Abstract
Nanofractured autologous matrix-induced chondrogenesis (NAMIC©) is a 1-step technique that combines nanofracture needling to induce bone marrow stimulation (BMS) and the use of cell-free collagen matrix to optimize cartilage regeneration. In this Technical Note, we describe a modification of the NAMIC procedure using mosaicplasty trephines to prepare the lesion surface and to shape collagen implants in an all-arthroscopic approach (A-NAMIC). This technique is indicated for the treatment of International Cartilage Repair Society grade III to IV knee chondral lesions of ≤4 cm2. After damaged cartilage is debrided, trephines are used to create a flat, circular lesion surfaces. Subsequently, BMS is performed with nanofracture, eliciting reproducible and stop-controlled subchondral bone perforations of 9-mm depth and 1-mm width. The collagen membrane is then cut to size with the trephine, placed over the prepared defect, and secured with fibrin glue, preventing loss of regenerating cells and growth factors to the joint space. Using trephines allows the rapid and precise creation of smooth defect surfaces with known dimensions, ensuring optimal lesion coverage. Additionally, nanofracture reduces trabecular compaction and allows for a deeper access to subchondral bone in comparison with conventional microfracture, improving lesion filling and production of cartilage with higher hyaline content.
Articular cartilage defects remain challenging clinical treatment problems for orthopaedic surgeons and, if left untreated, can progress to degenerative osteoarthritis, knee pain, and ultimately loss of function.1 The limited self-healing ability of articular cartilage is due to its avascular nature and slow extracellular matrix turnover, along with the limited capacity of resident chondrocytes to migrate to damaged areas.2 Cartilage repair techniques based on bone marrow mesenchymal stem cells (MSCs) have been widely used since first introduced in the 1950s.3 The most frequently used method for using stem cells is the microfracture technique, first described by Steadman et al.4 Microfracture introduces perforations in the exposed subchondral bone, inducing bleeding and recruitment of pluripotential stem cells to the joint surface that will differentiate into cartilage-producing chondrocytes.5 However, these procedures typically lack medium- to long-term durability, particularly in cases of larger lesions, because of progressive ossification and limited biomechanical properties of the regenerated tissue.6
An evolution of this method is autologous matrix-induced chondrogenesis (AMIC),7,8 which combines microfracture with the application of a collagen matrix membrane over the treated defect to serve as a scaffold for stem cells, allowing effective reconstruction of larger defects. The AMIC procedure can be performed with either an open approach7 or as an all-arthroscopic surgery.8 In the latter, the authors also introduced the use of mosaicplasty trephines to prepare the lesion surface and cut the matrix implant to size. Midterm studies on microfracturing and the AMIC have shown encouraging results.9
Recently, Benthien et al.10 introduced a further development with the nanofractured autologous matrix-induced chondrogenesis (NAMIC©) technique, in which a new subchondral needling procedure (Nanofracture®, Arthrosurface, Franklin, MA) is used instead of microfracture. Nanofracture creates smaller and deeper cell channels and in a more reproducible way than a standard microfracture, therefore increasing bone marrow access and achieving improved cartilage-resurfacing properties.2 In this Technical Note, we provide a detailed description of a trephine-assisted all-arthroscopic NAMIC (A-NAMIC) technique for the treatment of International Cartilage Repair Society (ICRS) Grade III/IV focal chondral lesions of the knee.
Technique
Indications for trephine-assisted A-NAMIC are symptomatic full-thickness ICRS grade III or IV focal chondral defects without extensive subchondral bony deficiency, and a lesion size of ≤4 cm2 and 5-mm depth,5 lying in regions of the joint allowing direct access to mosaicplasty trephines (coring reamer). Contraindications include partial-thickness defects, kissing lesions, and widespread articular and joint degeneration. Although the age limit for A-NAMIC is not yet determined, previous publications point to a decrease in bone marrow MSC cell number and regenerative capacity in older patients.5 Accordingly, the clinical success rate of bone marrow stimulation–based treatments has been most consistent in patients ≤40 years.5
Magnetic resonance imaging (MRI) T2 mapping and standard radiographic evaluation including standing anteroposterior and lateral radiographs are recommended. A Rosenberg view should also be performed to assess the main weightbearing area, and in case of clinical hints for leg axis deviation, an orthodiagram could be helpful.
