Abstract
BACKGROUND:
Obesity increases susceptibility to chronic pain, increases metabolism, and is associated with obstructive sleep apnea syndrome (OSAS), all which can complicate perioperative pain management of patients. In addition, obesity and OSAS can cause elevation of the adipose-derived hormone leptin, which increases metabolism. We hypothesized that obesity along with sleep apnea and leptin independently enhance morphine pharmacokinetics.
METHODS:
Children 5–12 years of age who were presenting for surgery were administered a morphine dose of 0.05 mg/kg. Blood was collected at baseline and at subsequent preset times for pharmacokinetic analysis of morphine and its metabolites. Three groups were studied: a nonobese group with severe OSAS, an obese group with severe OSAS, and a control group.
RESULTS:
Thirty-four patients consisting of controls (n = 16), nonobese/OSAS (n = 8), and obese/OSAS (n = 10) underwent analysis. The obese/OSAS group had a higher dose-adjusted mean maximum morphine concentration (CMAX) over 540 minutes compared to the controls (P < .001) and those with only OSAS (P = .014). The obese/OSAS group also had lower volume of distribution (Vd) when compared to OSAS-only patients (P = .007). In addition, those in the obese/OSAS group had a higher morphine 3-glucuronide (M3G) maximum concentration (P = .012) and a higher ratio of M3G to morphine than did the control group (P = .011). Time to maximum morphine 6-glucuronide (M6G) concentration was significantly lower in both nonobese/OSAS and obese/OSAS groups than in the control group (P < .005). C-reactive protein (CRP), interleukin (IL)-10, and leptin were all higher in the obese/OSAS group than in controls (P = .004, 0.026, and <0.001, respectively), and compared to OSAS-only patients, CRP (P = .013) and leptin (P = .002) levels were higher in the obese/OSAS group.
CONCLUSIONS:
The combination of obesity and OSAS was associated with an increase in morphine metabolism compared with that in normal-weight controls. Our previous study in mice demonstrated that obesity from leptin deficiency decreased morphine metabolism, but that metabolism normalized after leptin replacement. Leptin may be a cause of the increased morphine metabolism observed in obese patients.
Respiratory complications, including hypoxia and upper airway obstruction, remain among the leading causes of postoperative morbidity and mortality in the pediatric surgical population.1 Patients at highest risk for perioperative respiratory complications include children <3 years of age,2,3 obese patients,4 and those with obstructive sleep apnea syndrome (OSAS).5,6 Because opiate therapy with morphine is commonly administered perioperatively for surgical procedures that cause severe pain, a better understanding of how OSAS and obesity affect opioid metabolism will provide insight into appropriate dose concentrations and dosing frequency. Armed with such knowledge, anesthesiologists may be able to limit complications commonly associated with opioid therapy while still providing adequate postoperative pain management.
OSAS affects approximately 3% of the total pediatric population and is primarily treated by performing an adenotonsillectomy (AT). It has been reported that OSAS patients require less opioid medication than non-OSAS patients to meet equivalent pain scores7,8 and are more sensitive to the respiratory side effects of opioids.6 Pediatric patients diagnosed with severe OSAS based on hypoxia during sleep have been reported to have an increased sensitivity to opiates, and reducing the dose has been reported to reduce the incidence of respiratory complications by ;≥50%.9 These observations may result from hypoxia-related changes to opioid receptor expression. Intermittent hypoxia has been shown to upregulate mu-receptors and decrease pain sensitivity in rats,10 and studies have shown that children living at high altitude require less opioid for pain.11
Sensitivity to opioids may also be explained by hypoxia-induced changes to enzymatic function10 and thus to metabolism. Uridine diphosphate glucuronosyltransferase (UGT) 2B7 is the phase II enzyme responsible for glucuronidation metabolism of morphine sulfate to morphine 3-glucuronide (M3G) and morphine 6-glucuronide (M6G). Hypoxia has been shown to decrease UGT2B7 expression; however, few studies have evaluated morphine metabolism in patients who experience OSAS-related hypoxic episodes.
Pediatric obesity is reaching epidemic proportions. Approximately 33% of children in the United States are overweight, and 17% are obese. These statistics have shown little improvement since 2006. Obese adults are believed to have a low threshold for pain owing to chronic disease states and poor health. However, obese adults and children are also at a higher risk for postoperative respiratory complications, specifically after AT.12 Murine studies suggested that obesity decreased morphine metabolism, potentially via leptin.13,14 Obesity leads to multisystem diseases including a chronic inflammatory state, fatty liver, and defects in metabolism. Though glucuronidation is increased in obesity, studies evaluating the effects of UGT2B7 on morphine metabolism are limited.15
Both obesity and OSAS are multisystem, proinflammatory disorders16–18 that lead to multiorgan dysfunction, insulin and leptin resistance, pulmonary disease, and metabolic dysfunction18–22 that may play an integral role in altering opioid sensitivity. The mechanism for opioid sensitivity in these patient populations is poorly understood. Therefore, we examined effects of the interplay between obesity and OSAS on morphine pharmacokinetics in children. We hypothesized that morphine metabolism and clearance would be reduced in patients with (1) severe OSAS (diagnosed by polysomnography) and (2) obesity (defined as body weight >95th percentile by age). A better understanding of the interaction between obesity and OSAS-altered opioid metabolism will provide new insight into more effective and safer opioid treatment for these patients.
METHODS
This study was approved by the Johns Hopkins Institutional Review Board (IRB; No. IRB00059675) and was conducted in accordance with its guidelines. The trial was also registered with Clinicaltrials.gov (No. NCT02732795).
Patients selected were scheduled for otolaryngologic (ear/nose/throat [ENT]), orthopedic, genitourinary, and general surgery at the Johns Hopkins Hospital from February 5, 2016, to March 13, 2018. Children 5–12 years of age with a perioperative hospital stay ≥9 hours and for whom opioid therapy was indicated were eligible for this study. Informed consent/assent was obtained for all subjects in accordance with IRB protocol. Patients were excluded from the study if they were currently taking opioid medications, had an allergy or contraindication to morphine administration, took medications that altered liver metabolism (including seizure medications), or had end-stage liver or kidney disease. Initially, subjects were grouped into 4 classifications based on their weight and OSAS status as follows: nonobese and no OSAS (control), obese and no OSAS, nonobese and severe OSAS, and obese and severe OSAS. Severe OSAS (respiratory disturbance index ≥10) was diagnosed by a board-certified sleep physician who evaluated the polysomnogram using criteria established by the American Academy of Sleep Medicine.23 Obesity was defined as the child’s weight being greater than the 95th percentile, as delineated by the Centers for Disease Control.24 Patients defined to be nonobese were those below the 85th percentile for weight, thus excluding overweight children. Patients who did not have a polysomnogram were screened for exclusion secondary to sleep-disordered breathing with 2 questions: “Does your child snore at night?” and “Have you witnessed your child stop breathing during sleep?” If either question was answered in the affirmative, the child was not enrolled in the study. In addition to OSAS diagnosis and weight, patient demographics, including race, sex, age, comorbid diseases, and prematurity, were recorded.
