Key Points
Question
What is the association between perirectal hydrogel spacer placement and clinical outcomes of men receiving radiotherapy for prostate cancer?
Findings
This systematic review and meta-analysis, including results from 7 studies with 1011 patients receiving prostate cancer radiotherapy, found that perirectal hydrogel spacer placement was associated with less rectal irradiation, fewer rectal toxic effects, and higher bowel-related quality of life in long-term follow-up.
Meaning
Perirectal hydrogel spacer placement prior to prostate radiotherapy may be a prudent preventive strategy for reduction of radiotherapy-induced rectal complications.
Abstract
Importance
Perirectal spacers are intended to lower the risk of rectal toxic effects associated with prostate radiotherapy. A quantitative synthesis of typical clinical results with specific perirectal spacers is limited.
Objective
To evaluate the association between perirectal hydrogel spacer placement and clinical outcomes of men receiving radiotherapy for prostate cancer.
Data Sources
A systematic search was performed of the Cochrane Central Register of Controlled Trials, MEDLINE, and Embase for articles published through September 2019.
Study Selection
Studies comparing men who received a hydrogel spacer vs men who did not receive a spacer (controls) prior to prostate radiotherapy.
Data Extraction and Synthesis
Via random-effects meta-analysis, group comparisons were reported using the weighted mean difference for continuous measures and the risk ratio for binary measures.
Main Outcomes and Measures
Procedural results, the percentage volume of rectum receiving at least 70 Gy radiation (v70), early (≤3 months) and late (>3 months) rectal toxic effects, and early and late changes in bowel-related quality of life on the Expanded Prostate Cancer Index Composite (minimal clinically important difference, 4 points).
Results
The review included 7 studies (1 randomized clinical trial and 6 cohort studies) involving 1011 men (486 who received a hydrogel spacer and 525 controls), with a median duration of patient follow-up of 26 months (range, 3-63 months). The success rate of hydrogel spacer placement was 97.0% (95% CI, 94.4%-98.8% [5 studies]), and the weighted mean perirectal separation distance was 11.2 mm (95% CI, 10.1-12.3 mm [5 studies]). Procedural complications were mild and transient, occurring in 0% to 10% of patients within the studies. The hydrogel spacer group received 66% less v70 rectal irradiation compared with controls (3.5% vs 10.4%; mean difference, −6.5%; 95% CI, –10.5% to –2.5%; P = .001 [6 studies]). The risk of grade 2 or higher rectal toxic effects was comparable between groups in early follow-up (4.5% in hydrogel spacer group vs 4.1% in control group; risk ratio, 0.82; 95% CI, 0.52-1.28; P = .38 [6 studies]) but was 77% lower in the hydrogel spacer group in late follow-up (1.5% vs 5.7%; risk ratio, 0.23; 95% CI, 0.06-0.99; P = .05 [4 studies]). Changes in bowel-related quality of life were comparable between groups in early follow-up (mean difference, 0.2; 95% CI, –3.1 to 3.4; P = .92 [2 studies]) but were greater in the hydrogel spacer group in late follow-up (mean difference, 5.4; 95% CI, 2.8-8.0; P < .001 [2 studies]).
Conclusions and Relevance
For men receiving prostate radiotherapy, injection of a hydrogel spacer was safe, provided prostate-rectum separation sufficient to reduce v70 rectal irradiation, and was associated with fewer rectal toxic effects and higher bowel-related quality of life in late follow-up.
This systematic review and meta-analysis examines the association between perirectal hydrogel spacer placement and clinical outcomes of men receiving radiotherapy for prostate cancer.
Introduction
Radiotherapy (RT) is a primary management strategy for men who received a diagnosis of localized or locally advanced prostate cancer.1 Dose-escalated external-beam RT is a highly effective, curative treatment option in which higher radiation doses delivered to the prostate provide better biochemical control.2 The anterior rectal wall is particularly vulnerable to radiation-induced toxic effects given its anatomical proximity to the prostate, with 2 to 3 mm of distance typically separating the organs.3,4 Thus, the rectum is the dose-limiting structure with prostate RT. Greater rectal irradiation during RT increases the risk of both early and late gastrointestinal complications. Identification of strategies that safely lower rectal irradiation during prostate RT is warranted.
Several systematic reviews have provided qualitative evaluations of different perirectal spacer materials delivered prior to prostate RT,5,6,7 yet quantitative synthesis of clinical results with specific spacers is limited, to our knowledge. One method to reduce rectal toxic effects involves an absorbable polyethylene glycol hydrogel spacer (SpaceOAR; Boston Scientific), which is injected between the Denonvilliers fascia and anterior rectal wall prior to RT. The device provides perirectal separation through a typical 3-month course of RT and is completely metabolized after 6 months. We performed a systematic review and meta-analysis with the objective of evaluating the association of perirectal hydrogel spacer placement with the clinical outcomes of men receiving prostate RT.
Methods
We registered the protocol for this systematic review in the International Prospective Register of Systematic Reviews (PROSPERO) public database (CRD42020150087) and adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.8 This study was exempt from institutional review board approval because individual patient data were not used in this review, in accordance with 45 CFR §46.102(f).
Eligibility Criteria
We included randomized clinical trials or cohort studies of men who received the perirectal hydrogel spacer vs men who received no spacer prior to RT for localized or locally advanced prostate cancer. We excluded review articles and commentaries, studies with fewer than 10 patients, pre-post dosimetric studies, studies that failed to report a prespecified outcome of this review, and unpublished or gray literature study data.
