Abstract
Acupuncture has been used in China for thousands of years and concerned as a typical alternative medicine in inflammatory diseases nowadays. The nuclear factor-κB (NF-κB) transcription factor is an important regulator of inflammation. In this article, we discuss the role of acupuncture in NF-κB pathways and also present the acupoints selection, acupuncture administration, and related inflammation diseases and models from previous studies to bring readers close to a more complete understanding of the mechanisms between acupuncture and NF-κB in inflammatory diseases.
1. Introduction
Acupuncture is an oldest part of traditional Chinese medicine (TCM) with a history of more than three thousand years [1]. In recent decades, the evidence supported acupuncture which could be used as an efficient alternative therapy [2]. In the TCM theory, the combination of specific acupoints can relieve inflammatory symptoms, and it is widely studied in pain [3], obesity [4], and digestive system [5] diseases.
In mammals, the nuclear factor-κB (NF-κB) represents a family of inducible transcription factors [6]. Most of the inflammatory responses caused by infection, inflammatory cytokines, and engagement of antigen receptors are associated with the activation of NF-κB [7].
The canonical pathway of NF-κB activation consists of a series of steps [6]. First, TGFβ-activated kinase 1 could be activated in the intracellular signaling cascade and further activates a trimeric IκB kinase (IKK), which is composed of IKKα, β, and γ. In turn, the IKK complex will degrade IκB family members with ubiquitination and phosphorylation, resulting in NF-κB release and nuclear translocation. In general, the degradation of IκB, a key inhibition of NF-κB, is crucial for NF-κB activation and acts as a potential anti-inflammatory target [8].
MicroRNAs (miRNAs) are a kind of small endogenous RNAs, which could silence genes posttranscriptionally [9]. The communication of miRNAs and NF-κB was identified during inflammation [10]. In ulcerative colitis (UC), the contribution of miRNA in lowering IKKα expression levels could result in the activation of the NF-κB pathway [11].
Other classes of molecules could also act as a regulator of NF-κB. In inflammation and metabolic disorders, SIRT1 shows an antagonistic role of NF-κB [12]. P38, usually activated in proliferating cells, could act as an upstream agent of the NF-κB pathway in inflammatory response [13].
Not only in inflammation, but NF-κB could also contribute in many other cellular processes, including proliferation, apoptosis, and immune response [14], that may be a core of cellular homeostasis [15]. The TCM theory believes that harmony is the most important goal of health. Hence, we reviewed current literatures, trying to clarify the detail role of acupuncture and NF-κB in inflammatory diseases.
2. Methods
The research questions were combined into three key focus areas. (1) The mechanisms on how acupuncture could modulate NF-κB. (2) The detail of acupuncture regulating NF-κB in inflammation response. (3) The evidences that acupuncture could cure inflammatory diseases through NF-κB pathways.
Systematic searches were performed in the PubMed database with key words related to “acupuncture,” “electroacupuncture,” “NF-κB,” “IκB,” and “P65”. Papers published before 13 April 2020, present in English, containing clear intervention by acupuncture and providing objective outcome using randomized controlled trials were selected.
3. Results
This review contains 8 articles which reveal the mechanisms on how acupuncture could regulate NF-κB, 9 articles which introduce the detail of acupuncture regulating NF-κB in inflammation response, and 22 articles which show evidence that acupuncture could ameliorate inflammatory diseases through NF-κB pathways. All of them are animal studies.
3.1. Mechanisms of Acupuncture Regulating NF-κB
All articles in this review promote that acupuncture could inhibit the activation of NF-κB, but only a few of them investigated the detailed mechanisms. Based on current studies, acupuncture may modulate NF-κB through different ways (Figure 1).
Figure 1.