Intraoperative Management
General anesthesia was induced with sevoflurane and/or N2O, and the American Society of Anesthesiologists (ASA) standard monitors were placed. After induction, an intravenous (IV) catheter was inserted. At that time, blood was obtained to measure biomarker levels (including interleukin [IL]-1, IL-6, and IL-10, tumor necrosis factor [TNF]-α, C-reactive protein [CRP], glucose, insulin, and leptin) and morphine concentration at time = 0. A second IV line was placed in case the first failed. The duration of anesthesia, surgical procedure, and additional medication types and doses were recorded (Table 1).
Table 1.
Demographic Data of Enrolled Patients
| Total (n = 40) | Control (n = 18) | OSAS Only (n = 9) | Obese and OSAS (n = 13) | P Value | |
|---|---|---|---|---|---|
| Age, mean (SEM) (y) | 7.4 (0.4) | 8.3 (0.5) | 6.0 (0.6) | 7.0 (0.7) | .022 |
| Sex (%) | .840 | ||||
| Male | 26 (63.4) | 11 (61.1) | 5 (55.6) | 9 (69.2) | |
| Female | 15 (39.6) | 7 (38.9) | 4 (44.4) | 4 (30.7) | |
| Race (%) | .004 | ||||
| Caucasian | 19 (46.3) | 13 (72.2) | 2 (22.2) | 4 (30.8) | |
| Black/African American | 16 (39.0) | 3 (16.6) | 3 (33.3) | 9 (69.2) | |
| Asian | 2 (4.9) | 1 (5.6) | 1 (11.2) | 0 | |
| Other | 4 (9.8) | 1 (5.6) | 3 (33.3) | 0 | |
| Weight, mean (SEM) (kg) | 30.8 (2.6) | 27.3 (2.1) | 20.4 (1.1) | 42.9 (6.2) | <.001 |
| Morphine dose, mean (SEM), actual (mg/kg)a | 0.05 (0.002) | 0.05 (0.001) | 0.05 (0.001) | 0.04 (0.005) | .003 |
| Surgical procedure (%) | <.001 | ||||
| ENT (AT, SML/bronchoscopy) | 21 (51.2) | 0 | 8 (88.9) | 13 (100) | |
| General surgery (hernia, laparoscopy) | 3 (7.3) | 3 (16.7) | 0 | 0 | |
| Genitourinary (bladder extrophy) | 14 (34.2) | 13 (72.2) | 1 (11.1) | 0 | |
| Orthopedic (arthroscopy)b | 3 (7.3) | 2 (11.1) | 0 | 0 | |
| Anesthesia duration, mean (SD) (min) | 230.3 (243) | 426.4 (253.0)c | 59.4 (9.3) | 80.5 (44.5) | .001 |
Measures of association between groups were calculated using Fisher exact test for categorical and binomial variables. Pairwise comparisons were calculated by Kruskal-Wallis χ2 analysis for continuous variables.
Abbreviations: AT, adenotonsillectomy; ENT, ear/nose/throat; OSAS, obstructive sleep apnea syndrome; SD, standard deviation; SEM, standard error of the mean; SML, suspension microlaryngoscopy.
Actual mg/kg dosing for morphine reflects the dose the patients received if one were to administer morphine on a mg/kg basis based on their actual body weight.
Patients undergoing surgery who required blood product transfusion, including spinal fusion, were not enrolled.
Patients in the control group underwent short procedures including ENT procedures (mean anesthesia duration 73.1 minutes, SD = 36.1), as well as long procedures including bladder extrophy repair (mean anesthesia duration = 458.6 minutes, SD = 259.9).
After the initial blood draw, a standard dose of 0.05 mg/kg morphine hydrochloride (molecular weight [MW] = 375.9; 10 mg/mL; West-Ward, Eatontown, NJ) was administered. For all obese patients, the morphine dose was based on the patient’s ideal body weight (http://nccd.cdc.gov/dnpabmi/Calculator.aspx) as recommended for morphine dosing.15,25 After this initial dose of morphine, no additional morphine was administered intraoperatively or postoperatively. Other pain medications, including nonopioid analgesics such as acetaminophen and ketorolac, as well as nonmorphine opioids, including fentanyl and hydromorphone, were administered as deemed necessary to ensure that each patient’s pain was controlled.
Blood Collection
Blood samples were collected at 10, 30, 45, 60, 90, 120, 180, 220, 360, 400, 480, and 540 minutes after the single morphine dose. After 1–2 mL of blood was aspirated and discarded, approximately 0.5 mL of blood was collected in a green-top Lithium Heparin Vacutainer tube (BD, Franklin Lakes, NJ). The 9-hour sampling duration allowed for 3 metabolic halflives of morphine (T1/2 = 3 hours) to elapse, which enabled robust estimation of the critical pharmacokinetic parameters for morphine and morphine metabolites.25
Blood Sample Storage and Analysis
Whole blood samples were centrifuged immediately after collection, or the Vacutainer was stored in a 0°C refrigerator for processing the next day. Processing consisted of centrifuging the samples at 3000 rpm at 4°C for 20 minutes. The 0.5–1.0 mL aliquots of plasma were then stored at −80°C. We analyzed the samples in batches by high-performance liquid chromatography-tandem mass spectroscopy26 to identify concentrations (ng/mL) of morphine, M3G, and M6G. Mass spectrometry was performed in the positive multiple reaction monitoring mode (MRM). Dynamic range of the assay was 0.25–1000 ng/mL (r2 > 0.99) for morphine and 1–1000 ng/mL (r2 > 0.99) for M3G and M6G. Accuracy and precision were within 15% of the nominal values.26
Pharmacokinetic Determinations
Morphine, M3G, and M6G concentration data were analyzed by noncompartmental methods (Phoenix WinNonlin version 6.4 software; Pharsight A Certara Company, Cary, NC). Individual maximum concentration (CMAX) and time to reach CMAX (TMAX) values were obtained by visual inspection of the semilogarithmic plots of concentration versus time (Figure 1). We calculated area under the curve (AUC) for plots of morphine, M6G, and M3G concentration versus time over 540 minutes (AUC540) by the log-linear trapezoidal method.27 We planned for 9-hour duration of blood sampling, approximately 3 T1/2 lives of morphine.28 Studies have shown that only 8% of the morphine AUC is extrapolated beyond the 6-hour observation, and only 6% is extrapolated beyond our planned 9-hour terminal observation making for very robust AUC estimates.25 Due to the low rate of metabolism during the terminal phase (or third halflife), time between blood draws was extended to minimize patient discomfort. We determined the terminal elimination rate constant (λz) from the slope of the terminal phase of concentration versus time curve. Both AUC540 and CMAX were dose adjusted to a 0.05 mg/kg equivalent for comparison across groups by multiplying the ratio of 0.05 mg/kg to the actual individual mg/kg dose received based on the patients’ ideal body weight. The elimination T1/2 was calculated as ln(2) divided by λz. Standard equations for apparent volume of distribution (Vd) and total clearance adjusting for weight were used, utilizing AUC0-inf to determine clearance.29 For terminal elimination end points, an adjusted r2 ≥0.80 was used as a quality metric. This model is fitted to the elimination phase, using elimination rate constant (Kel; terminal phase constant) and fitting a linear model to the time versus log concentration curve. We did not report the M6G and M3G λz, Vd, and clearance because the number of terminal concentration points was insufficient to accurately characterize these pharmacokinetic parameters. M3G-to-morphine and M6G-to-morphine AUC540 ratios were calculated based on morphine, M3G, and M6G levels detected.