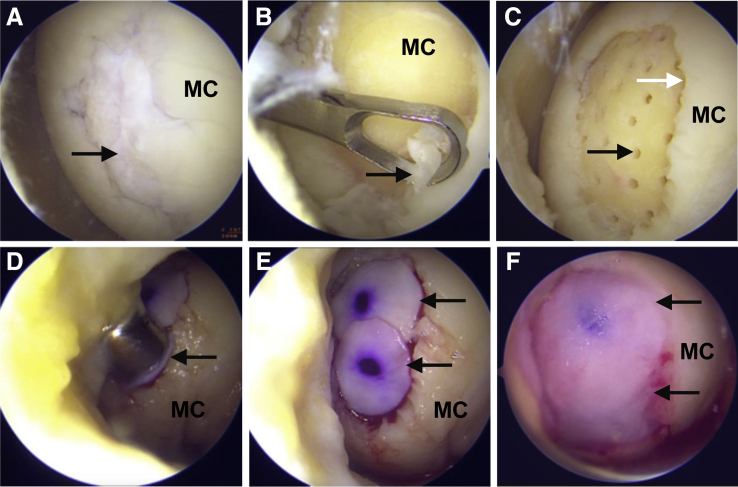
Surgery on the knee is performed under general anesthesia with the patient positioned supine on a standard operation table. A pneumatic tourniquet is placed at the root of the thigh, and a padded lateral support is used to allow the application of a valgus force to open the medial joint. Standard anterolateral and anteromedial portals are used as viewing and working portals, respectively, with the latter being positioned depending on the location of the articular cartilage defect to provide optimal access to the cartilage lesion. A thorough examination of the knee joint is then performed to evaluate the extent of the cartilage lesion as well as meniscal and cruciate ligament defects (Fig 1A). After assessment of the associated defects, all the damaged cartilage is debrided down to the subchondral bone plate with an open curette and a shaver, creating a contained lesion with well-defined healthy vertical edges surrounding the defect (Fig 1B). At this point, the chondral defect is addressed as described by Piontek et al.8 Accordingly, the lesion is sized with a 5-mm probe or a mosaicplasty trephine of the appropriate diameter (6-, 8-, or 10-mm-diameter mosaicplasty trephine; Arthrex, Naples, FL). The diameter-selected trephine is then used to create a flat, circularly shaped defect surface with known dimensions that will ultimately accommodate the membrane implant. For defect surfaces >10 mm, additional reconstruction areas are prepared using appropriate trephines, trying to prevent the unnecessary debridement of surrounding healthy cartilage.
Fig 1.
All-arthroscopic nanofractured autologous matrix-induced chondrogenesis (A-NAMIC) treatment of focal chondral lesions of the knee. Surgery was performed in the left knee joint with the patient positioned supine and using anterolateral and anteromedial portals as visualization and working portals, respectively. (A) Localization of a grade III full-thickness knee chondral lesion (black arrow). (B) Removal of damaged cartilage (black arrow) and sclerotic bone with an open curette. (C) Image showing the spiral configuration of the 9-mm-depth and 1-mm-diameter nanofracture-performed channels (black arrow). The white arrow indicates the well-defined healthy cartilage margins after debridement of damaged cartilage. (D) Implantation of the 3D I/III collagen membrane with 8- and 10-mm-diameter mosaicplasty trephines (black arrow). (E) Final examination to ensure that the lesion is fully covered by the membrane. Black arrows indicate the 2 implants in their final position. (F) Fibrin sealing. The black arrow indicates the final appearance of the lesion after application of the fibrin glue. MC, medial condyle.
Once the defect preparation is completed, nanofracture is performed by placing the 1-mm needle (PleuriStik Guide Wire; Arthrosurface) into the lumen of a 15°-angled, cannulated pick (Arthrosurface), and the distal tip of the pick is then placed onto the target site. With a mallet, multiple taps are made on the proximal head of the needle, driving the tip into the subchondral bone and obtaining standardized and stop-controlled 9-mm-depth and 1-mm-diameter channels. The needle is then removed with the thumb tab accessory (Arthrosurface), and the process is repeated at 2- to 3-mm intervals, allowing adequate bone bridges between each channel to protect the mechanical stability of the subchondral bone. Subchondral needling is started from the periphery of the lesion and then continued toward the center of the lesion following a systematic, spiral pattern (Fig 1C).