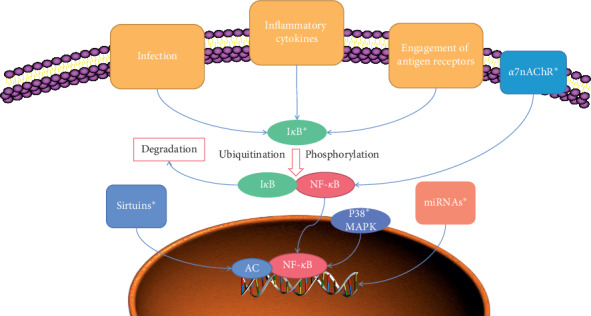
Mechanisms of acupuncture regulating NF-κB. ∗Acupuncture could inhibit NF-κB through sirtuins, P38, α7nAChR, miRNAs, and IκB in this review. Infection, inflammatory cytokines, and engagement of antigen receptors can activate NF-κB leading to inflammation responses. IκB is a key to inhibit this progress. Current evidence showed that acupuncture could modulate IKKα and sirtuins to decrease the activation of NF-κB. The role of miRNAs in inflammation is multiple, and acupuncture could dual-regulate the level of different miRNAs based on their function. As a result, acupuncture could be concerned as an efficient NF-κB antagonist, and there may be other complex mechanisms which are still unclear.
In the upstream of NF-κB, α7nAChR [16], Sirt1 [17], and P38 [18] were identified as targets of acupuncture in NF-κB pathways. Meanwhile, miRNAs also play a great role in this process. The regulation of acupuncture on miRNAs may be different depending on their function. Studies observed that acupuncture decreases miR-155 and miR-21 and increase miR-146a, which leads to inhibition of NF-κB [19], and the decrease of miR-155 by acupuncture is also observed [20].
When it comes to the canonical pathway of NF-κB activation, acupuncture shows great potential in inhibiting the degradation of IκB [21]. Wei et al. observed that acupuncture enhances the expression of IKKα against the activation of NF-κB [22].
3.2. Further Intervention of Acupuncture on Inflammation Responses after Regulating NF-κB
In most studies, NF-κB has been used as a marker to measure the efficacy of acupuncture in inflammation. Meanwhile, some research studies suggest that the regulation of NF-κB by acupuncture could induce the further intervention of inflammatory cells or inflammatory cytokines to relieve inflammatory response (Figure 2).
Figure 2.
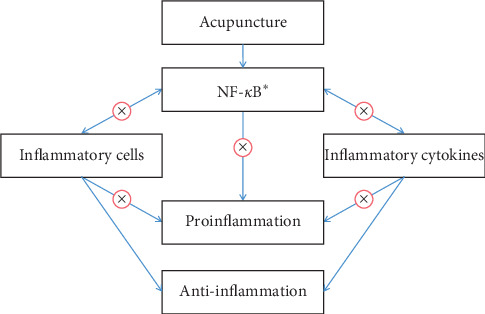
Further intervention of acupuncture on inflammation responses after regulating NF-κB. ∗Acupuncture could inhibit the activation of NF-κB and further regulates inflammatory cells and inflammatory cytokines to relieve inflammatory response in this review. Inflammation cells include Th17 and Treg cells, macrophages 1 and macrophages 2 cells, and mast cells. Inflammatory cytokines include IL-1, IL-1β, IL-6, IL-10, IL-12, IL-13, TNF-α, and MCP-1. These inflammation factors have pro- or anti-inflammation function. After downregulation of NF-κB by acupuncture, these inflammatory factors present a tendency of anti-inflammation.
Wei et al. investigated that acupuncture could modulate the activity of Th17 and Treg cells and the number of CD4 + IL-17A + cells and CD4 + Foxp3 + cells in asthma rats [22]. Wang et al. identified that the anti-inflammation effect of acupuncture on allergic diseases is targeting inflammatory cytokines including IL-6, TNF-α, IL-13, and MCP-1 in mast cells [20]. In a study performed by Wu et al., acupuncture attenuates IL-1β, IL-6, TNF-α, and MMP-3 on knee osteoarthritis [23]. In research studies about obesity, acupuncture could regulate inflammatory cytokines, such as TNF-α, IL-6, and IL-1β [24] and inflammatory cells, such as macrophages [17]. Similar phenomenon was observed in acupuncture treating pruritus [25]. Liu et al. [26] and Xue et al. [27] also found that acupuncture could act against the release of proinflammatory cytokines by the regulation of NF-κB.
3.3. Inflammatory Diseases Could Be Ameliorated by Acupuncture through NF-κB Pathways
The current evidence supports that acupuncture could be administrated as an obvious NF-κB inhibitor to treat extensive inflammatory diseases (Table 1).
