubMed] [Google Scholar]
- 44.Simonini G, Ferrara G, Pontikaki I, Scoccimarro E, Giani T, Taddio A, et al. Flares after withdrawal of biologic therapies in juvenile idiopathic arthritis: Clinical and laboratory correlates of remission duration. Arthritis care & research. 2018. October 3. [DOI] [PubMed] [Google Scholar]
- 45.Su Y, Yang YH, Chiang BL. Treatment response to etanercept in methotrexate refractory juvenile idiopathic arthritis: an analysis of predictors and long-term outcomes. Clin Rheumatol. 2017. September;36(9):1997–2004. [DOI] [PubMed] [Google Scholar]
- 46.Remesal A, J DEI, Merino R, Garcia-Consuegra J. Discontinuation of etanercept after successful treatment in patients with juvenile idiopathic arthritis. J Rheumatol. 2010. September;37(9):1970–1. [DOI] [PubMed] [Google Scholar]
- 47.Pratsidou-Gertsi P, Trachana M, Pardalos G, Kanakoudi-Tsakalidou F. A follow-up study of patients with juvenile idiopathic arthritis who discontinued etanercept due to disease remission. Clin Exp Rheumatol. 2010. Nov-Dec;28(6):919–22. [PubMed] [Google Scholar]
- 48.Hissink Muller P, Brinkman DMC, Schonenberg-Meinema D, van den Bosch WB, Koopman-Keemink Y, Brederije ICJ, et al. Treat to target (drug-free) inactive disease in DMARD-naive juvenile idiopathic arthritis: 24-month clinical outcomes of a three-armed randomised trial. Ann Rheum Dis. 2018. October 11. [DOI] [PubMed] [Google Scholar]
- 49.Ter Haar NM, van Dijkhuizen EHP, Swart JF, van Royen-Kerkhof A, El Idrissi A, Leek AP, et al. Treatment to Target Using Recombinant Interleukin-1 Receptor Antagonist as First-Line Monotherapy in New-Onset Systemic Juvenile Idiopathic Arthritis: Results From a Five-Year Follow-Up Study. Arthritis & rheumatology (Hoboken, NJ). 2019. July;71(7):1163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saboo US, Metzinger JL, Radwan A, Arcinue C, Parikh R, Mohamed A, et al. Risk factors associated with the relapse of uveitis in patients with juvenile idiopathic arthritis: a preliminary report. J AAPOS. 2013. October;17(5):460–4. [DOI] [PubMed] [Google Scholar]
- 51.Acharya NR, Patel S, Homayounfar G, Enanoria WTA, Shakoor A, Chakrabarti A, et al. Relapse of Juvenile Idiopathic Arthritis-Associated Uveitis after Discontinuation of Immunomodulatory Therapy. Ocular immunology and inflammation. 2018. February 16:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lerman MA, Lewen MD, Kempen JH, Mills MD. Uveitis Reactivation in Children Treated With Tumor Necrosis Factor Alpha Inhibitors. American journal of ophthalmology. 2015. July;160(1):193–200.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalinina Ayuso V, van de Winkel EL, Rothova A, de Boer JH. Relapse rate of uveitis post-methotrexate treatment in juvenile idiopathic arthritis. Am J Ophthalmol. 2011. February;151(2):217–22. [DOI] [PubMed] [Google Scholar]
- 54.Simonini G, Bracaglia C, Cattalini M, Taddio A, Brambilla A, De Libero C, et al. Predictors of Relapse after Discontinuing Systemic Treatment in Childhood Autoimmune Chronic Uveitis. J Rheumatol. 2017. June;44(6):822–6. [DOI] [PubMed] [Google Scholar]
- 55.Shakoor A, Esterberg E, Acharya NR. Recurrence of uveitis after discontinuation of infliximab. Ocular immunology and inflammation. 2014. April;22(2):96–101. [DOI] [PubMed] [Google Scholar]