Literature Search
We systematically searched the Cochrane Central Register of Controlled Trials, MEDLINE, and Embase for potentially eligible studies. The search strategy included combinations of anatomical-specific (prostat*), disease-specific (cancer and carcinoma), and device-specific (hydrogel, perirectal spacer, polyethylene glycol, rectal spacer, and SpaceOAR) key words. No language or date restrictions were applied to the searches. We purposely used a broad literature search strategy to maximize sensitivity. We also performed supplemental searches of the Directory of Open Access Journals, Google Scholar, and the reference lists of included articles and relevant meta-analyses. The final search was performed in September 2019.
Study Selection and Data Extraction
Two experienced systematic reviewers (including L.E.M.) independently screened records for eligibility. References were retrieved from the electronic databases and consolidated in a deduplicated bibliographic file that was used for study screening and classification. After the exclusion of irrelevant records, we obtained the full texts of remaining articles and reviewed them for eligibility. Non–English-language manuscripts were translated to English by a medical translator. The same 2 reviewers independently extracted data from included studies; discrepancies between the reviewers were resolved by discussion. For articles in which outcome data were unclear, we attempted to obtain the data by contacting the corresponding author. When multiple articles included overlapping series of patients, we preferentially extracted outcome data from the primary article with the largest sample size for early outcomes and from the article with the longest follow-up duration for late outcomes. We used a predesigned data extraction form to record the following data from each study: manuscript metadata, study characteristics, risk of bias, patient characteristics, and outcomes.
Outcomes
Outcomes of this review were procedural results, rectal irradiation, rectal toxic effects, and bowel-related quality of life (QoL). Procedural results included the success of hydrogel spacer placement, perirectal separation distance, and procedural complications. A procedural complication was defined as the inability to inject the hydrogel spacer into the perirectal space or any complication, regardless of severity, occurring during the procedure. We preferentially extracted rectal irradiation data from studies using external-beam RT that reported the percentage volume of the rectum receiving at least 70 Gy radiation (v70) because this threshold is highly correlated with late rectal toxic effects.9 When dosimetric v70 results were not explicitly reported, we selected the reported value closest to v70. Rectal toxic effects were reported as the risk of a grade 2 or higher bowel complication in early follow-up (≤3 months) and late follow-up (>3 months). Supplemental analyses were performed on the risk of early and late rectal toxic effects of any severity (grade ≥1). For studies that reported the frequency of individual (but not overall) rectal toxic effect types by grade, the most common symptom was included in the analysis. For studies in which rectal toxic effect grade was not reported, we defined mild bowel complications as grade 1, moderate complications as grade 2, and severe complications as grade 3 rectal toxic effects. Bowel-related QoL was reported on the Expanded Prostate Cancer Index Composite (range, 0-100, where higher values indicate better bowel-related QoL), with a 4-point change from baseline considered a minimal clinically important difference.10
Risk of Bias
The Cochrane Collaboration tool was used to assess risk of bias in individual studies, which included evaluations of sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias.11 A judgement as to the possible risk of bias on each of the domains was made from the extracted information, rated as high risk or low risk. If there was insufficient detail reported in the study, we judged the risk of bias as unclear.
Statistical Analysis
A random-effects meta-analysis model with inverse variance weighting was used for all outcomes owing to anticipated heterogeneity among studies in design, patient characteristics, and prostate RT protocols. Patient follow-up data were analyzed as reported, and no adjustments were made for missing data. The success of hydrogel spacer placement and the perirectal separation distance were reported as the weighted mean and 95% CI. For rectal toxic effects, we calculated the risk ratio and 95% CI, where values less than 1 indicated lower risk with hydrogel spacer and values greater than 1 indicated higher risk with hydrogel spacer. For rectal irradiation and bowel-related QoL, we calculated the weighted mean difference between groups. Individual study results and pooled meta-analysis data were displayed with forest plots. We investigated the potential for publication bias by visually inspecting funnel plots for asymmetry and with the Egger regression test.12 Heterogeneity of outcomes among studies was estimated with the I2 statistic, where values of 25% or less represented low inconsistency, 50% represented moderate inconsistency, and 75% or greater represented high inconsistency.13 Significant heterogeneity was defined by a Cochran Q test P < .10 or I2 > 50%. For outcomes with significant heterogeneity, we explored sources of heterogeneity with predefined subgroup analyses of study-level factors, including study design, method of patient enrollment, number of sites, and sample size. We performed a 1-study-removed sensitivity analysis, in which the meta-analysis for each outcome was recalculated after removing 1 study at a time to determine the association of individual studies with meta-analysis results. All tests were 2-sided, and the threshold for statistical significance was P < .05. Statistical analyses were conducted by a statistician using Review Manager, version 5.3 (Cochrane Collaboration).
Results
Systematic Review Results and Study Identification
The literature search retrieved 473 unique records, and manual searches identified 2 additional records. After screening titles and abstracts for eligibility, 73 full-text articles were reviewed. We contacted the corresponding author of 4 articles who provided unpublished data.14,15,16,17 Ultimately, 7 studies (1 randomized clinical trial and 6 cohort studies) were included in the analysis.4,14,18,19,20,21,22 A PRISMA flow diagram depicting the study identification and selection is shown in eFigure 1 in the Supplement.