Table 1.
Inflammatory diseases could be ameliorated by acupuncture through NF-κB pathways.
| Disease | Models | Acupoints | Acupuncture administration | Main results | Reference |
|---|---|---|---|---|---|
| Asthma | OVA-induced mouse asthma model | GV14, BL12, BL13 | MA, 30 mins each day, every other day for 4 weeks | Acupuncture attenuated inflammation and inhibited Th17 and the Treg activity | [22] |
| Allergic contact dermatitis | DNCB-induced mouse atopic dermatitis | LI11 | MA, 8 days | Acupuncture treatment is effective in alleviating allergic contact dermatitis by reducing proinflammatory cytokines and proteins | [28] |
| DNFB-induced mouse atopic dermatitis | ST36 | EA, continuous waves, 2 Hz and 1 mA for 5 min, 2 Hz and 1.5 mA for 5 min, and 2 Hz and 2 mA for 20 min each day, 7 days | EA treatment inhibits NF-κB and AP-1 activation, as well as promotes the negative feedback regulation of IL-33 signaling via targeting miR-155 in mast cells | [20] | |
| CAG | MNNG-induced CAG rat model | ST36, CV12 | MA, 15 mins each day, 60 days | Acupuncture downregulate NF-κB p65, miR-155, and miR-21 and upregulate miR-146a expression in CAG rats | [19] |
| Cognitive impairment | Cerebral I/R-injured rat model | DU20, DU24 | EA, disperse waves, 1 and 20 Hz, 30 mins each day, 10 days | Electroacupuncture ameliorates cognitive impairment through inhibition of NF-κB-mediated neuronal cell apoptosis | [29] |
| COPD | Smoking-induced COPD rat model | ST36, BL13 | EA, alternating waves, 10/50 Hz and 2 mA for 30 mins each day, 7 days | EA treatment can reduce the lung inflammatory response and improve the lung function in COPD | [16] |
| Depression | Chronic unpredictable stress rat model of depression | GV20, PC6 | MA, 10 mins each day, every other day for 4 weeks | Acupuncture markedly inhibited the activation of NF-κB in the brain regions | [30] |
| GV20, GV29 | MA, 20 mins each day, 28 days | The antidepressant effect of acupuncture is effective and has a multitarget characteristic, which may be related to amino acid metabolism and inflammatory pathways | [31] | ||
| HIBD | HIBD rat model | DU14, DU20 | EA, 2–100 Hz and 3 mA for 30 mins each day, 14 days | EA against hypoxic-ischemic brain damage in rats via NF-κB/neuronal nitric oxide synthase | [32] |
| Neuropathic pain | PTX-induced neuropathic pain rat model | ST36 | EA, continuous waves, 10 Hz and 1 mA for 10 mins each day, every other day for 15 days | EA treatment attenuates PTX-induced neuropathic pain via inhibiting spinal glia and the TLR4/NF-κB pathway | [33] |
| Neurodegeneration disease | Telomerase-deficient mice | ST36 | MA or EA, 7 days | EA could specifically ameliorate the spatial learning and memory capability for telomerase-deficient mice through the activation of TrkB and NF-κB than MA | [34] |
| Obesity | Leptin deficient mice | ST36 | EA, continuous waves, 2 Hz, 0.5 and 1 mA for 10 mins each day, three times weekly for one or two consecutive weeks | EA prevents weight gain through modulation of HIF-1α-dependent pathways and inflammatory response in obese adipose tissues | [17] |
| High fat diet-induced obesity rat model | ST36, ST40, CV3, CV4 | EA, continuous waves, 2 Hz and 1 mA for 10 mins each day, three times weekly for 8 weeks | EA prevents inflammation through activation of Sirt1 | [24] | |
| OA | Surgery-induced OA rabbit model | ST35, EX-LE5 | EA, square waves, 2 Hz and 100 Hz alternating polarity for 30 mins each day, 8 weeks | EA treatment may delay cartilage degeneration by downregulating inflammatory factors through the NF-κB signaling pathway | [23] |
| Pruritus | Morphine-induced pruritus mouse model | LI11, SP10 | EA, square waves, 2/15 Hz and 2 mA for 30 mins each day, 5 days | EA preconditioning improved pruritus through the TLR2/4-MyD88-NF-κB pathway | [25] |