Figure 1.
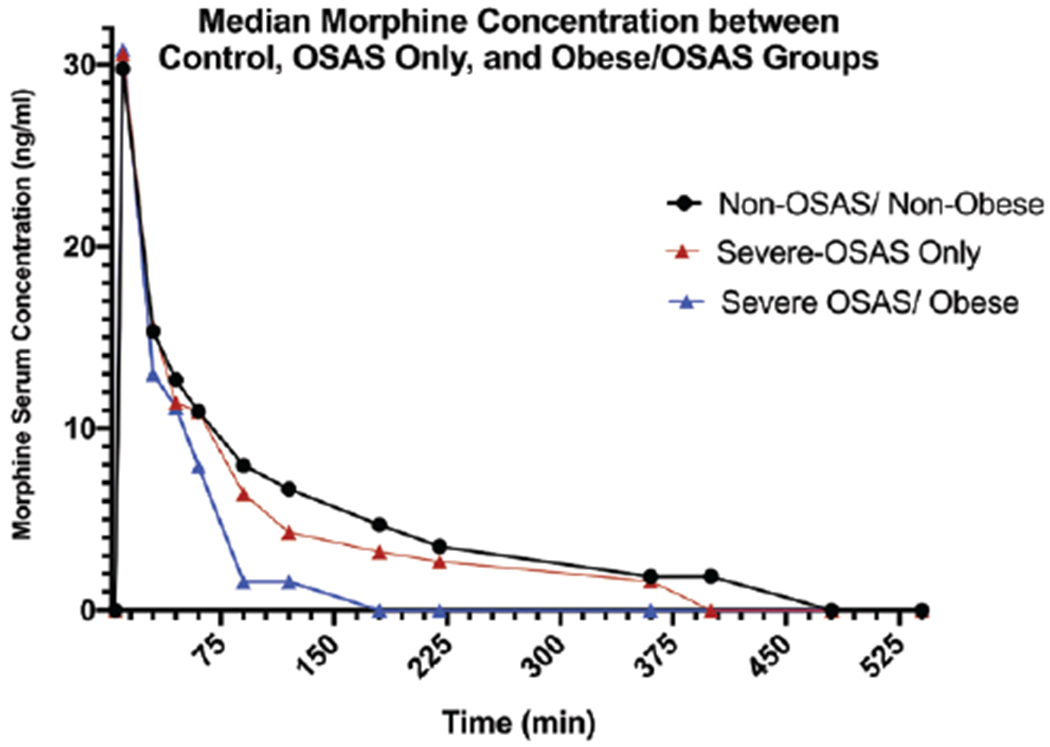
Comparison of mean serum morphine concentrations measured at preset time points after morphine injection in normal-weight patients without OSAS, patients with severe OSAS only, and obese patients with severe OSAS. OSAS indicates obstructive sleep apnea syndrome.
Statistical Analysis
A sample size calculation was performed based on our previous AUC calculations in obese and wild-type mice. With a sample size of 7 per group, we will have to have 90% power (type 1 error = 0.02). All pharmacokinetic parameters are summarized by descriptive statistics. Initially, univariate linear regression analysis was used to determine significance between pharmacokinetic parameters and demographic covariates, including anesthesia duration, sex, and race. These variables have been shown to affect liver metabolism and why they were chosen for univariate analysis. Weight was not analyzed because all obese patients had severe OSAS as a comorbid condition. Differences in demographic variables among groups were initially tested by the nonparametric Kruskal-Wallis test. In addition, a Dunn post hoc pairwise comparison with Bonferroni correction between groups was performed. Inflammatory biomarker concentration differences were analyzed using a Kruskal-Wallis test with a Dunn post hoc pairwise comparison test as well as multivariable linear regression models adjusting for weight, sex, and duration of anesthesia. These analyses were repeated for M3G and M6G pharmacokinetics (PK) parameters. Statistical analysis was performed using Stata v15.1 for Mac (StataCorp, College Station, TX), which adjusted P values in the results for an experiment-wise error rate of 0.05 for 3 comparison groups. Therefore, Bonferroni-adjusted P values <0.05 were considered statistically significant.
Results
A total of 331 patients were prescreened for obesity and OSAS. After those who met exclusion criteria were eliminated, the 2 primary reasons that patients were not enrolled were surgery occurring too late in the day and absence of polysomnogram to diagnose OSAS severity. Only 1 patient was recruited to the obese/no OSAS group because the families of most obese patients reported on the screening questionnaire that their child snored and/or had nocturnal apneas. This patient, and thus the obese/no OSAS group, was not included in the final analysis.
Excluding the 1 obese/no OSAS patient, 40 children were enrolled into the study as follows: nonobese/no OSAS (control, n = 18), OSAS only (n = 9), and obese with severe OSAS (n = 13). Overall, the mean age was 7.4 ± 0.4 years (mean ± standard error of the mean [SEM]), with slightly more males (63.4%) than females with predominantly Caucasians (46.3%) and African American (39%) participants. On average, ideal body weight dosing provided lower doses to the obese/OSAS group (0.04 mg/kg ±0.0005) than the other groups (0.05 ± 0.001; Table 1). Surgical procedures differed significantly between the groups. Patients in the control group primarily underwent genitourinary surgeries (92.9%), which were significantly longer in duration (426.4 ± 253 minutes) than the surgeries of the OSAS-only group (59.4 ± 9.3 minutes; P < .001) and obese/OSAS group (80.4 ± 44.5 minutes; P < .001). Patients with OSAS only underwent primarily ENT procedures, and those in the obese/OSAS group all underwent ENT procedures.