After perforation of the subchondral bone, the joint is washed to remove any cartilage debris and loose bony particles. The irrigation pump is turned off, and the joint is dried with the help of a small gauze pad. The prepared cartilage defect is then covered with a biodegradable, cell-free, type I/III collagen and elastine membrane of porcine origin (Cartimaix; Matricel, Herzogenrath, Germany) to keep released bone marrow cells within the defect area. For this purpose, the collagen membrane is removed from the sterile packaging, rehydrated with physiological saline, and cut to size with the same mosaicplasty trephine used to shape the defect surface. The membrane has a 2-sided appearance: the smooth side (which is marked with a sterile pen to facilitate side identification within the joint) faces the joint, whereas the fibrous, rough side faces the cartilage defect. The circular implant is positioned in the trephine with the porous layer facing the lesion, and the trephine pusher is slid down to the lesion surface (Fig 1D). The membrane is pressed into the defect. If the diameter of the cartilage defect does not exceed 10 mm, only 1 patch is used. For larger defects, the operation is repeated with subsequent trephine-sized membrane patches until the whole cartilage lesion is fully covered. It is important that the edges of the lesion are vertical and that the membrane implant is slightly undersized in the surface of the cartilage defect, as this will retain the membrane in place. The implants can be repositioned with the help of an arthroscopic probe to ensure they fit the defect without overlapping onto the surrounding healthy cartilage (Fig 1E). Finally, the lesion is sealed by applying a fibrin glue (Tissel 4 ml; Baxter, Deerfield, IL) to all membrane-covered areas via a 19-gauge needle (Fig 1F). The glue is left to set for 5 min. Once the membrane is fixed, 10 knee movements (consisting of flexion and extension) are performed to check that the membrane remains in place.
All-arthroscopic nanofractured autologous matrix-induced chondrogenesis (A-NAMIC) treatment of an ICRS Grade III focal chondral lesion of the knee is shown in the Video and summarized in Table 1.
Table 1.
Step-by-Step Summary of A-NAMIC Technique
| Step | Description | Observations |
|---|---|---|
| 1 | Position patient supine on a standard operation table. | Use a padded lateral support to apply valgus force. |
| 2 | Place the pneumatic tourniquet at the roof of the thigh. | |
| 3 | Establish anterolateral and anteromedial portals as viewing and working portals, respectively. | The working portal is positioned depending on the location of the articular cartilage defect to provide optimal, direct access to the cartilage lesion. |
| 4 | Evaluate the extent of the cartilage lesion as well as meniscal and cruciate ligament defects. | |
| 5 | Debride damaged cartilage and remove sclerotic bone plate. | Use sharp curette and a shaver. |
| 6 | Give a circular shape to the defect surface. | Use a trephine of adequate diameter. Create well-defined, healthy vertical edges surrounding the defect. |
| 7 | Perform nanofracture. | Place the 1-mm Nitinol needle into the lumen of a 15°-angled, cannulated pick. Perform multiple taps with a mallet. Follow spiral pattern to cover the whole lesion area. |
| 8 | Repeat the process at 2- to 3-mm intervals. | Allow adequate bone bridges between each channel to protect the mechanical stability of subchondral bone. |
| 9 | Wash the joint to remove any cartilage debris and loose bony particles. | |
| 10 | Turn the irrigation pump off and dry the joint. | Use a small gauze pad. |
| 11 | Rehydrate the type I/III collagen and elastine membrane with physiological saline and cut to size. | Use a 10-mm mosaicplasty trephine (coring reamer) to cut the membrane. |
| 12 | Place the circular implant in the trephine and slide down the trephine pusher to the lesion surface. | The membrane has a 2-sided appearance; the smooth side faces the joint, and the fibrous, rough side faces the cartilage defect. |
| 13 | Press the membrane into the defect. | The membrane implant must be slightly undersized on the surface of the cartilage defect to retain the membrane in place. |
| 14 | For larger defects (>10 mm), repeat the operation until the cartilage lesion is fully covered. | The implants can be repositioned with the help of an arthroscopic probe. |
| 15 | Seal the lesion with fibrin glue. | Use a 19-gauge needle to apply the glue. |
| 16 | Perform 10 knee movements to check that the membrane remains in place. | Perform flexion and extension. |
Weightbearing is restricted for 6 weeks after surgery, since this may lead to displacement of the collagen membrane and poorer tissue integration, and moving around with crutches is encouraged. Continuous passive movement (CPM) is recommended daily to prevent joint stiffness and improve early motion, starting 48 h after surgery, with initial settings ranging from 0° to 40°. The range of motion (ROM) is then daily increased as tolerated until 90° of flexion is restored. Partial weightbearing is initiated at 7 weeks. High-impact sports are not recommended, but light sporting activities (swimming, cycling) are allowed. Isotonic and isometric exercises to potentiate quadriceps and hamstrings strength are also recommended. High-impact sports are allowed at 12 months.