| RA | Surgery-induced RA rabbit mode | ST35, EX-LE5 | EA, continuous waves, 2 Hz and 2 mA for 30 mins each day, 4 weeks | EA can reduce the expression of TLR4, MYD88, and NF-κB, which play an important role in treatment of adjuvant arthritis | [35] |
| Stroke | MACo rat model | GV20, GV14 | EA, amplitude-modulated waves, 5 Hz and 2.7–3.0 mA for 25 mins each day, 6 days | EA subacute phase cerebral I/R injuries by reducing S100B-mediated neurotoxicity | [18] |
| LI11, ST36 | EA, dilatational waves, 1–20 Hz and 2.7–3.0 mA for 30 mins each day, 3 days | EA improves motor impairment via inhibition of microglia-mediated neuroinflammation in the sensorimotor cortex after ischemic stroke | [26] | ||
| SAP | Sodium taurocholate-induced SAP rat model | ST25 | MA or EA, 2–100 Hz and 2 mA, twice after SAP induction | Both MA and EA might have a therapeutic effect on rats with SAP through inhibition of NF-κB expression and a reduction in the release of proinflammatory cytokines | [27] |
| Traumatic injury | Surgical trauma rat model | ST36, EX-LE7 | EA, 2 Hz and 60 Hz alternating polarity for 30 mins, once after surgery | EA inhibits apoptosis of splenic lymphocytes in traumatized rats through modulation of the TNF-α/NF-κB signaling pathway | [36] |
| Feeney's free fall epidural impact method, TBI rat model | GV20, GV25, GV16, GV15, LI4 | MA, 15 mins, thrice | Acupuncture has a bidirectional regulatory effect on the TLR2/4-NF-κB signaling pathway-related genes TLR2, TLR4, and NF-κB in the TBI rat cortex, promoting their expression in the early stage and inhibiting it in the later stage | [37] | |
| VD | CMi rat model | ST36 | Verum acupuncture | Acupuncture could protect cognitive function against oxidative stress induced by CMi, which is partially associated with suppression of NF-κB-p53 activation | [38] |
MA: manual acupuncture; EA: electroacupuncture; OVA: ovalbumin; DNCB: 1-chloro-2,4-dini-trobenzene; DNFB: 2,4-dinitrofluorobenzene; CAG: chronic atrophic gastritis; MNNG: N-methyl-N′-nitro-N-nitrosoguanidine; I/R: ischemia/reperfusion; COPD: chronic obstructive pulmonary disease; HIBD: hypoxic-ischemic brain damage; PTX: paclitaxel; OA: osteoarthritis; RA: rheumatoid arthritis; MACo: middle cerebral artery occlusion; SAP: severe acute pancreatitis; TBI: traumatic brain injury; VD: vascular dementia; CMi: cerebral multi-infarction.
The strategy of acupoints selection in different diseases was basically according to the TCM theory. But, some specific acupoints with high frequency were used. For example, due to its enhanced immune function in the TCM theory, ST36 was widely applied in different studies with the same effect on inhibiting NF-κB and relieving inflammation.
In acupuncture delivery, manual acupuncture (MA) or electroacupuncture (EA) was adopted in different studies. The overall data indicate that both MA and EA could decrease NF-κB and suppress inflammation. There is not enough evidence to show a significant difference in regulating NF-κB between MA and EA, including different waveform, frequency, and current.
Analyzing all the results as a whole, acupuncture has an optimistic effect on the inflammatory response in various organs, tissues, and cells. Whether it is a 'long-term or short-term treatment, all subjects are benefited from acupuncture treatment. Since all the studies were conducted on animals, the side effects of acupuncture are unclear.
4. Discussion
Nowadays, the curative effect of acupuncture is recognized all around the world. There are plenty of clinical trials, which demonstrate acupuncture as a useful alternative medical therapy in inflammatory diseases [39] or inflammatory responses in diseases [40].