Pharmacokinetics
Pharmacokinetic analysis could not be completed for 6 patients who had too few data points (≤3 recorded morphine concentrations) or incomplete data. Data were incomplete if blood was not collected. Reasons for a missing blood draw included the inability to aspirate blood from the IV line, patient refusal, or IV line inaccessible during surgery. These missing data points appeared to be at random. Pharmacokinetic parameters for morphine could not be determined for 2 patients from the control group, 1 from the OSAS-only group, and 3 from the obese/OSAS group, leaving a total of 34 patients for analysis. When performing noncompartmental calculations, 1 subject in the control group and 1 in the OSAS-only group did not have all 3 analyte (MS, M3G, and M6G) curves fit within the established r2 ≥0.80 metric. In the control patient, there were several missing data points (due to the angiocatheter not drawing back), leading to a less accurate fit for M3G (r2 = 0.78) and M6G (r2 = 0.52). In the OSAS patient, there was a value at T0 (likely due to a contaminant) leading to a more poorly fit curve for morphine analysis (r2 = 0.71). Both subjects were retained in the analysis because data acquired were not outliers from the data points collected. PK parameters (except for AUC540) were not conducted for the M3G and M6G analytes.
Pharmacokinetic parameters of the control, OSAS only, and obese/OSAS groups are summarized in Table 2. Univariate linear regression analysis revealed no significant differences in morphine pharmacokinetic parameters when we evaluated the individual effects of race, sex, or anesthesia duration.
Table 2.
Pharmacokinetic Analysis for Morphine Sulfate
| Parameter | Control (N = 16) |
OSAS Only (N = 8) |
Obese and OSAS (N = 10) |
OSAS Only Versus Control (P Value) |
Obese and OSAS Versus Control (P Value) |
Obese and OSAS Versus OSAS Only (P Value) |
|---|---|---|---|---|---|---|
| Morphine | ||||||
| AUC540-adjust (ng·min/mL) | 2505.6 (±149.7) | 2083.0 (±194.6) | 2206.6 (±316.0) | .17 | .72 | .60 |
| CMAX-adjust | 30.1 (±3.4) | 29.0 (±2.6) | 47.6 (±4.0) | 1.0 | <.001 | .01 |
| AUC540 (ng·min/mL) | 2505.6 (±598.6) | 2083.0 (±550.5) | 1537.9 (±764.6) | .16 | .004 | .41 |
| CMAX (ng/mL) | 30.1 (±13.5) | 29.0 (±7.4) | 33.5 (±12.3) | .87 | .55 | 1.0 |
| TMAX (min) | 10 (10, 60) | 10 (10, 45) | 10 (10, 10) | .70 | .14 | .67 |
| T1/2 (min) | 138.0 (27.1, 780.1) | 171.9 (53.3, 898.8) | 61.8 (22.9, 392.9) | 1.0 | .13 | .13 |
| Clearance (mL/min/kg) | 0.02 (±0.001) | 0.02 (±0.003) | 0.02 (±0.004) | .70 | .28 | .96 |
| Vd (mL/kg) | 0.18 (±0.03) | 0.29 (±0.09) | 0.09 (±0.03) | .32 | .07 | .01 |
| M3G | ||||||
| CMAX (ng/mL) | 41.4 (±4.6) | 45.5 (±3.3) | 56.4.0 (±4.1) | .52 | .01 | .27 |
| TMAX (min) | 90 (30,220) | 90 (45, 180) | 60 (30,120) | .82 | .03 | .30 |
| M6G | ||||||
| TMAX (min) | 120 (90,480) | 90 (45, 90) | 60 (45, 120) | <.002 | <.001 | 1.0 |
| M3G:MS ratio | 5.5 (±0.76) | 6.0 (±0.79) | 10.1 (±1.83) | .5 | .01 | .24 |
| M6G:MS ratio | 0.001 (±0.0004) | 0.0007 (±0.0002) | 0.0009 (±0.0002) | .67 | .05 | .46 |
Pharmacokinetic data are expressed as mean (±SEM) with exception of TMAX and T1/2, which is reported as median (min, max). Comparisons were made by Kruskal-Wallis rank sums test, and family-wise error rate is controlled using Dunn post hoc test with Bonferroni adjustment (STATA SE v15.1). Only statistically significant M3G and M6G PK parameters are shown in the table.
Abbreviations: AUC, area under curve; AUC-adjust, AUC calculation dose adjusted in obese group; CMAX, maximum concentration; CMAX-adjust, CMAX calculation dose adjusted in obese group; M3G, morphine 3-glucuronide; M6G, morphine 6-glucuronide; MS, morphine sulfate; OSAS, obstructive sleep apnea syndrome; PK, pharmacokinetics; SEM, standard error of the mean; T1/2, half-life; TMAX, time to maximum concentration; Vd, volume of distribution.
Morphine Pharmacokinetics
There was evidence that patients with obesity and OSAS have different morphine pharmacokinetics compared to that of controls. PK parameters evaluated include area under curve (AUC), clearance, CMAX, Kel, TMAX, and Vd. The mean morphine AUC540 of the obese/OSAS group (1537.9 ng-min/mL) was significantly lower than that of the control group (2505.6 ng·min/mL, χ2 = 9.4, P = .004). However, after dose adjusting for AUC540 in the obese/OSAS group (2206.6 ng·min/mL), there was no longer a significant difference in AUC540 compared to the controls (χ2 = 2.5, P = .72). After dose adjusting for CMAX calculations, the CMAX for the obese/OSAS group (47.6 ng/mL) was found to be significantly higher than both the control (30.1 ng/mL, χ2 = 12.7, P < .001) and the OSAS-only groups (29.0 ng/mL, χ2 = 12.7, P = .01; Figure 2). The Vd also differed between the groups, with the obese/OSAS group having a significantly lower Vd (0.09 mL/kg) than the OSAS only (0.29 mL/kg, χ2 = 8.5, P = .007).
Figure 2.