- 56.Angeles-Han ST, Ringold S, Beukelman T, Lovell D, Cuello CA, Becker ML, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Screening, Monitoring, and Treatment of Juvenile Idiopathic Arthritis-Associated Uveitis. Arthritis care & research. 2019. June;71(6):703–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerss J, Roth J, Holzinger D, Ruperto N, Wittkowski H, Frosch M, et al. Phagocyte-specific S100 proteins and high-sensitivity C reactive protein as biomarkers for a risk-adapted treatment to maintain remission in juvenile idiopathic arthritis: a comparative study. Ann Rheum Dis. 2012. December;71(12):1991–7. [DOI] [PubMed] [Google Scholar]
- 58.Anink J, Van Suijlekom-Smit LW, Otten MH, Prince FH, van Rossum MA, Dolman KM, et al. MRP8/14 serum levels as a predictor of response to starting and stopping anti-TNF treatment in juvenile idiopathic arthritis. Arthritis Res Ther. 2015;17:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rothmund F, Gerss J, Ruperto N, Dabritz J, Wittkowski H, Frosch M, et al. Validation of relapse risk biomarkers for routine use in patients with juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). 2014. June;66(6):949–55. [DOI] [PubMed] [Google Scholar]
- 60.Yamasaki Y, Takei S, Imanaka H, Nerome Y, Kubota T, Nonaka Y, et al. Prediction of long-term remission of oligo/polyarticular juvenile idiopathic arthritis with S100A12 and vascular endothelial growth factor. Mod Rheumatol. 2016. July;26(4):551–6. [DOI] [PubMed] [Google Scholar]
- 61.Hinze CH, Foell D, Johnson AL, Spalding SJ, Gottlieb BS, Morris PW, et al. Serum S100A8/A9 and S100A12 Levels in Children with Polyarticular Forms of Juvenile Idiopathic Arthritis: Relationship to Maintenance of Clinical Inactive Disease During and Flare after Discontinuation of Anti-TNF Therapy. Arthritis & rheumatology (Hoboken, NJ). 2018. September 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mor-Vaknin N, Rivas M, Legendre M, Mohan S, Yuanfan Y, Mau T, et al. High Levels of DEK Autoantibodies in Sera of Patients With Polyarticular Juvenile Idiopathic Arthritis and With Early Disease Flares Following Cessation of Anti–Tumor Necrosis Factor Therapy. Arthritis and Rheumatology. 2018;70(4):594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Magni-Manzoni S, Scire CA, Ravelli A, Klersy C, Rossi S, Muratore V, et al. Ultrasound-detected synovial abnormalities are frequent in clinically inactive juvenile idiopathic arthritis, but do not predict a flare of synovitis. Ann Rheum Dis. 2013. February;72(2):223–8. [DOI] [PubMed] [Google Scholar]
- 64.Zhao Y, Rascoff NE, Iyer RS, Thapa M, Reichley L, Oron AP, et al. Flares of Disease in Children with Clinically Inactive Juvenile Idiopathic Arthritis Were Not Correlated with Ultrasound Findings. J Rheumatol. 2018. June;45(6):851–7. [DOI] [PubMed] [Google Scholar]
- 65.Miotto ESVB, Mitraud SAV, Furtado RNV, Natour J, Len CA, Terreri M. Patients with juvenile idiopathic arthritis in clinical remission with positive power Doppler signal in joint ultrasonography have an increased rate of clinical flare: a prospective study. Pediatr Rheumatol Online J. 2017. November 13;15(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Lucia O, Ravagnani V, Pregnolato F, Hila A, Pontikaki I, Gattinara M, et al. Baseline ultrasound examination as possible predictor of relapse in patients affected by juvenile idiopathic arthritis (JIA). Ann Rheum Dis. 2018. October;77(10):1426–31. [DOI] [PubMed] [Google Scholar]
- 67.van Gulik EC, Hemke R, Welsink-Karssies MM, Schonenberg-Meinema D, Dolman KM, Barendregt AM, et al. Normal MRI findings of the knee in patients with clinically active juvenile idiopathic arthritis. Eur J Radiol. 2018. May;102:36–40. [DOI] [PubMed] [Google Scholar]