NF-κB has been studied in inflammation for a long time, and it is found closely associated with multiple signaling pathways [8]. NF-κB could regulate a variety of cellular mechanisms, making it a key factor in inflammation, immunity, and even tumors [41].
In this article, we discussed current studies about acupuncture, which regulate NF-κB pathways in inflammatory diseases. The evidence supplies that acupuncture effectively inhibits the activation of NF-κB. However, compared with the complexity of NF-κB pathways, the mechanism of acupuncture in inhibiting NF-κB in current studies is still superficial. The regulation of acupuncture on NF-κB was only identified on miRNAs, sirtuins, and other upstream agents and IκB in the canonical pathway. This information may explain how acupuncture could modulate NF-κB pathways. But considering future research, there are much more unknown mechanisms, such as the noncanonical pathway, subunit target of NF-κB, and other combined signal pathways. In addition, the subsequent modification on inflammatory cytokines and inflammatory cells may be crucial to explain the detail of acupuncture and NF-κB on inflammation. Some cell experiments, which investigated the complex mechanisms of acupuncture regulating NF-κB pathways, provide us a deeper understanding [42]. But there was no acupuncture intervention in their protocol, making them unsuitable to this review.
The intervention of acupuncture on NF-κB pathways is effective on treating various inflammatory diseases in animal models. We also searched clinical trials with this topic. Although present trials did not involve the NF-κB pathway, the efficacy of acupuncture in some inflammatory diseases, including asthma [43], chronic atrophic gastritis [44], cognitive impairment [45], chronic obstructive pulmonary disease [46], and brain damage [47], was recognized.
One of the objectives of this article is to better apply acupuncture in the clinical practice of inflammatory diseases. The selection of acupoints is crucial for the acupuncture therapy in TCM. There may be some broad-spectrum anti-inflammation acupoints such as ST-36, which could be used in various inflammatory diseases. To our knowledge, different waveform, frequency, and current of EA may have different therapeutic effects. We have detailed the setting of EA delivery, but it is hard to recommend an optimal option of EA for treating inflammation based on the current findings.
There are some limitations that need to be considered. First, in order to ensure the authenticity and reliability of the conclusions, only a small number of studies were identified based on our searching criteria. Although the inhibitory effect of acupuncture on NF-κB was significant, there were insufficient duplication experiments to explain the detail of this process. Many unknown mechanisms need to be investigated. Second, publication bias may also exist. Last, all studies in this review were based on animals. When it comes to human, higher levels of evidence and treatment protocols are necessary. And because of the diversity and particularity of inflammatory diseases, all possible adverse reactions in acupuncture treatment should be noticed.
5. Conclusion
This article provided an overview of the crosstalk between acupuncture and NF-κB in inflammatory diseases. A variety of studies with different diseases and models support acupuncture as an efficient NF-κB antagonist and modulate inflammatory cytokines and inflammatory cells to relieve inflammation. It may help us in understanding the role of acupuncture and NF-κB in inflammatory diseases better. More studies are still needed in the future to provide high-level evidence.
Acknowledgments
This review was supported by the National Natural Science Foundation of China (No. 81774401) and Wuhan Medical Research Project (WX19Y18).
Contributor Information
Dan Luo, Email: luodanchina@163.com.