Study and Patient Characteristics
The characteristics of the 7 primary studies4,14,18,19,20,21,22 are reported in Table 1.4,14,15,16,17,18,19,20,21,22,23,24,25,26,27 There was 1 randomized clinical trial,4 1 prospective cohort study,22 1 cohort study with prospective enrollment in the hydrogel spacer group and retrospective enrollment for patients who received no spacer,21 and 4 retrospective cohort studies.14,18,19,20 In the study by Wolf et al22 that compared outcomes with the hydrogel spacer, biodegradable balloon, and no spacer treatment, we excluded results of the balloon group from the analysis. Among 1011 patients receiving RT, 486 received hydrogel spacer injection prior to RT, and 525 received no perirectal spacer (controls). Radiotherapy protocols included external-beam RT with a total therapeutic dose ranging from 76 to 81 Gy (5 studies), brachytherapy with or without external-beam RT (1 study), or combination therapy (1 study). The median duration of patient follow-up was 26 months (range, 3-63 months). The primary sources of bias were owing to a lack of randomization and blinding in most studies (eTable 1 in the Supplement). Late follow-up outcomes were also susceptible to attrition bias, with 18% of patients (117 of 650) missing data reported for late grade 2 or higher rectal toxic effect outcomes over a median of 38 months and 48% patients (187 of 389) missing data for late bowel-related QoL over a median of 48 months. The characteristics of the patients in the primary studies are reported in Table 2.4,14,18,19,20,21,22 The mean patient age in each study ranged from 67 to 74 years, prostate-specific antigen levels ranged from 5.6 to 10.2 ng/mL (to convert to micrograms per liter, multiply by 1.0), and use of androgen deprivation therapy varied considerably between studies. Patients received a diagnosis of localized or locally advanced prostate cancer (clinical stages T1-T3) and presented variably across all risk categories.
Table 1. Characteristics of Primary Studies of Radiotherapy With vs Without Hydrogel Spacer for Prostate Cancer.
| Primary studya | Secondary studies | Design | No. of sites | Country | No. of patients who received HGS/No. of controls | Radiotherapy protocol | Follow-up for patients who received HGS/controls, mo |
|---|---|---|---|---|---|---|---|
| Chao et al,18 2019 | Chao et al,23 2019 | RCS | 1 | Australia | 32/65 | BT: 18 Gy (3 fx) or 16 Gy (2 fx); IMRT: 50.4 Gy (28 fx) | 42/65 |
| Mariados et al,4 2015 | Pieczonka et al,24 2016; Hamstra et al,25 2017; Hamstra et al,26 2018 | RT | 20 | United States | 149/73 | IMRT: 79.2 Gy (44 fx) | 37/37b |
| Pinkawa et al,14 2017 | Pinkawa et al,15 2017; Pinkawa et al,16 2012; Pinkawa et al,17 2013 | RCS | 1 | Germany | 101/66 | IMRT: 76-80 Gy (38-40 fx) | 63/63c |
| Taggar et al,19 2018 | None | RCS | 1 | United States | 79/136 | BT with or without EBRT | <12d |
| te Velde et al,20 2019 | te Velde et al,27 2017 | RCS | 3 | Australia | 65/56 | IMRT: 81 Gy (45 fx) | <36d |
| Whalley et al,21 2016 | None | PCSe | 1 | Australia | 30/110 | IMRT: 80 Gy (40 fx) | 28/26 |
| Wolf et al,22 2015 | None | PCS | 1 | Austria | 30/19 | IMRT: 75.85 Gy (41 fx) | 3d |
Abbreviations: BT, brachytherapy; EBRT, external-beam radiotherapy; fx, fraction; Gy, gray; HGS, hydrogel spacer; IMRT, intensity-modulated radiation therapy; PCS, prospective cohort study; RCS, retrospective cohort study; RT, randomized trial.
Early outcomes were preferentially extracted from primary studies. For studies in which multiple articles were developed using overlapping patients, late outcomes were extracted from the article with the longest follow-up duration.
Late outcomes derived from Hamstra et al.25
Late outcomes derived from Pinkawa et al.15
Values by treatment group not reported.
Prospective enrollment in HGS group; retrospective enrollment in control group.
Table 2. Patient Characteristics in Primary Studies of Radiotherapy With vs Without Hydrogel Spacer for Prostate Cancer.
| Source | Age, ya | Prostate volume, mLa | PSA, ng/mLa | ADT, %b | Clinical stage, %b | Risk category, %b | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | Recurrent | Low | Intermediate | High | |||||
| Chao et al,18 2019 | 77/73 | 47/43 | 11.6/9.5 | 100/85 | 16/20 | 34/57 | 50/23 | NR | 0c | 43c | 57c |
| Mariados et al,4 2015 | 66/68 | 47/50 | 5.6/5.7 | 0/0 | 64/69 | 36/32 | 0/0 | 0/0 | 65/51d | 36/49d | 0d |
| Pinkawa et al,14 2017 | 72/73 | 48/48e | 7.6/7.3 | 25/25e | 72/73 | 25/25 | 3/2 | 0/0 | 33/33 | 37/42 | 30/26 |
| Taggar et al,19 2018 | 69/69 | 29/35 | 7.2/6.6 | NR | 61/53 | 24/28 | 1/5 | 14/14 | 100d | 0d | 0d |
| te Velde et al,20 2019 | 72/72 | 39/33 | 8.8/9.7 | 97/96 | NR | NR | NR | NR | 2/0 | 31/34 | 68/66 |
| Whalley et al,21 2016 | 72/NR | NR | 9.9/NR | 50/49 | NR | NR | 17/NR | NR | 0/0 | 47/44 | 53/56 |
| Wolf et al,22 2015 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
Abbreviations: ADT, androgen deprivation therapy; NR, data not reported; PSA, prostate-specific antigen.