- 68.Schett G, Emery P, Tanaka Y, Burmester G, Pisetsky DS, Naredo E, et al. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann Rheum Dis. 2016. August;75(8):1428–37. [DOI] [PubMed] [Google Scholar]
- 69.Edwards CJ, Fautrel B, Schulze-Koops H, Huizinga TWJ, Kruger K. Dosing down with biologic therapies: a systematic review and clinicians’ perspective. Rheumatology (Oxford). 2017. November 1;56(11):1847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verhoef LM, van den Bemt BJ, van der Maas A, Vriezekolk JE, Hulscher ME, van den Hoogen FH, et al. Down-titration and discontinuation strategies of tumour necrosis factor-blocking agents for rheumatoid arthritis in patients with low disease activity. The Cochrane database of systematic reviews. 2019. May 24;5:Cd010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rech J, Hueber AJ, Finzel S, Englbrecht M, Haschka J, Manger B, et al. Prediction of disease relapses by multibiomarker disease activity and autoantibody status in patients with rheumatoid arthritis on tapering DMARD treatment. Ann Rheum Dis. 2016. September;75(9):1637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tweehuysen L, van den Ende CH, Beeren FM, Been EM, van den Hoogen FH, den Broeder AA. Little Evidence for Usefulness of Biomarkers for Predicting Successful Dose Reduction or Discontinuation of a Biologic Agent in Rheumatoid Arthritis: A Systematic Review. Arthritis & rheumatology (Hoboken, NJ). 2017. February;69(2):301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bouman CAM, van der Maas A, van Herwaarden N, Sasso EH, van den Hoogen FHJ, den Broeder AA. A multi-biomarker score measuring disease activity in rheumatoid arthritis patients tapering adalimumab or etanercept: predictive value for clinical and radiographic outcomes. Rheumatology (Oxford). 2017. June 1;56(6):973–80. [DOI] [PubMed] [Google Scholar]
- 74.Tweehuysen L, den Broeder N, van Herwaarden N, Joosten LAB, van Lent PL, Vogl T, et al. Predictive value of serum calprotectin (S100A8/A9) for clinical response after starting or tapering anti-TNF treatment in patients with rheumatoid arthritis. RMD Open. 2018;4(1):e000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Inciarte-Mundo J, Ramirez J, Hernandez MV, Ruiz-Esquide V, Cuervo A, Cabrera-Villalba SR, et al. Calprotectin strongly and independently predicts relapse in rheumatoid arthritis and polyarticular psoriatic arthritis patients treated with tumor necrosis factor inhibitors: a 1-year prospective cohort study. Arthritis research & therapy. 2018. December 13;20(1):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ye W, Tucker LJ, Coates LC. Tapering and Discontinuation of Biologics in Patients with Psoriatic Arthritis with Low Disease Activity. Drugs. 2018. November;78(16):1705–15. [DOI] [PubMed] [Google Scholar]
- 77.Ward MM, Deodhar A, Gensler LS, Dubreuil M, Yu D, Khan MA, et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis & rheumatology (Hoboken, NJ). 2019. August 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guzman J, Oen K, Tucker LB, Huber AM, Shiff N, Boire G, et al. The outcomes of juvenile idiopathic arthritis in children managed with contemporary treatments: results from the ReACCh-Out cohort. Ann Rheum Dis. 2015;74(10):1854–60. [DOI] [PubMed] [Google Scholar]
- 79.UCAN CAN-DU. 2019; Available from: http://www.ucan-u.org/blog/homepage-block-2/ucan-can-du-a-unique-collaborative-network-of-dutch-and-canadian-researchers