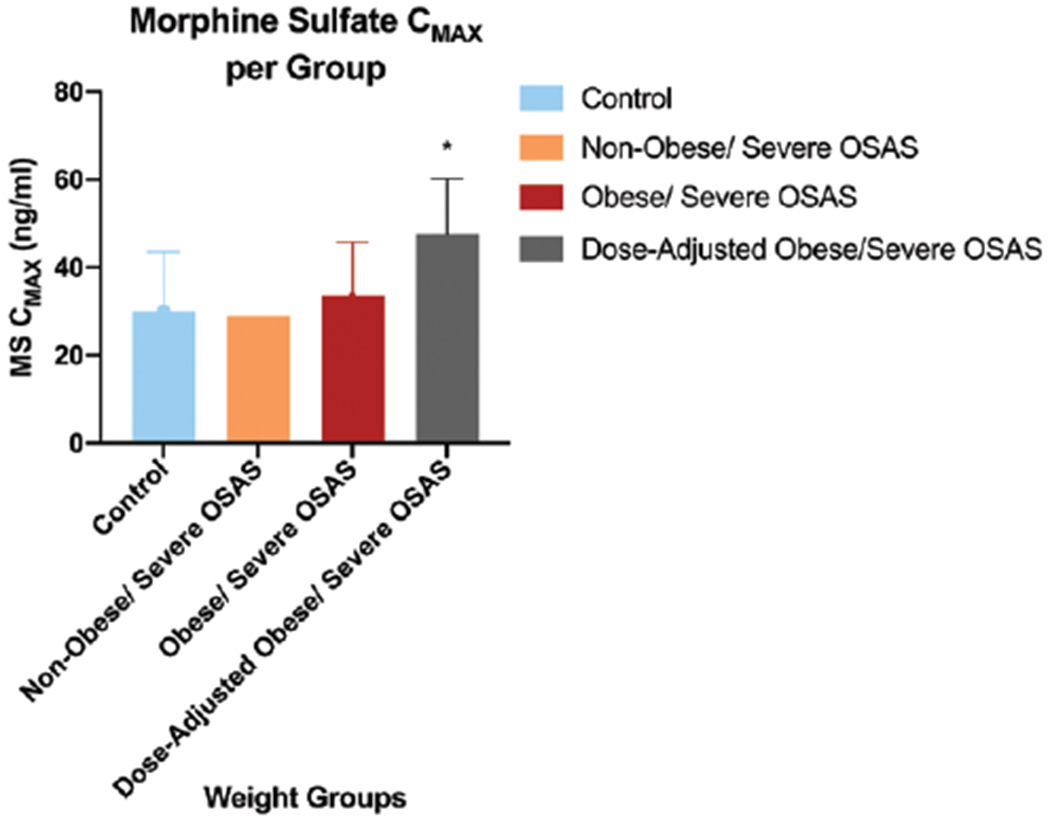
CMAX of MS. Comparison of mean (±SD) CMAX for plasma morphine during 540 minutes of blood sampling time between normal-weight, non-OSAS controls, nonobese OSAS, and obese patients with OSAS. Dose-adjusted mean CMAX in obese patients with OSAS is also presented. *P < .05 versus the nonobese/non-OSAS control group. CMAX indicates maximum concentration; MS, morphine sulfate; OSAS, obstructive sleep apnea syndrome; SD, standard deviation.
M3G Pharmacokinetics
Patients with obesity and OSAS also had significant changes in M3G CMAX and M3G-to-morphine sulfate ratio when compared to controls (Table 2). Patients in the obese/OSAS group had a higher CMAX (56.4 ng/mL) than did control patients (41.4, χ2 = 7.1, P = .01). The ratio of M3G to morphine was also higher in the obese/OSAS group (10.1) than in the control group (5.5, χ2 = 7.1, P = .01).
M6G Pharmacokinetics
One patient from the control group was removed for M6G evaluation owing to an extremely high level. TMAX significantly differed among the groups, whereby the median TMAX was significantly lower in both OSAS-only (90 minutes) and obese/OSAS (60 minutes) groups than in the control group (120 minutes, χ2 = 18.6, P < .005). The M6G:morphine ratio was also lower in the obese/OSAS group (0.0009) compared to controls, but was not significant (0.001, χ2 = 4.4, P = .053).
Biomarker Analysis
Insulin was measured in every patient, and leptin levels were measured in all but 2 patients (both in the OSAS-only group). Levels of CRP were not analyzed in 5 patients from the obese/OSAS group, and inflammatory biomarkers, including IL-6, IL-10, and TNF-α, failed to be analyzed in 7 patients (2 in the OSAS-only group and 5 in the obese/OSAS group), and IL-1β failed to be measured in 13 patients (6 in the control group, 2 in the OSAS-only group, and 5 in the obese/OSAS group) owing to sample processing error.
The obese/OSAS group had significantly higher mean values of the inflammatory markers CRP (χ2 = 10.1, P = .004), IL-6, (χ2 = 8.4, P = .006), and IL-10 (χ2 = 6.4, P = .03) than did controls. In addition, the obese/OSAS group had higher CRP (χ2 = 10.1, P = .01), insulin (χ2 = 8.5, P = .03), and leptin (χ2 = 14.4, P = .003) levels than did the OSAS-only group. Insulin levels were significantly lower in the OSAS-only group than in the control group (χ2 = 8.5, P = .01; Table 3 and Figure 3).
Table 3.
Comparison of Inflammatory Biomarker Levels Between Patients With Obstructive Sleep Apnea Only and Those With Obesity and Obstructive Sleep Apnea
| Biomarker | Control | OSAS Only | Obese and OSAS |
|---|---|---|---|
| CRP (μg/mL) | 0.85 (±1.3) | 1.78 (±3.8) | 4.53 (±4.2)a,b |
| n = 18 | n = 9 | n = 8 | |
| IL-1β (pg/mL) | 0.22 (±0.41) | 0.17 (±0.07) | 0.18 (±0.16) |
| n = 12 | n = 7 | n = 8 | |
| IL-10 (pg/mL) | 0.46 (±0.16) | 0.59 (±0.17) | 1.0 (±0.70)a |
| n = 18 | n = 7 | n = 8 | |
| IL-6 (pg/mL) | 0.59 (±0.26) | 0.74 (±0.29) | 1.02 (±0.25)a |
| n = 18 | n = 7 | n = 8 | |
| Insulin (μU/mL) | 10.2 (±10.4) | 3.6 (±2.8)c | 7.6 (±3.6) |
| n = 18 | n = 9 | n = 13 | |
| Leptin (ng/mL) | 7.4 (±8.8) | 4.5 (±2.1) | 28.8 (±21.3)a,b |
| n = 18 | n = 7 | n = 13 | |
| TNF-α (pg/mL) | 3.0 (±1.2) | 2.6 (±0.7) | 3.5 (±1.1) |
| n = 18 | n = 7 | n = 8 |
Results are expressed as mean (SD). A total of 40 participants underwent biomarker analysis; however, due to lab error or insufficient sampling, not all biomarkers were performed on all subjects. The sample size for each biomarker per group is listed below mean (SD). Comparisons were made by Kruskal-Wallis rank sums test and Dunn post hoc method using Bonferroni correction.
Abbreviations: CRP C-reactive protein; IL, interleukin; OSAS, obstructive sleep apnea syndrome; SD, standard deviation; TNF, tumor necrosis factor.
P < .05, control versus obese and OSAS.
P < .05, OSAS only versus obese and OSAS.
P < .05, control versus OSAS only.
Figure 3.