SI conversion factors: To convert PSA to micrograms per liter, multiply by 1.0.
Data reported as mean values for patients receiving hydrogel spacer/mean values for controls.
Data reported as percentage of patients receiving hydrogel spacer/percentage of controls.
Values by treatment group not reported.
Data not reported; estimated from Gleason score where 6 is low risk, 7 is intermediate risk, and 8 or higher is high risk.
Data reported in a secondary study.
Procedural Results
In 5 studies, the hydrogel spacer was placed in 97.0% (95% CI, 94.4%-98.8%) of attempted cases. Causes of delivery failure were unsuccessful hydrodissection (n = 5), inadvertent needle entry into the rectal lumen with no clinical sequelae (n = 3), and unspecified cause (n = 1). The weighted mean perirectal separation distance after hydrogel spacer placement was 11.2 mm (95% CI, 10.1-12.3 mm [5 studies]). Procedural complications were uncommon but reported inconsistently. In the hydrogel spacer pivotal trial,4 10% of patients experienced mild and transient complications that did not delay RT. Whalley et al21 reported a single case (3%) of inadvertent injection into the rectal lumen without adverse sequelae. Pinkawa et al14 and Taggar et al19 reported no procedural complications among treated patients. The frequency of procedural complications was not reported in 3 studies.18,20,22
Association of Hydrogel Spacer Placement With Clinical Outcomes
Compared with controls, men who received the hydrogel spacer prior to external-beam RT received 66% less v70 rectal irradiation (3.5% vs 10.4%; mean difference, −6.5%; 95% CI, –10.5% to –2.5%; P = .001 [6 studies]; Figure 1). There was no difference between the hydrogel spacer and control groups in the risk of early grade 2 or higher rectal toxic effects (4.5% vs 4.1%; risk ratio, 0.82; 95% CI, 0.52-1.28; P = .38 [6 studies]; eFigure 2 in the Supplement). However, in late follow-up (median, 38 months; range, 28-60 months), risk of grade 2 or higher rectal toxic effects was associated with a 77% reduction in the hydrogel spacer group relative to controls (1.5% vs 5.7%; risk ratio, 0.23; 95% CI, 0.06-0.99; P = .05 [4 studies]; Figure 2). When expanding the analysis to include rectal toxic effects of any severity (grade ≥1), men treated with the hydrogel spacer were associated with a lower risk of early rectal toxic effects (20.5% vs 29.5%; risk ratio, 0.72; 95% CI, 0.58-0.91; P = .005 [7 studies]; eFigure 3 in the Supplement) and late (median, 40 months; range, 28-60 months) rectal toxic effects (4.8% vs 16.2%; risk ratio, 0.38; 95% CI, 0.22-0.65; P < .001 [5 studies]; eFigure 4 in the Supplement).
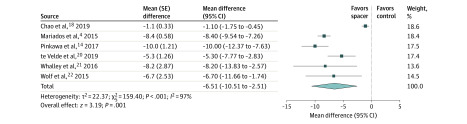
Figure 1. Rectal Irradiation With vs Without Perirectal Hydrogel Spacer.
The mean difference and 95% CI between hydrogel spacer and control groups in the percentage of rectal volume receiving at least 70 Gy irradiation are plotted for each study (with inverse-variance weighting method and random-effect model). The size of the square is proportional to the sample size of the study. The pooled mean difference denoted by the diamond apex, and the 95% CI is denoted by the diamond width. A pooled mean difference of less than 0 indicates less rectal irradiation with hydrogel spacer; a value greater than 0 indicates more rectal irradiation with hydrogel spacer. The pooled percentage of rectal volume receiving at least 70 Gy irradiation was 3.5% with hydrogel spacer and 10.4% with controls (mean difference, −6.5%; P < .001). Significant heterogeneity among studies was identified (I2 = 97%; P < .001).
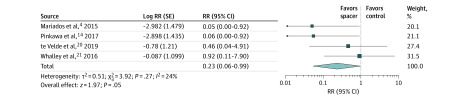
Figure 2. Late Grade 2 or Higher Rectal Toxic Effects With vs Without Perirectal Hydrogel Spacer.
The risk ratio (RR) and 95% CI between hydrogel spacer and control groups are plotted for each study (with inverse-variance weighting method and random-effect model). The size of the square is proportional to the sample size of the study. The pooled RR is denoted by the diamond apex, and the 95% CI is denoted by the diamond width. A pooled RR of greater than 1 indicates higher risk with controls. A pooled RR of less than 1 indicates lower risk with hydrogel spacer. Late grade 2 or higher rectal toxic effects were significantly lower in the hydrogel spacer group (1.5% vs 5.7%; RR, 0.23; P = .05). Significant heterogeneity among studies was not identified (I2 = 24%; P = .27).
Changes in bowel-related QoL were not different between the groups at 3-month follow-up (mean difference, 0.2; 95% CI, –3.1 to 3.4; P = .92 [2 studies]; eFigure 5 in the Supplement) but were greater in the hydrogel spacer group in late follow-up (median, 48 months; range, 36-60 months) and exceeded the threshold for a minimal clinically importance difference (mean difference, 5.4; 95% CI, 2.8-8.0; P < .001 [2 studies]; Figure 3).