- 80.Cleary A, Murphy H, & Davidson J Intra-articular corticosteroid injections in juvenile idiopathic arthritis.. Archives of Disease in Childhood,. 2003;88(3):192–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Falvey S, Shipman L, Ilowite N, & Beukelman T Methotrexate-induced nausea in the treatment of juvenile idiopathic arthritis.. Pediatric Rheumatology. 2017;15(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wallace C, Giannini E, Spalding S, Hashkes P, O’Neil K, Zeft A,… Lovell D Predictors and Sustainability Of Clinical Inactive Disease In Polyarticular Juvenile Idiopathic Arthritis Given Aggressive Therapy Very Early In The Disease Course. Arthritis And Rheumatism. 2013;65:S334–S5. [Google Scholar]
- 83.Kwok LW, Tam LS, Zhu T, Leung YY, Li E. Predictors of maternal and fetal outcomes in pregnancies of patients with systemic lupus erythematosus. Lupus. 2011;20(8):829–36. [DOI] [PubMed] [Google Scholar]
- 84.Lahiri M, Lim AY. Angioedema and systemic lupus erythematosus--a complementary association? Annals of the Academy of Medicine, Singapore. 2007;36(2):142–5. [PubMed] [Google Scholar]
- 85.Lahutte B, Cornic F, Bonnot O, Consoli A, An-Gourfinkel I, Amoura Z, et al. Multidisciplinary approach of organic catatonia in children and adolescents may improve treatment decision making. Progress in neuro-psychopharmacology & biological psychiatry. 2008;32(6):1393–8. [DOI] [PubMed] [Google Scholar]
- 86.Gottlieb BS, Keenan GF, Lu T, Ilowite NT. Discontinuation of methotrexate treatment in juvenile rheumatoid arthritis. Pediatrics. 1997. December;100(6):994–7. [DOI] [PubMed] [Google Scholar]
- 87.Minden K, Horneff G, Niewerth M, Seipelt E, Aringer M, Aries P, et al. Time of Disease-Modifying Antirheumatic Drug Start in Juvenile Idiopathic Arthritis and the Likelihood of a Drug-Free Remission in Young Adulthood. Arthritis care & research. 2019. April;71(4):471–81. [DOI] [PubMed] [Google Scholar]
- 88.Iglesias E, Torrente-Segarra V, Bou R, Ricart S, González MI, Sánchez J, et al. Non-systemic juvenile idiopathic arthritis outcome after reaching clinical remission with anti-TNF-α therapy: A clinical practice observational study of patients who discontinued treatment. Rheumatology International. 2014;34(8):1053–7. [DOI] [PubMed] [Google Scholar]
- 89.Postepski J, Kobusinska K, Olesinska E, Osinska V, Opoka-Winiarska V. Clinical remission in juvenile idiopathic arthritis after termination of etanercept. Rheumatol Int. 2013. October;33(10):2657–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baszis K, Garbutt J, Toib D, Mao J, King A, White A, et al. Clinical outcomes after withdrawal of anti-tumor necrosis factor alpha therapy in patients with juvenile idiopathic arthritis: a twelve-year experience. Arthritis Rheum. 2011. October;63(10):3163–8. [DOI] [PubMed] [Google Scholar]
- 91.Aquilani A, Marafon DP, Marasco E, Nicolai R, Messia V, Perfetti F, et al. Predictors of flare following etanercept withdrawal in patients with rheumatoid factor–negative juvenile idiopathic arthritis who reached remission while taking medication. Journal of Rheumatology. 2018;45(7):956–61. [DOI] [PubMed] [Google Scholar]
- 92.Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature Working G. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005. September;140(3):509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Breitbach M, Tappeiner C, Bohm MR, Zurek-Imhoff B, Heinz C, Thanos S, et al. Discontinuation of long-term adalimumab treatment in patients with juvenile idiopathic arthritis-associated uveitis. Graefes Arch Clin Exp Ophthalmol. 2017. January;255(1):171–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.