Rui Chen, Email: unioncr@163.com.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Lai H. C., Chang Q. Y., Hsieh C. L. Signal transduction pathways of acupuncture for treating some nervous system diseases. Evidence-Based Complementary and Alternative Medicine. 2019;2019:37. doi: 10.1155/2019/2909632.2909632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu X. K., Stener-Victorin E., Kuang H. Y., et al. Effect of acupuncture and clomiphene in Chinese women with polycystic ovary syndrome: a randomized clinical trial. JAMA. 2017;317(24):2502–2514. doi: 10.1001/jama.2017.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato K. L., Sanada L. S., Silva M. D. d., Okubo R., Sluka K. A. Transcutaneous electrical nerve stimulation, acupuncture, and spinal cord stimulation on neuropathic, inflammatory, and noninflammatory pain in rat models. The Korean Journal of Pain. 2020;33(2):121–130. doi: 10.3344/kjp.2020.33.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu Y., Li G. Auricular acupuncture induces FNDC5/irisin and attenuates obese inflammation in mice. Acupuncture in Medicine. 2020 doi: 10.1136/acupmed-2017-011405.d2017011405 [DOI] [PubMed] [Google Scholar]
- 5.Yang N. N., Ye Y., Tian Z. X., et al. Effects of electroacupuncture on the intestinal motility, and local inflammation are modulated by acupoint selection and stimulation frequency in postoperative ileus mice. Neurogastroenterology & Motility. 2020;32(5) doi: 10.1111/nmo.13808.e13808 [DOI] [PubMed] [Google Scholar]
- 6.Sun S.-C. The noncanonical NF-κB pathway in immunity and inflammation. Nature Reviews Immunology. 2017;17(9):545–558. doi: 10.1038/nri.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayden M. S., Ghosh S. NF-κB in immunobiology. Cell Research. 2011;21(2):223–244. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oeckinghaus A., Hayden M. S., Ghosh S. Crosstalk in NF-κB signaling pathways. Nature Immunology. 2011;12(8):695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 9.Lu T. X., Rothenberg M. E. MicroRNA. Journal of Allergy and Clinical Immunology. 2018;141(4):1202–1207. doi: 10.1016/j.jaci.2017.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong L., Simard M. J., Huot J. Endothelial microRNAs regulating the NF‐κB pathway and cell adhesion molecules during inflammation. The Faseb Journal. 2018;32(8):4070–4084. doi: 10.1096/fj.201701536r. [DOI] [PubMed] [Google Scholar]
- 11.Valmiki S., Ahuja V., Puri N., Paul J. miR-125b and miR-223 contribute to inflammation by targeting the key molecules of the NF-kappaB pathway. Frontiers in Medicine. 2019;6:p. 313. doi: 10.3389/fmed.2019.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kauppinen A., Suuronen T., Ojala J., Kaarniranta K., Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cellular Signalling. 2013;25(10):1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Ulivi V., Giannoni P., Gentili C., Cancedda R., Descalzi F. p38/NF-kB-dependent expression of COX-2 during differentiation and inflammatory response of chondrocytes. Journal of Cellular Biochemistry. 2008;104(4):1393–1406. doi: 10.1002/jcb.21717. [DOI] [PubMed] [Google Scholar]
- 14.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441(7092):431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 15.Chen J., Stark L. A. Crosstalk between NF-kappaB and nucleoli in the regulation of cellular homeostasis. Cells. 2018;7(10) doi: 10.3390/cells7100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X.-f., Xiang S.-y., Geng W.-y., et al. Electroacupuncture regulates the cholinergic anti-inflammatory pathway in a rat model of chronic obstructive pulmonary disease. Journal of Integrative Medicine. 2018;16(6):418–426. doi: 10.1016/j.joim.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Luo D., Liu L., Liang F. X., Yu Z. M., Chen R. Electroacupuncture: a feasible Sirt1 promoter which modulates metainflammation in diet-induced obesity rats. Evidence-Based Complementary and Alternative Medicine. 2018;2018:10. doi: 10.1155/2018/5302049.5302049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng C. Y., Lin J. G., Tang N. Y., Kao S. T., Hsieh C. L. Electroacupuncture-like stimulation at the Baihui (GV20) and Dazhui (GV14) acupoints protects rats against subacute-phase cerebral ischemia-reperfusion injuries by reducing S100B-mediated neurotoxicity. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0091426.e91426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J., Huang K., Zhong G., et al. Acupuncture decreases NF-kappaB p65, miR-155, and miR-21 and increases miR-146a expression in chronic atrophic gastritis rats. Evidence-Based Complementary and Alternative Medicine. 2016;2016:9. doi: 10.1155/2016/9404629.9404629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z., Yi T., Long M., Ding F., Ouyang L., Chen Z. Involvement of the negative feedback of IL-33 signaling in the anti-inflammatory effect of electroacupuncture on allergic contact dermatitis via targeting MicroRNA-155 in mast cells. Inflammation. 2018;41(3):859–869. doi: 10.1007/s10753-018-0740-8. [DOI] [PubMed] [Google Scholar]
- 21.Liu F., Fang J., Shao X., Liang Y., Wu Y., Jin Y. Electroacupuncture exerts an anti-inflammatory effect in a rat tissue chamber model of inflammation via suppression of NF-kappaB activation. Acupuncture in Medicine. 2014;32(4):340–345. doi: 10.1136/acupmed-2013-010460. [DOI] [PubMed] [Google Scholar]
- 22.Wei Y., Dong M., Zhang H., et al. Acupuncture attenuated inflammation and inhibited Th17 and Treg activity in experimental asthma. Evidence-Based Complementary and Alternative Medicine. 2015;2015:8. doi: 10.1155/2015/340126.340126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu G.-w., Chen J., Huang Y.-m., et al. Electroacupuncture delays cartilage degeneration by modulating nuclear factor-κb signaling pathway. Chinese Journal of Integrative Medicine. 2019;25(9):677–683. doi: 10.1007/s11655-018-2916-8. [DOI] [PubMed] [Google Scholar]
- 24.Wen C. K., Lee T. Y. Electroacupuncture prevents white adipose tissue inflammation through modulation of hypoxia-inducible factors-1alpha-dependent pathway in obese mice. BMC Complement Altern Med. 2015;15:p. 452. doi: 10.1186/s12906-015-0977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye Y. S., Pan A. Z., Zhen Y., Kang M. R., Zhang B., Yi W. M. Antipruritic effects of electroacupuncture on morphine-induced pruritus model mice through the TLR2/4-MyD88-NF-κB pathway. Neuroreport. 2019;30(5):331–337. doi: 10.1097/wnr.0000000000001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W., Wang X., Yang S., et al. Electroacupunctre improves motor impairment via inhibition of microglia-mediated neuroinflammation in the sensorimotor cortex after ischemic stroke. Life Sciences. 2016;151:313–322. doi: 10.1016/j.lfs.2016.01.045. [DOI] [PubMed] [Google Scholar]
- 27.Xue Q. M., Pan H., Huang L., Li N. Effects of acupuncture at ST25 on inflammatory mediators and nuclear factor-kappaB activation in a rat model of severe acute pancreatitis. Acupuncture in Medicine. 2015;33(4):299–304. doi: 10.1136/acupmed-2014-010609. [DOI] [PubMed] [Google Scholar]
- 28.Park J. Y., Park H. J., Choi Y. Y., Kim M. H., Kim S. N., Yang W. M. Effects of acupuncture on 1-chloro-2,4-dinitrochlorobenzene-induced atopic dermatitis. Evidence-Based Complementary and Alternative Medicine. 2013;2013:8. doi: 10.1155/2013/982095.982095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng X., Yang S., Liu J., et al. Electroacupuncture ameliorates cognitive impairment through inhibition of NF-κB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats. Molecular Medicine Reports. 2013;7(5):1516–1522. doi: 10.3892/mmr.2013.1392. [DOI] [PubMed] [Google Scholar]
- 30.Lu J., Shao R.-H., Jin S.-Y., Hu L., Tu Y., Guo J.-Y. Acupuncture ameliorates inflammatory response in a chronic unpredictable stress rat model of depression. Brain Research Bulletin. 2017;128:106–112. doi: 10.1016/j.brainresbull.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y., Jiang H., Meng H., et al. Antidepressant mechanism research of acupuncture: insights from a genome-wide transcriptome analysis of frontal cortex in rats with chronic restraint stress. Evidence-Based Complementary and Alternative Medicine. 2017;2017:13. doi: 10.1155/2017/1676808.1676808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y., Li W., Hu L., et al. Downregulation of nitric oxide by electroacupuncture against hypoxic-ischemic brain damage in rats via nuclear factor-κB/neuronal nitric oxide synthase. Molecular Medicine Reports. 