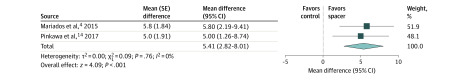
Figure 3. Change in Late Bowel-Related Quality of Life (QoL) With vs Without Perirectal Hydrogel Spacer.
The mean difference and 95% CI between hydrogel spacer and control groups are plotted for each study (with inverse-variance weighting method and random-effect model). The size of the square is proportional to the sample size of the study. The pooled mean difference denoted by the diamond apex, and the 95% CI is denoted by the diamond width. A pooled mean difference of less than 0 indicates lower bowel-related QoL with hydrogel spacer; a value greater than 0 indicates higher bowel-related QoL with hydrogel spacer. Bowel-related QoL reported on a 0 to 100 scale, where higher values indicate better QoL. Late bowel-related QoL was significantly higher in the hydrogel spacer group (mean difference, 5.4; P < .001). Significant heterogeneity among studies was not identified (I2 = 0%; P = .76).
Heterogeneity, Publication Bias, and Sensitivity Analyses
The only outcome for which we identified significant heterogeneity among studies was rectal irradiation (v70). Heterogeneity for all other outcomes was negligible or low, with the I2 values ranging from 0% to 24%. We explored potential sources of heterogeneity among studies in rectal irradiation with a subgroup analysis. No study-level factor was significantly associated with the results; the hydrogel spacer group was associated with a 5% to 8% reduction in v70 among all subgroups (eTable 2 in the Supplement). Funnel plot asymmetry was not evident for any outcome, and the results of the Egger regression test did not indicate publication bias (eFigures 6-12 in the Supplement). Meta-analysis conclusions were largely unchanged in a 1-study-removed sensitivity analysis in which the meta-analysis was recalculated after removing 1 study at a time (eTable 3 in the Supplement).
Discussion
The rectum is the dose-limiting structure in men receiving RT for prostate cancer; therefore, strategies that allow dose escalation while decreasing rectal irradiation may optimize local tumor control with fewer bothersome bowel symptoms. In this meta-analysis of studies including men receiving RT for localized or locally advanced prostate cancer, injection of a hydrogel spacer was acceptably safe and achieved prostate-rectum separation of approximately 11 mm. Compared with no treatment with a perirectal spacer during RT for prostate cancer, hydrogel spacer placement was associated with a 77% lower risk for late grade 2 or higher rectal toxic effects and higher bowel-related QoL scores during late follow-up that exceeded the threshold for a minimal clinically important change. Overall, these results suggest that injection of an absorbable perirectal hydrogel spacer prior to RT for prostate cancer may reduce rectal irradiation and the associated rectal toxic effects that manifest clinically after longer-term follow-up.
Despite the observed results in late follow-up with the hydrogel spacer, it is plausible that the duration of individual studies was insufficient to fully characterize the true magnitude of rectal toxic effects after RT. Several studies28,29 have reported that, among patients receiving intensity-modulated RT for prostate cancer, the frequency of rectal toxic effects significantly increased for at least 5 years before plateauing. For comparison, the median follow-up duration among studies reporting late rectal toxic effects in this review was 3.3 years. Thus, the clinical benefit of the perirectal spacer may potentially be underestimated in this review owing to limited duration of follow-up. Unfortunately, the number of studies providing results was insufficient to explore the association between follow-up duration and late grade 2 or higher rectal toxic effects.
The results of this meta-analysis are the first, to our knowledge, to convey the typical results experienced by men who received a hydrogel spacer vs those who did not receive a spacer prior to initiating RT for localized or locally advanced prostate cancer. Although these findings are comparable to those reported in the randomized clinical trial by Mariados et al,4 the results of this meta-analysis allow for a more reliable estimate of the effect size of the association between the hydrogel spacer procedure and clinical outcomes than would be obtained in a single study. Furthermore, the inclusion of the results obtained from clinical trials as well as from commercial use improves the generalizability of the findings.
To achieve optimal results, we advise that hydrogel spacer placement procedure be performed by radiation oncologists, urologists, or interventional radiologists with experience in transperineal procedures and transrectal ultrasonography. It is possible that a learning curve must be overcome before clinical benefit to the patient is maximized. In a case series of 64 patients treated with a hydrogel spacer prior to prostate RT, Pinkawa et al30 reported that the last 32 patients had an improved and more symmetrical spacer placement, improved treatment planning, and less treatment-related early toxic effects compared with the first 32 patients in the series. However, the clinical importance of hydrogel spacer placement symmetry remains unclear because others have reported significant reductions in rectal irradiation dose even with asymmetric device placement.31 Standardized definitions for hydrogel spacer accuracy and symmetry should be developed, which could help determine the optimal hydrogel spacer positioning to achieve maximal rectal dose reduction. Hydrogel spacer injection should only be attempted after adequate hydrodissection to expand the perirectal space.
There was no statistical difference between groups in the risk of early grade 2 or higher rectal toxic effects. Although this risk was 18% lower in those treated with the hydrogel spacer, the analysis was underpowered to statistically detect this modest benefit because early grade 2 or higher rectal toxic effects occurred in only 4% of patients. Over the long term, the benefits of the hydrogel spacer became more apparent, with significant reductions in grade 2 or higher rectal toxic effects and clinically meaningful improvements in bowel-related QoL.