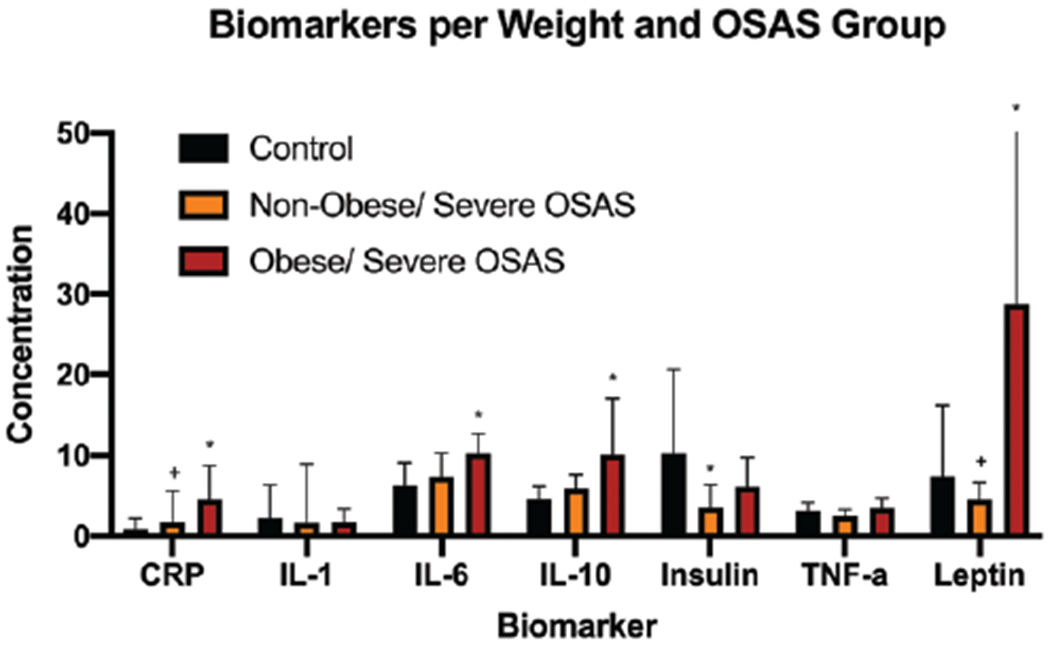
Comparison of biomarker concentrations between normal-weight and obese patients with and without OSAS. *P < .05 versus nonobese/non-OSAS control group. +P < .05 versus obese/OSAS group. Units of measurement are as follows: CRP (μg/mL), IL-1β (pg/mL), IL-10 (pg/mL), insulin (μU/mL), leptin (ng/mL), TNF-α (pg/mL). Concentrations of IL-1, IL-6, and IL-10 were multiplied by 10 to optimize visual comparisons on the graph. CRP indicates C-reactive protein; IL, interleukin; OSAS, obstructive sleep apnea syndrome; TNF, tumor necrosis factor.
Leptin was significantly higher in the obese/OSAS group (28.8 ± 21.3) than in the control (7.4 ± 8.8, χ2 = 18.7, P < .001) or OSAS-only group (4.5 ± 2.1, χ2 = 18.7, P < .002). A multivariable linear regression established that leptin could predict dose-adjusted morphine AUC540 (F(4, 28) = 3.3; P = .002; 95% confidence interval [CI], −64.1 to −16.0) and that leptin accounted for 22.1% of the variability in morphine AUC540. Leptin levels did not predict morphine Vd in this analysis (P = .66).
DISCUSSION
Obesity with a comorbid diagnosis of severe OSAS has a pronounced effect on morphine pharmacokinetics. Our findings show that obese patients with severe OSAS have higher peak intravascular morphine concentrations compared to normal-weight patients and those with OSAS. Also, obese patients with severe OSAS had a lower morphine Vd than patients with OSAS alone. In addition, the M3G-to-morphine ratio was significantly higher in the obese/OSAS group than in the control group, providing evidence of a more rapid metabolism.
Reports from previous studies have been conflicting with regard to the effects of obesity on liver enzyme function and morphine pharmacokinetics. Murine and rat studies showed decreases in UGT RNA, and UGT enzyme activity was decreased by as much as 60% in mice fed a high-fat diet as compared to that in lean mice. One study in adult humans showed that clearance of morphine metabolites was delayed in obese patients compared with that in lean controls.30 Lloret-Linares et al31 studied the effects of weight change, specifically after gastric bypass, on oral morphine pharmacokinetics and showed that morphine absorption increased drastically after weight loss, increasing AUC and morphine exposure in these patients. Changes to the gut microbiome were suggested as a possible cause for these observations.32,33 By contrast, several studies in obese humans showed increased UGT activity leading to higher morphine glucuronidation and clearance.34 Studies comparing obese to nonobese adults and children showed increased clearance of UGT-mediated medications, including paracetamol, oxazepam, and lorazepam.35,36 Our results are consistent with these later studies, showing that morphine pharmacokinetics was enhanced in obese subjects compared to normal-weight controls.
The mechanisms by which obesity affects UGT activity are not well known. One potential regulatory element is leptin, an adipokine secreted by adipose tissue. In an obese murine model, Miyamoto et al37 showed reduction in 5-adenosine monophosphate (AMP)-activated protein kinase and reduced triglyceride levels in the liver. In addition, we previously showed that leptin-deficient obese (ob/ob) mice had decreased morphine metabolism that normalized to that of wild-type lean mice after they received leptin replacement.13 Diet-induced obese mice did not show substantial changes to morphine metabolism when compared to wild-type mice, suggesting that leptin deficiency and not obesity alone predominantly affected morphine metabolism. UGT activity has been shown to decrease with weight loss.31 How obesity affects the specific subunits of UGT involved in morphine metabolism, however, remains unclear. In our current study, leptin levels directly predicted morphine AUC540 and clearance suggesting that it might activate phase I and/or phase II enzymes, including UGT, as 1 possible mechanism for increasing morphine metabolism.
Our results have several clinical implications for the care of obese children with OSAS who require opioid therapy. We have shown that when dosing obese patients based on the recommended ideal body weight, a significantly lower amount of morphine is present in the vasculature (lower AUC). Patients with obesity have a higher incidence of chronic pain,38 and murine models of obesity indicate a higher incidence of postoperative pain. However, by dose-adjusting PK parameters, we can compare morphine exposure among groups, mimicking what would happen if an obese child were dosed based on their actual body weight. Our results show that higher morphine concentrations are achieved in obese patients with OSAS, as described by higher CMAX with similar clearance, which is likely caused by the hydrophilic properties of morphine and its metabolites. By dosing on actual body weight, our calculations suggest that a higher morphine concentration will be achieved; however, due to morphine’s hydrophilicity, more of the drug will stay within the vasculature, distribute to the brain, and may increase the risk for respiratory depression. Obese patients are at a higher risk of respiratory complications and upper airway obstruction after anesthesia and higher intravascular morphine concentrations may be a significant contributor to this observation.