2015;11(2):837–842. doi: 10.3892/mmr.2014.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y.-X., Yao M.-J., Liu Q., Xin J.-J., Gao J.-H., Yu X.-C. Electroacupuncture treatment attenuates paclitaxel-induced neuropathic pain in rats via inhibiting spinal glia and the TLR4/NF-kappaB pathway. Journal of Pain Research. 2020;13:239–250. doi: 10.2147/jpr.s241101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin D., Zhang J., Zhuang W., et al. The effect of electroacupuncture versus manual acupuncture through the expression of TrkB/NF-kappaB in the subgranular zone of the dentate gyrus of telomerase-deficient mice. Evidence-Based Complementary and Alternative Medicine. 2018;2018:11. doi: 10.1155/2018/1013978.1013978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong Z. Q., Zhu J., Lu D. Z., Chen Q., Xu Y. L. Effect of electroacupuncture in “”zusanli”” and “kunlun” acupoints on TLR4 signaling pathway of adjuvant arthritis rats. American Journal of Therapeutics. 2018;25(3):e314–e319. doi: 10.1097/mjt.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 36.Wang K., Wu H., Chi M., Zhang J., Wang G., Li H. Electroacupuncture inhibits apoptosis of splenic lymphocytes in traumatized rats through modulation of the TNF-alpha/NF-kappaB signaling pathway. Molecular Medicine Reports. 2015;11(1):237–241. doi: 10.3892/mmr.2014.2740. [DOI] [PubMed] [Google Scholar]
- 37.Lin S. J., Cao L. X., Cheng S. B., et al. Effect of acupuncture on the TLR2/4-NF-kappaB signalling pathway in a rat model of traumatic brain injury. Acupuncture in Medicine. 2018;36(4):247–253. doi: 10.1136/acupmed-2017-011472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J. W., Wang X. R., Ma S. M., Yang N. N., Li Q. Q., Liu C. Z. Acupuncture attenuates cognitive impairment, oxidative stress, and NF-kappaB activation in cerebral multi-infarct rats. Acupuncture in Medicine. 2019;37(5):283–291. doi: 10.1136/acupmed-2017-011491. [DOI] [PubMed] [Google Scholar]
- 39.Song G., Fiocchi C., Achkar J.-P. Acupuncture in inflammatory bowel disease. Inflammatory Bowel Diseases. 2019;25(7):1129–1139. doi: 10.1093/ibd/izy371. [DOI] [PubMed] [Google Scholar]
- 40.Meng J.-b., Jiao Y.-n., Xu X.-j., et al. Electroacupuncture attenuates inflammatory responses and intra-abdominal pressure in septic patients. Medicine. 2018;97(17):p. e0555. doi: 10.1097/md.0000000000010555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taniguchi K., Karin M. NF-κB, inflammation, immunity, and cancer: coming of age. Nature Reviews Immunology. 2018;18(5):309–324. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 42.Wang J. Y., Li H., Ma C. M., Wang J. L., Lai X. S., Zhou S. F. Acupuncture may exert its therapeutic effect through microRNA-339/Sirt2/NF-kappaB/FOXO1 axis. Biomed Research International. 2015;2015:9. doi: 10.1155/2015/249013.249013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brinkhaus B., Roll S., Jena S., et al. Acupuncture in patients with allergic asthma: a randomized pragmatic trial. The Journal of Alternative and Complementary Medicine. 2017;23(4):268–277. doi: 10.1089/acm.2016.0357. [DOI] [PubMed] [Google Scholar]
- 44.Gao X., Yuan J., Li H., Ren S. Clinical research on acupuncture and moxibustion treatment of chronic atrophic gastritis. Journal of Traditional Chinese Medicine. 2007;27(2):87–91. [PubMed] [Google Scholar]
- 45.Yang J.-W., Shi G.-X., Zhang S., et al. Effectiveness of acupuncture for vascular cognitive impairment no dementia: a randomized controlled trial. Clinical Rehabilitation. 2019;33(4):642–652. doi: 10.1177/0269215518819050. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki M., Muro S., Fukui M., et al. Effects of acupuncture on nutritional state of patients with stable chronic obstructive pulmonary disease (COPD): reanalysis of COPD acupuncture trial, a randomized controlled trial. BMC Complementary and Alternative Medicine. 2018;18(1):p. 287. doi: 10.1186/s12906-018-2341-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang S. B., Cho S. Y., Kwon S., et al. Acupuncture attenuates postoperative inflammation in patients after craniotomy: a prospective, open-labeled, controlled trial. Medicine. 2020;99(11) doi: 10.1097/md.0000000000019071.e19071 [DOI] [PMC free article] [PubMed] [Google Scholar]