With the advent of newer technologies such as intensity modulation and image guidance, it has been argued that the risk of grade 2 or higher rectal toxic effects over the long term is acceptably low with RT for prostate cancer and that any benefit derived from prophylactic measures to further decrease this risk may be clinically insignificant.32 The results of this meta-analysis, however, counter this argument because a statistically greater bowel-related QoL was associated with receiving the hydrogel spacer, and the magnitude of the benefit exceeded the threshold for a clinically meaningful difference. Although it is possible that the performance of the hydrogel spacer may differ according to RT protocols or patient characteristics, there was no evidence of important differences among subgroups. Admittedly, owing to the small number of studies included in this review, analyses intended to undercover potential associations of covariates with outcome measures were underpowered. Overall, we suggest that the hydrogel spacer has a favorable risk-benefit profile for patients receiving RT for prostate cancer. Furthermore, we recognize that additional studies with adequate follow-up durations may be informative to provide more reliable estimates regarding the safety and effectiveness of hydrogel spacers.
Strengths and Limitations
This review has several strengths, including adherence to the PRISMA guidelines, the prospective registration of the systematic review protocol, the careful identification and handling of studies with overlapping patients, and the evaluation of the association of heterogeneity and certain biases with outcomes. There were also several limitations of the included studies. First, this review included only 7 studies, and each outcome was not reported in all of these studies. Second, the small number of eligible studies afforded low statistical power to detect publication bias or to adequately explore sources of heterogeneity in subgroup analyses. Significant heterogeneity was identified among studies for v70 rectal irradiation, which could not be explained by the subgroup analysis findings. Although v70 rectal irradiation statistically favored the hydrogel spacer group in each study, factors that were not measured in this review were likely associated with this variability. Third, the review included mainly nonrandomized comparisons, which may confound the interpretation of the results. Fourth, the follow-up durations of most studies were insufficient to fully evaluate late-developing rectal toxic effects and associated bowel-related QoL. Fifth, while procedural complications were uncommon and minor in severity, the reporting of these outcomes was inconsistent among studies. Sixth, no studies of hydrogel spacer placement in men receiving stereotactic body RT were eligible for inclusion in this review. Although several studies have reported promising results with hydrogel spacer placement prior to stereotactic body RT,33,34,35,36 the current evidence regarding the clinical utility of hydrogel spacer placement in this treatment setting remains inconclusive.
Conclusions
Among men planning to receive RT for localized or locally advanced prostate cancer, injection of a hydrogel spacer was safe, provided prostate-rectum separation sufficient to reduce v70 rectal irradiation, and was associated with lower rectal toxic effects and higher bowel-related QoL in late follow-up. The limitations of this review that may confound interpretation were a small number of eligible studies, the predominance of nonrandomized study designs with associated risks of bias, and follow-up durations that may be inadequate to detect long-term clinical manifestations of rectal irradiation.
eFigure 1. PRISMA Flow Diagram
eFigure 2. Early Grade ≥2 Rectal Toxicity With vs Without Perirectal Hydrogel Spacer
eFigure 3. Early Grade ≥1 Rectal Toxicity With vs Without Perirectal Hydrogel Spacer
eFigure 4. Late Grade ≥1 Rectal Toxicity With vs Without Perirectal Hydrogel Spacer
eFigure 5. Change in Early Bowel Quality of Life (QoL) With vs Without Perirectal Hydrogel Spacer
eFigure 6. Funnel Plot for the Meta-Analysis of Rectal Irradiation With vs Without Perirectal Hydrogel Spacer
eFigure 7. Funnel Plot for the Meta-Analysis of Early Grade ≥2 Rectal Toxicity With vs Without Perirectal Hydrogel Spacer
eFigure 8. Funnel Plot for the Meta-Analysis of Early Grade ≥1 Rectal Toxicity With vs Without Perirectal Hydrogel Spacer
eFigure 9. Funnel Plot for the Meta-Analysis of Late Grade ≥2 Rectal Toxicity With vs Without Perirectal Hydrogel Spacer
eFigure 10. Funnel Plot for the Meta-Analysis of Late Grade ≥1 Rectal Toxicity With vs Without Perirectal Hydrogel Spacer
eFigure 11. Funnel Plot for the Meta-Analysis of Change in Early Bowel Quality of Life (QoL) With vs Without Perirectal Hydrogel Spacer
eFigure 12. Funnel Plot for the Meta-Analysis of Change in Late Bowel Quality of Life (QoL) With vs Without Perirectal Hydrogel Spacer
eTable 1. Cochrane Risk of Bias Assessment Among Individual Studies
eTable 2. Subgroup Analysis of Study-Level Factors on Rectal Irradiation (v70) During External Beam Radiotherapy With vs Without Hydrogel Spacer for Prostate Cancer
eTable 3. One-Study-Removed Sensitivity Analyses of Radiotherapy With vs Without Hydrogel Spacer for Prostate Cancer