Discussion
Therapies based on AMIC combine BMS with lesion coverage by a commercially available collagen I/III matrix.7 These techniques have been proven to improve treatment success.9 Microfracture is the most widespread technique to induce MSC recruitment to the joint surface. However, microfracturing is not a reproducible procedure, making it difficult to compare outcomes of different studies and establish optimal treatment strategies. Accordingly, there is great variability in the depth and diameter of the microfracture perforations depending on factors such as the shape and size of the awl tip or the force exerted upon it by the surgeon. This is specially relevant considering that shallow subchondral bone penetration could result in suboptimal bone marrow stimulation,2 and excessive hole diameter11 could produce trabecular fragmentation and intrachannel bone compaction. In this sense, compared with cone-shaped 1.5- to 2-mm-wide and 2- to 4-mm-depth perforations observed in conventional microfracture, nanofracture showed better bone marrow access with standardized stop-controlled 1-mm-wide channels extending 9 mm into the subchondral bone.5 This defined length limit is set by the Nitinol needle, as cadaver studies showed that longer needles would have a higher risk of wire fracture without necessarily resulting in a better outcome. After nanofracture, deeper channels improved defect filling, with more hyaline cartilage containing a greater amount of type II collagen and less type I collagen compared with shallow marrow access. In an ovine model, Zedde et al.12 showed that nanofracture resulted in better restoration of the normal subchondral bone architecture at 6 months compared with microfracture.
Autologous chondrocyte implantation (ACI) and matrix-induced autologous chondrocyte implantation (MACI) are multistage, complex (timely and costly) procedures that consist of chondrocyte harvesting from a low-weightbearing region of the joint, its expansion in vitro, and its reimplantation into the debrided cartilage defect either directly (ACI) or by previously seeding chondrocytes on an absorbable porcine-derived mixed collagen (type I and III) membrane before implantation.13 In comparison with these techniques, AMIC/NAMIC is a simple, cost-effective cell-free technique that can be performed in a single surgery and excludes the need for in vitro culturing. In AMIC/NAMIC, the blood clot containing microfracture-released MSCs is stabilized by a mixed collagen type I and type III matrix, helping to promote early mechanical stability and cartilage regeneration. In addition, the use of fibrin glue to prevent collagen membrane relocation can provide a supplementary advantage over sutures, as fibrin glue acts as an additional scaffold for chondrocytes.7
Finally, the use of mosaicplasty trephines provides a rapid and precise method to create smooth defect surfaces with known dimensions. This results in optimal lesion coverage with the matrix implant, because the defect area has been reshaped with the same mosaicplasty trephine that is used to cut the collagen matrix.
The purpose of this Technical Note is to provide a step-by-step description of the trephine-assisted A-NAMIC surgical procedure for the treatment of knee cartilage defects. This technique represents a step forward in the treatment of ICRS grade III/IV focal chondral lesions of the knee with direct access. The main advantages of this technique are its reproducibility and cost-efficiency and that it can be performed all-arthroscopically in a single-step procedure. Patients have a higher clinical success and a much shorter rehabilitation time compared with the traditional AMIC,10 which may be related to a higher rate of defect filling and hyaline properties of the regenerated cartilage. This is especially relevant in lesions >2 cm2. The main inconveniences are that nanofracturing needles sometimes tend to bend and may not be applied at the correct angle. In addition, the location of the chondral lesion could be a limiting factor, considering that direct access is needed to place the membrane over the defect with the trephines. A summary of the key risks and limitations of this technique is shown in Table 2. Finally, it would be advisable to perform prospective long-term randomized trials that compare NAMIC with other cartilage repair techniques.
Table 2.