A higher M3G:morphine ratio also suggests increased morphine metabolism in patients who are obese with OSAS. Because morphine is metabolized more quickly in obese children with OSAS undergoing surgery, multimodal pain therapies should be implemented that include medications with minimal to no respiratory side effects. In addition to a multimodal approach, morphine dose reduction with a more frequent dosing schedule may optimize postoperative pain while minimizing postoperative respiratory events.
Leptin was significantly higher in obese/OSAS patients than in either controls or those with only OSAS, suggesting that it might mediate the increase in morphine metabolism. Because leptin is also a respiratory stimulant, postoperative leptin administration may be an ideal adjunctive medication with morphine for nonobese patients at increased risk for postoperative respiratory complications. In addition, leptin administration could increase morphine metabolism in patients experiencing an overdose and, unlike naloxone, increase morphine metabolism without causing complete opioid pain blockade that may lead to hyperalgesia. Future studies are necessary to better understand the mechanism by which leptin affects morphine pharmacokinetics as well as the potential role of leptin as a short-term adjunctive therapy.
Our study had several limitations. First, we were only able to recruit 1 patient with obesity but no OSAS due to high frequency of snoring in obese children. To evaluate the effects of obesity alone, 1 possible population to study would be obese patients presenting for AT who have been diagnosed by preoperative polysomnogram with mild OSAS. However, these patients do not have prolonged postoperative hospital stays that would allow for adequate blood sampling time. Second, patients from the control group underwent anesthesia for longer periods of time than those in the other groups. Although having sedated patients facilitated blood draws, inhalational anesthesia can affect hepatic blood flow, primarily reducing portal blood flow,39 and may affect morphine metabolism. Our findings indicated that morphine metabolism was faster in those undergoing longer surgery durations. Shorter surgeries in otherwise healthy patients often did not warrant hospitalization postoperatively, limiting the pool of available control patients with shorter duration surgeries. Also, we were unable to assess some pharmacokinetic parameters, specifically elimination parameters, for M3G and M6G owing to an insufficient period of postdose sampling.
CONCLUSIONS
Obesity and OSAS increased morphine metabolism compared to that in normal-weight controls. A previous study from our laboratory showed in a murine model that obesity from leptin deficiency decreased morphine metabolism, which normalized after leptin replacement. In the current study, obesity caused elevated leptin levels, suggesting that leptin may be the cause of increased morphine metabolism observed in obese patients with OSAS. Additional studies are required to determine more optimal regimens for opioid administration in obese pediatric patients to avoid the higher risk of respiratory depression with higher peak concentrations and inadequate pain management with altered clearance.
KEY POINTS.
Question: Does obesity and obstructive sleep apnea affect morphine pharmacokinetics in children?
Findings: In obese children with obstructive sleep apnea syndrome (OSAS), lower dosed morphine concentrations reach higher intravascular levels than nonobese, non-OSAS children.
Meaning: Morphine dose adjustments should be made for patients with obstructive sleep apnea and obesity.
Acknowledgments
Funding: This work received funding from Society of Pediatric Anesthesiology (SPA) Young Investigator Award (No. 123836). Principle Investigator: Nicholas M. Dalesio, MD, MPH.
GLOSSARY
- AMP
adenosine monophosphate
- ASA
American Society of Anesthesiologists
- AT
adenotonsillectomy
- AUC
area under curve
- CI
confidence interval
- CMAX
maximum concentration
- CRP
C-reactive protein
- ENT
ear/nose/throat
- IL
interleukin
- IRB
institutional review board
- IV
intravenous
- Kel
elimination rate constant
- M3G
morphine 3-glucuronide
- M6G
morphine 6-glucuronide
- MRM
multiple reaction monitoring mode
- MW
molecular weight
- OSAS
obstructive sleep apnea syndrome
- PK
pharmacokinetics
- SEM
standard error of the mean
- SML
suspension microlaryngoscopy
- T1/2
half-life
- TMAX
time to maximum concentration
- TNF
tumor necrosis factor
- UGT
uridine diphosphate glucuronosyltransferase
- Vd
volume of distribution
Footnotes
The authors declare no conflicts of interest.
Institutional review board: Approved by the Johns Hopkins Institutional Review Board (No. IRB00059675) and conducted in accordance with its guidelines. Tel: 410-502-2092; 1620 McElderry St, Reed Hall - B130, Baltimore, MD 21205-1911; jhmeirb@jhmi.edu.
Reprints will not be available from the authors.
REFERENCES
- 1.Jimenez N, Posner KL, Cheney FW, Caplan RA, Lee LA, Domino KB. An update on pediatric anesthesia liability: a closed claims analysis. Anesth Analg. 2007;104:147–153. [DOI] [PubMed] [Google Scholar]
- 2.Statham MM, Elluru RG, Buncher R, Kalra M. Adenotonsillectomy for obstructive sleep apnea syndrome in young children: prevalence of pulmonary complications. Arch Otolaryngol Head Neck Surg. 2006;132:476–480. [DOI] [PubMed] [Google Scholar]
- 3.Cheng J, Elden L. Outcomes in children under 12 months of age undergoing adenotonsillectomy for sleep-disordered breathing. Laryngoscope. 2013;123:2281–2284. [DOI] [PubMed] [Google Scholar]
- 4.Gleich SJ, Olson MD, Sprung J, et al. Perioperative outcomes of severely obese children undergoing tonsillectomy. Paediatr Anaesth. 2012;22:1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwengel DA, Sterni LM, Tunkel DE, Heitmiller ES. Perioperative management of children with obstructive sleep apnea. Anesth Analg. 2009;109:60–75. [DOI] [PubMed] [Google Scholar]
- 6.Sanders JC, King MA, Mitchell RB, Kelly JP. Perioperative complications of adenotonsillectomy in children with obstructive sleep apnea syndrome. Anesth Analg. 2006;103:1115–1121. [DOI] [PubMed] [Google Scholar]
- 7.Brown KA, Laferrière A, Moss IR. Recurrent hypoxemia in young children with obstructive sleep apnea is associated with reduced opioid requirement for analgesia. Anesthesiology. 2004;100:806–810; discussion 5A. [DOI] [PubMed] [Google Scholar]