eReferences
References
- 1.Chen J, Oromendia C, Halpern JA, Ballman KV. National trends in management of localized prostate cancer: a population based analysis 2004-2013. Prostate. 2018;78(7):512-520. doi: 10.1002/pros.23496 [DOI] [PubMed] [Google Scholar]
- 2.Viani GA, Stefano EJ, Afonso SL. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys. 2009;74(5):1405-1418. doi: 10.1016/j.ijrobp.2008.10.091 [DOI] [PubMed] [Google Scholar]
- 3.Kang MH, Yu YD, Shin HS, Oh JJ, Park DS. Difference in the rate of rectal complications following prostate brachytherapy based on the prostate-rectum distance and the prostate longitudinal length among early prostate cancer patients. Korean J Urol. 2015;56(9):637-643. doi: 10.4111/kju.2015.56.9.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mariados N, Sylvester J, Shah D, et al. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92(5):971-977. doi: 10.1016/j.ijrobp.2015.04.030 [DOI] [PubMed] [Google Scholar]
- 5.Mok G, Benz E, Vallee JP, Miralbell R, Zilli T. Optimization of radiation therapy techniques for prostate cancer with prostate-rectum spacers: a systematic review. Int J Radiat Oncol Biol Phys. 2014;90(2):278-288. doi: 10.1016/j.ijrobp.2014.06.044 [DOI] [PubMed] [Google Scholar]
- 6.Tang Q, Zhao F, Yu X, Wu L, Lu Z, Yan S. The role of radioprotective spacers in clinical practice: a review. Quant Imaging Med Surg. 2018;8(5):514-524. doi: 10.21037/qims.2018.06.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afkhami Ardekani M, Ghaffari H. Optimization of prostate brachytherapy techniques with polyethylene glycol–based hydrogel spacers: a systematic review. Brachytherapy. 2020;19(1):13-23. doi: 10.1016/j.brachy.2019.08.009 [DOI] [PubMed] [Google Scholar]
- 8.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomita N, Soga N, Ogura Y, et al. Preliminary analysis of risk factors for late rectal toxicity after helical tomotherapy for prostate cancer. J Radiat Res. 2013;54(5):919-924. doi: 10.1093/jrr/rrt025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skolarus TA, Dunn RL, Sanda MG, et al. ; PROSTQA Consortium . Minimally important difference for the Expanded Prostate Cancer Index Composite Short Form. Urology. 2015;85(1):101-105. doi: 10.1016/j.urology.2014.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinkawa M, Berneking V, König L, Frank D, Bretgeld M, Eble MJ. Hydrogel injection reduces rectal toxicity after radiotherapy for localized prostate cancer. Strahlenther Onkol. 2017;193(1):22-28. doi: 10.1007/s00066-016-1040-6 [DOI] [PubMed] [Google Scholar]
- 15.Pinkawa M, Berneking V, Schlenter M, Krenkel B, Eble MJ. Quality of life after radiation therapy for prostate cancer with a hydrogel spacer: 5-year results. Int J Radiat Oncol Biol Phys. 2017;99(2):374-377. doi: 10.1016/j.ijrobp.2017.05.035 [DOI] [PubMed] [Google Scholar]
- 16.Pinkawa M, Piroth MD, Holy R, et al. Quality of life after intensity-modulated radiotherapy for prostate cancer with a hydrogel spacer: matched-pair analysis. Strahlenther Onkol. 2012;188(10):917-925. doi: 10.1007/s00066-012-0172-6 [DOI] [PubMed] [Google Scholar]
- 17.Pinkawa M, Piroth MD, Holy R, et al. Spacer stability and prostate position variability during radiotherapy for prostate cancer applying a hydrogel to protect the rectal wall. Radiother Oncol. 2013;106(2):220-224. doi: 10.1016/j.radonc.2012.11.010 [DOI] [PubMed] [Google Scholar]
- 18.Chao M, Ow D, Ho H, et al. Improving rectal dosimetry for patients with intermediate and high-risk prostate cancer undergoing combined high-dose-rate brachytherapy and external beam radiotherapy with hydrogel space. J Contemp Brachytherapy. 2019;11(1):8-13. doi: 10.5114/jcb.2019.82836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taggar AS, Charas T, Cohen GN, et al. Placement of an absorbable rectal hydrogel spacer in patients undergoing low-dose-rate brachytherapy with palladium-103. Brachytherapy. 2018;17(2):251-258. doi: 10.1016/j.brachy.2017.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.te Velde BL, Westhuyzen J, Awad N, Wood M, Shakespeare TP. Late toxicities of prostate cancer radiotherapy with and without hydrogel SpaceAOR insertion. J Med Imaging Radiat Oncol. 2019;63(6):836-841. doi: 10.1111/1754-9485.12945 [DOI] [PubMed] [Google Scholar]
- 21.Whalley D, Hruby G, Alfieri F, Kneebone A, Eade T. SpaceOAR hydrogel in dose-escalated prostate cancer radiotherapy: rectal dosimetry and late toxicity. Clin Oncol (R Coll Radiol). 2016;28(10):e148-e154. doi: 10.1016/j.clon.2016.05.005 [DOI] [PubMed] [Google Scholar]
- 22.Wolf F, Gaisberger C, Ziegler I, et al. Comparison of two different rectal spacers in prostate cancer external beam radiotherapy in terms of rectal sparing and volume consistency. Radiother Oncol. 2015;116(2):221-225. doi: 10.1016/j.radonc.2015.07.027 [DOI] [PubMed] [Google Scholar]
- 23.Chao M, Bolton D, Lim Joon D, et al. High dose rate brachytherapy boost for prostate cancer: biochemical control and the impact of transurethral resection of the prostate and hydrogel spacer insertion on toxicity outcomes. J Med Imaging Radiat Oncol. 2019;63(3):415-421. doi: 10.1111/1754-9485.12882 [DOI] [PubMed] [Google Scholar]