Risks and Limitations
| Risks |
|
|
|
|
| Limitations |
|
|
|
|
|
|
Footnotes
The authors report that they have no conflicts of interest in the authorship and publication of this article. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
The following is a detailed description of the trephine-assisted all-arthroscopic nanofractured autologous matrix-induced chondrogenesis (A-NAMIC) surgical technique to treat International Cartilage Repair Society grade III/IV osteochondral lesions. This video presents the case of a 41-year-old male patient with continuous pain and functional limitation in the left knee after failed 1-year conservative treatment with nonsteroidal anti-inflammatory drugs. Complementary explorations included Rosenberg view, clinical high-frequency ultrasound system, and long-leg weightbearing radiographs, which yielded normal results. A 3-Tesla magnetic resonance image showed an osteochondral lesion of 2 cm2 in the load-bearing area of the medial condyle. To perform the A-NAMIC surgery, the following instruments and implant were required: the nanofracture device, consisting of a reusable hand instrument, a single-use disposable PleuriStik guide wire, and a single-use thumb tab accessory (Arthrosurface, Franklin, MA); an open 4-mm curette; an arthroscopy bur; 8- and 10-mm mosaicplasty trephines; collagen I/III biphasic matrix (Cartimaix; Matricel, Herzogenrath, Germany); fibrin glue (Tissel, Baxter, Deerfield, IL); ruler; and pen. The patient was positioned supine decubitus, with artificial ischemia in the upper limb. Two arthroscopic portals were placed: the anterolateral portal, for visualization, and the anteromedial portal, which allows for direct perpendicular access to the chondral lesion. The lesion was localized, and the functional integrity of the meniscus and the anterior cruciate ligament was assessed, yielding normal results. All the damaged cartilage and sclerotic bone layer was debrided using a 4-mm open curette. Lesion edges were reshaped with mosaicplasty trephines, leaving vertical edges. Bone marrow stimulation (BMS) was performed using the nanofracture device, guiding reproducible 1-mm-diameter and 9-mm-depth holes. Holes were placed in a spiral configuration and leaving a minimum 2-mm space between holes. After BMS was completed, the lesion was sized with a 5-mm probe. Water flow was stopped, and the lesion was dried as much as possible. The collagen membrane was prepared. Once the porous face was localized and marked with a pen, the membrane was hydrated with physiological saline and cut to lesion size with the same trephine that was used to shape the lesion. The membrane was then positioned in the trephine with the porous layer facing the lesion and implanted by pushing the trephine into the lesion. The porosity of the implant facilitates adhesion to the lesion surface. To ensure full coverage, the membrane can be repositioned with a probe. It is of utmost importance that the implant sits flat in the lesion area and that it covers all gaps without passing the lesion edges. In this particular case, 2 rounded implants of 10-mm diameter were used to fully cover the lesion. After that, the lesion was sealed with fibrin glue, followed by a waiting period of 5 minutes to ensure correct gelification and implant stability. the patient’s recommended rehabilitation protocol consisted of non-weightbearing and continuous passive motion movement during the first 6 weeks. At 7 weeks, the patient started with partial weightbearing, full active motion, and no impact sports such as cycling or swimming. Twelve months after surgery, the new cartilage should be mature, allowing high-impact sports.Finally, it is important to emphasize that the AMIC technique has a >10-year follow-up with good results. Previous publications from Piontek et al.8 and Benthien and Behrens10 define previous steps of this new technique.
References
- 1.Redondo M.L., Naveen N.B., Liu J.N., Tauro T.M., Southworth T.M., Cole B.J. Preservation of knee articular cartilage. Sports Med Arthrosc. 2018;26:e23–e30. doi: 10.1097/JSA.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 2.Chen H., Hoemann C.D., Sun J. Depth of subchondral perforation influences the outcome of bone marrow stimulation cartilage repair. J Orthop Res. 2011;29:1178–1184. doi: 10.1002/jor.21386. [DOI] [PubMed] [Google Scholar]
- 3.Pridie K. A method of resurfacing osteoarthritic knee joints. J Bone Jt Surg Br. 1959;41:618–619. [Google Scholar]
- 4.Steadman J.R., Rodkey W.G., Rodrigo J.J. Microfracture: Surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391:S362–S369. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- 5.Benthien J.P., Behrens P. Reviewing subchondral cartilage surgery: Considerations for standardised and outcome predictable cartilage remodelling: A technical note. Int Orthop. 2013;37:2139–2145. doi: 10.1007/s00264-013-2025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gobbi A., Karnatzikos G., Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22:1986–1996. doi: 10.1007/s00167-013-2676-8. [DOI] [PubMed] [Google Scholar]
- 7.Benthien J.P., Behrens P. The treatment of chondral and osteochondral defects of the knee with autologous matrix-induced chondrogenesis (AMIC): Method description and recent developments. Knee Surg Sports Traumatol Arthrosc. 2011;19:1316–1319. doi: 10.1007/s00167-010-1356-1. [DOI] [PubMed] [Google Scholar]