- 8.Savransky V, Reinke C, Jun J, et al. Chronic intermittent hypoxia and acetaminophen induce synergistic liver injury in mice. Exp Physiol. 2009;94:228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raghavendran S, Bagry H, Detheux G, Zhang X, Brouillette RT, Brown KA. An anesthetic management protocol to decrease respiratory complications after adenotonsillectomy in children with severe sleep apnea. Anesth Analg. 2010;110:1093–1101. [DOI] [PubMed] [Google Scholar]
- 10.Wu J, Li P, Wu X, Chen W. Chronic intermittent hypoxia decreases pain sensitivity and increases the expression of HIF1α and opioid receptors in experimental rats. Sleep Breath. 2015;19:561–568. [DOI] [PubMed] [Google Scholar]
- 11.Rabbitts JA, Groenewald CB, Dietz NM, Morales C, Räsänen J. Perioperative opioid requirements are decreased in hypoxic children living at altitude. Paediatr Anaesth. 2010;20:1078–1083. [DOI] [PubMed] [Google Scholar]
- 12.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–1532. [DOI] [PubMed] [Google Scholar]
- 13.Dalesio NM, Hendrix CW, McMichael DH, et al. Effects of obesity and leptin deficiency on morphine pharmacokinetics in a mouse model. Anesth Analg. 2016;123:1611–1617. [DOI] [PubMed] [Google Scholar]
- 14.Dalesio NM, Lee CKK, Hendrix CW. In response. Anesth Analg. 2017;125:362–363. [DOI] [PubMed] [Google Scholar]
- 15.Lloret Linares C, Declèves X, Oppert JM, et al. Pharmacology of morphine in obese patients: clinical implications. Clin Pharmacokinet. 2009;48:635–651. [DOI] [PubMed] [Google Scholar]
- 16.Nasrallah MP, Ziyadeh FN. Overview of the physiology and pathophysiology of leptin with special emphasis on its role in the kidney. Semin Nephrol. 2013;33:54–65. [DOI] [PubMed] [Google Scholar]
- 17.Gozal D. Sleep, sleep disorders and inflammation in children. Sleep Med. 2009;10(suppl 1):S12–S16. [DOI] [PubMed] [Google Scholar]
- 18.Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med. 2008;177:1142–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cullen A, Ferguson A. Perioperative management of the severely obese patient: a selective pathophysiological review. Can J Anaesth. 2012;59:974–996. [DOI] [PubMed] [Google Scholar]
- 20.Tauman R, Serpero LD, Capdevila OS, et al. Adipokines in children with sleep disordered breathing. Sleep. 2007;30:443–449. [DOI] [PubMed] [Google Scholar]
- 21.Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118:580–586. [DOI] [PubMed] [Google Scholar]
- 22.Capdevila OS, Kheirandish-Gozal L, Dayyat E, Gozal D. Pediatric obstructive sleep apnea: complications, management, and long-term outcomes. Proc Am Thorac Soc. 2008;5:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iber C; Medicine AAOS. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Winchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 24.Barlow SE; Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164–S192. [DOI] [PubMed] [Google Scholar]
- 25.Meineke I, Freudenthaler S, Hofmann U, et al. Pharmacokinetic modelling of morphine, morphine-3-glucuronide and morphine-6-glucuronide in plasma and cerebrospinal fluid of neurosurgical patients after short-term infusion of morphine. Br J Clin Pharmacol. 2002;54:592–603. [DOI] [PubMed] [Google Scholar]
- 26.Sartori D, Lewis T, Breaud A, Clarke W. The development of a high-performance liquid chromatography-tandem mass spectrometric method for simultaneous quantification of morphine, morphine-3-β-glucuronide, morphine-6-β-glucuronide, hydromorphone, and normorphine in serum. Clin Biochem. 2015;48:1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabrielsson J, Weiner D. Non-compartmental analysis. Methods Mol Biol. 2012;929:377–389. [DOI] [PubMed] [Google Scholar]
- 28.Stanski DR, Greenblatt DJ, Lowenstein E. Kinetics of intravenous and intramuscular morphine. Clin Pharmacol Ther. 1978;24:52–59. [DOI] [PubMed] [Google Scholar]
- 29.Gibaldi M, Perrier D. Noncompartmental analysis based on statistical moment theory In: Pharmacokinetics, Revised and Expanded. 2nd ed. New York, NY: Marcel Dekker; 1982:409–419. [Google Scholar]
- 30.de Hoogd S, Välitalo PAJ, Dahan A, et al. Influence of morbid obesity on the pharmacokinetics of morphine, morphine-3-glucuronide, and morphine-6-glucuronide. Clin Pharmacokinet. 2017;56:1577–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lloret-Linares C, Hirt D, Bardin C, et al. Effect of a roux-en-y gastric bypass on the pharmacokinetics of oral morphine using a population approach. Clin Pharmacokinet. 2014;53:919–930. [DOI] [PubMed] [Google Scholar]
- 32.Lloret-Linares C, Scherrmann JM, Declèves X. The effect of morbid obesity on morphine glucuronidation. Pharmacol Res. 2016;114:299–300. [DOI] [PubMed] [Google Scholar]
- 33.Kang M, Mischel RA, Bhave S, et al. The effect of gut microbiome on tolerance to morphine mediated antinociception in mice. Sci Rep. 2017;7:42658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brill MJ, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CA. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51:277–304. [DOI] [PubMed] [Google Scholar]
- 35.Mutlib AE, Goosen TC, Bauman JN, Williams JA, Kulkarni S, Kostrubsky S. Kinetics of acetaminophen glucuronidation by UDP-glucuronosyltransferases 1A1, 1A6, 1A9 and 2B15. Potential implications in acetaminophen-induced hepatotoxicity. Chem Res Toxicol. 2006;19:701–709. [DOI] [PubMed] [Google Scholar]
- 36.Court MH, Duan SX, Guillemette C, et al. Stereoselective conjugation of oxazepam by human UDP-glucuronosyltransferases (UGTs): S-oxazepam is glucuronidated by UGT2B15, while R-oxazepam is glucuronidated by UGT2B7 and UGT1A9. Drug Metab Dispos. 2002;30:1257–1265. [DOI] [PubMed] [Google Scholar]
- 37.Miyamoto L, Ebihara K, Kusakabe T, et al. Leptin activates hepatic 5′-AMP-activated protein kinase through sympathetic nervous system and α1-adrenergic receptor: a potential mechanism for improvement of fatty liver in lipodystrophy by leptin. J Biol Chem. 2012;287:40441–40447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raebel MA, Newcomer SR, Reifler LM, et al. Chronic use of opioid medications before and after bariatric surgery. JAMA. 2013;310:1369–1376. [DOI] [PubMed] [Google Scholar]
- 39.Frink EJ Jr, Morgan SE, Coetzee A, Conzen PF, Brown BR Jr. The effects of sevoflurane, halothane, enflurane, and isoflurane on hepatic blood flow and oxygenation in chronically instrumented greyhound dogs. Anesthesiology. 1992;76:85–90. [DOI] [PubMed] [Google Scholar]