- 24.Pieczonka CM, Mariados N, Sylvester JE, et al. Hydrogel spacer application technique, patient tolerance and impact on prostate intensity modulated radiation therapy: results from a prospective, multicenter, pivotal randomized controlled trial. Urol Pract. 2016;3:141-146. doi: 10.1016/j.urpr.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 25.Hamstra DA, Mariados N, Sylvester J, et al. Continued benefit to rectal separation for prostate radiation therapy: final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017;97(5):976-985. doi: 10.1016/j.ijrobp.2016.12.024 [DOI] [PubMed] [Google Scholar]
- 26.Hamstra DA, Mariados N, Sylvester J, et al. Sexual quality of life following prostate intensity modulated radiation therapy (IMRT) with a rectal/prostate spacer: secondary analysis of a phase 3 trial. Pract Radiat Oncol. 2018;8(1):e7-e15. doi: 10.1016/j.prro.2017.07.008 [DOI] [PubMed] [Google Scholar]
- 27.te Velde BL, Westhuyzen J, Awad N, Wood M, Shakespeare TP. Can a peri-rectal hydrogel spaceOAR programme for prostate cancer intensity-modulated radiotherapy be successfully implemented in a regional setting? J Med Imaging Radiat Oncol. 2017;61(4):528-533. doi: 10.1111/1754-9485.12580 [DOI] [PubMed] [Google Scholar]
- 28.Dearnaley D, Syndikus I, Mossop H, et al. ; CHHiP Investigators . Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi: 10.1016/S1470-2045(16)30102-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(4):464-474. doi: 10.1016/S1470-2045(15)00567-7 [DOI] [PubMed] [Google Scholar]
- 30.Pinkawa M, Klotz J, Djukic V, et al. Learning curve in the application of a hydrogel spacer to protect the rectal wall during radiotherapy of localized prostate cancer. Urology. 2013;82(4):963-968. doi: 10.1016/j.urology.2013.07.014 [DOI] [PubMed] [Google Scholar]
- 31.Fischer-Valuck BW, Chundury A, Gay H, Bosch W, Michalski J. Hydrogel spacer distribution within the perirectal space in patients undergoing radiotherapy for prostate cancer: impact of spacer symmetry on rectal dose reduction and the clinical consequences of hydrogel infiltration into the rectal wall. Pract Radiat Oncol. 2017;7(3):195-202. doi: 10.1016/j.prro.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 32.Lawrie TA, Green JT, Beresford M, et al. Interventions to reduce acute and late adverse gastrointestinal effects of pelvic radiotherapy for primary pelvic cancers. Cochrane Database Syst Rev. 2018;1:CD012529. doi: 10.1002/14651858.CD012529.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang ME, Black PJ, Elliston CD, et al. A novel model to correlate hydrogel spacer placement, perirectal space creation, and rectum dosimetry in prostate stereotactic body radiotherapy. Radiat Oncol. 2018;13(1):192. doi: 10.1186/s13014-018-1135-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang ME, Mayeda M, Liz M, et al. Stereotactic body radiotherapy with periprostatic hydrogel spacer for localized prostate cancer: toxicity profile and early oncologic outcomes. Radiat Oncol. 2019;14(1):136. doi: 10.1186/s13014-019-1346-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones RT, Hassan Rezaeian N, Desai NB, et al. Dosimetric comparison of rectal-sparing capabilities of rectal balloon vs injectable spacer gel in stereotactic body radiation therapy for prostate cancer: lessons learned from prospective trials. Med Dosim. 2017;42(4):341-347. doi: 10.1016/j.meddos.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 36.Zelefsky MJ, Pinitpatcharalert A, Kollmeier M, et al. Early tolerance and tumor control outcomes with high-dose ultrahypofractionated radiation therapy for prostate cancer. Eur Urol Oncol. 2019;S2588-9311(19)30147-6. Published online October 23, 2019. doi: 10.1016/j.euo.2019.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. PRISMA Flow Diagram
eFigure 2. Early Grade ≥2 Rectal Toxicity With vs Without Perirectal Hydrogel Spacer
eFigure 3. Early Grade ≥1 Rectal Toxicity With vs Without Perirectal Hydrogel Spacer
eFigure 4. Late Grade ≥1 Rectal Toxicity With vs Without Perirectal Hydrogel Spacer
eFigure 5. Change in Early Bowel Quality of Life (QoL) With vs Without Perirectal Hydrogel Spacer
eFigure 6. Funnel Plot for the Meta-Analysis of Rectal Irradiation With vs Without Perirectal Hydrogel Spacer
eFigure 7. Funnel Plot for the Meta-Analysis of Early Grade ≥2 Rectal Toxicity With vs Without Perirectal Hydrogel Spacer
eFigure 8. Funnel Plot for the Meta-Analysis of Early Grade ≥1 Rectal Toxicity With vs Without Perirectal Hydrogel Spacer
eFigure 9. Funnel Plot for the Meta-Analysis of Late Grade ≥2 Rectal Toxicity With vs Without Perirectal Hydrogel Spacer
eFigure 10. Funnel Plot for the Meta-Analysis of Late Grade ≥1 Rectal Toxicity With vs Without Perirectal Hydrogel Spacer
eFigure 11. Funnel Plot for the Meta-Analysis of Change in Early Bowel Quality of Life (QoL) With vs Without Perirectal Hydrogel Spacer
eFigure 12. Funnel Plot for the Meta-Analysis of Change in Late Bowel Quality of Life (QoL) With vs Without Perirectal Hydrogel Spacer
eTable 1. Cochrane Risk of Bias Assessment Among Individual Studies
eTable 2. Subgroup Analysis of Study-Level Factors on Rectal Irradiation (v70) During External Beam Radiotherapy With vs Without Hydrogel Spacer for Prostate Cancer
eTable 3. One-Study-Removed Sensitivity Analyses of Radiotherapy With vs Without Hydrogel Spacer for Prostate Cancer
eReferences