- 8.Piontek T., Ciemniewska-Gorzela K., Szulc A., Naczk J., Słomczykowski M. All-arthroscopic AMIC procedure for repair of cartilage defects of the knee. Knee Surg Sports Traumatol Arthrosc. 2012;20:922–925. doi: 10.1007/s00167-011-1657-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinwachs M.R., Gille J., Volz M. Systematic review and meta-analysis of the clinical evidence on the use of autologous matrix-induced chondrogenesis in the knee. Cartilage. 2019 doi: 10.1177/1947603519870846. 194760351987084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benthien J.P., Behrens P. Nanofractured autologous matrix induced chondrogenesis (NAMIC©)—Further development of collagen membrane aided chondrogenesis combined with subchondral needling: A technical note. Knee. 2015;22:411–415. doi: 10.1016/j.knee.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Eldracher M., Orth P., Cucchiarini M., Pape D., Madry H. Small subchondral drill holes improve marrow stimulation of articular cartilage defects. Am J Sports Med. 2014;42:2741–2750. doi: 10.1177/0363546514547029. [DOI] [PubMed] [Google Scholar]
- 12.Zedde P., Cudoni S., Giachetti G. Subchondral bone remodeling: Comparing nanofracture with microfracture. An ovine in vivo study. Joints. 2016;4:87–93. doi: 10.11138/jts/2016.4.2.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makris E.A., Gomoll A.H., Malizos K.N., Hu J.C., Athanasiou K.A. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11:21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following is a detailed description of the trephine-assisted all-arthroscopic nanofractured autologous matrix-induced chondrogenesis (A-NAMIC) surgical technique to treat International Cartilage Repair Society grade III/IV osteochondral lesions. This video presents the case of a 41-year-old male patient with continuous pain and functional limitation in the left knee after failed 1-year conservative treatment with nonsteroidal anti-inflammatory drugs. Complementary explorations included Rosenberg view, clinical high-frequency ultrasound system, and long-leg weightbearing radiographs, which yielded normal results. A 3-Tesla magnetic resonance image showed an osteochondral lesion of 2 cm2 in the load-bearing area of the medial condyle. To perform the A-NAMIC surgery, the following instruments and implant were required: the nanofracture device, consisting of a reusable hand instrument, a single-use disposable PleuriStik guide wire, and a single-use thumb tab accessory (Arthrosurface, Franklin, MA); an open 4-mm curette; an arthroscopy bur; 8- and 10-mm mosaicplasty trephines; collagen I/III biphasic matrix (Cartimaix; Matricel, Herzogenrath, Germany); fibrin glue (Tissel, Baxter, Deerfield, IL); ruler; and pen. The patient was positioned supine decubitus, with artificial ischemia in the upper limb. Two arthroscopic portals were placed: the anterolateral portal, for visualization, and the anteromedial portal, which allows for direct perpendicular access to the chondral lesion. The lesion was localized, and the functional integrity of the meniscus and the anterior cruciate ligament was assessed, yielding normal results. All the damaged cartilage and sclerotic bone layer was debrided using a 4-mm open curette. Lesion edges were reshaped with mosaicplasty trephines, leaving vertical edges. Bone marrow stimulation (BMS) was performed using the nanofracture device, guiding reproducible 1-mm-diameter and 9-mm-depth holes. Holes were placed in a spiral configuration and leaving a minimum 2-mm space between holes. After BMS was completed, the lesion was sized with a 5-mm probe. Water flow was stopped, and the lesion was dried as much as possible. The collagen membrane was prepared. Once the porous face was localized and marked with a pen, the membrane was hydrated with physiological saline and cut to lesion size with the same trephine that was used to shape the lesion. The membrane was then positioned in the trephine with the porous layer facing the lesion and implanted by pushing the trephine into the lesion. The porosity of the implant facilitates adhesion to the lesion surface. To ensure full coverage, the membrane can be repositioned with a probe. It is of utmost importance that the implant sits flat in the lesion area and that it covers all gaps without passing the lesion edges. In this particular case, 2 rounded implants of 10-mm diameter were used to fully cover the lesion. After that, the lesion was sealed with fibrin glue, followed by a waiting period of 5 minutes to ensure correct gelification and implant stability. the patient’s recommended rehabilitation protocol consisted of non-weightbearing and continuous passive motion movement during the first 6 weeks. At 7 weeks, the patient started with partial weightbearing, full active motion, and no impact sports such as cycling or swimming. Twelve months after surgery, the new cartilage should be mature, allowing high-impact sports.Finally, it is important to emphasize that the AMIC technique has a >10-year follow-up with good results. Previous publications from Piontek et al.8 and Benthien and Behrens10 define previous steps of this new technique.