Key Points
Question
From 2007 to 2017, an era of focus on reducing heart failure readmissions, how did readmission and mortality rates after heart failure admission change in the Veterans Affairs Health Care System?
Findings
This cohort study of 304 374 heart failure hospital admissions from 164 566 patients from January 2007 to September 2017 shows that after risk adjustment, the odds of readmission steadily declined, but there was no significant change in mortality rates.
Meaning
Per this analysis, patients in the Veterans Affairs Health Care System have experienced similar heart failure readmission rate reductions as Medicare patients in Inpatient Prospective Payment System Hospitals, without a change in mortality despite use of nonfinancial incentives.
This cohort study evaluates trends in readmission and mortality after heart failure admission in the Veterans Affairs Health Care System, which had no financial penalties, from 2007 to 2017.
Abstract
Importance
The Centers for Medicare & Medicaid Services and the Veterans Affairs Health Care System provide incentives for hospitals to reduce 30-day readmission and mortality rates. In contrast with the large body of evidence describing readmission and mortality in the Medicare system, it is unclear how heart failure readmission and mortality rates have changed during this period in the Veterans Affairs Health Care System.
Objectives
To evaluate trends in readmission and mortality after heart failure admission in the Veterans Affairs Health Care System, which had no financial penalties, in a decade involving focus on heart failure readmission reduction (2007-2017).
Design, Setting, and Participants
This cohort study used data from all Veterans Affairs–paid heart failure admissions from January 2007 to September 2017. All Veterans Affairs–paid hospital admissions to Veterans Affairs and non–Veterans Affairs facilities for a primary diagnosis of heart failure were included, when the admission was paid for by the Veterans Affairs. Data analyses were conducted from October 2018 to March 2020.
Exposures
Admission for a primary diagnosis of heart failure at discharge.
Main Outcomes and Measures
Thirty-day all-cause readmission and mortality rates.
Results
A total of 164 566 patients with 304 374 hospital admissions were included. Among the 304 374 hospital admissions between 2007 and 2017, 298 260 (98.0%) were for male patients, and 195 205 (64.4%) were for white patients. The mean (SD) age was 70.8 (11.5) years. The adjusted odds ratio of 30-day readmission declined throughout the study period to 0.85 (95% CI, 0.83-0.88) in 2015 to 2017 compared with 2007 to 2008. The adjusted odds ratio of 30-day mortality remained stable, with an adjusted odds ratio of 1.01 (95% CI, 0.96-1.06) in 2015 to 2017 compared with 2007 to 2008. Stratification by left ventricular ejection fraction showed similar readmission reduction trends and no significant change in mortality, regardless of strata.
Conclusions and Relevance
In this analysis of an integrated health care system that provided guidance and nonfinancial incentives for reducing readmissions, such as public reporting of readmission rates, risk-adjusted 30-day readmission declined despite inclusion of clinical variables in risk adjustment, but mortality did not decline. Future investigations should focus on evaluating the effectiveness of specific approaches to readmission reduction to inform efficient and effective application in individual health systems, hospitals, and practices.
Introduction
In the past decade, reducing readmission rates after heart failure (HF) hospitalization has become a national priority. Heart failure is a highly prevalent and costly disease, with 6.5 million Americans affected between 2011 and 2014 and an annual cost of $30.7 billion, 80% of which is associated with hospitalizations.1 In October 2012, the US Centers for Medicare & Medicaid Services (CMS) initiated the Hospital Readmissions Reduction Program (HRRP), which targets HF readmission reduction through financial penalties on hospitals with high readmission rates.2 Readmission for HF decreased following the implementation of HRRP; however, studies have raised concern for an associated mortality increase.3,4,5,6,7,8
Around the same time, the Veterans Affairs Health Care System (VAHCS) made decreasing HF readmissions a priority. Interventions included public reporting, beginning in July 2012; incorporation of HF 30-day readmission rate into hospitals’ overall star ratings, beginning in July 2014; and encouragement of participation in transitions of care programs, such as the American College of Cardiology Hospital to Home Initiative (most VAHCS hospitals participated by 2009).9,10 The goal of our study was to evaluate trends in HF readmissions and mortality over the past decade in the VAHCS, an integrated health care system that prioritized reducing readmissions without introducing financial penalties.
Methods
We used patient-level data from all VA-paid HF admissions (VA and non-VA facilities) in January 2007 through September 2017. A Stanford University institutional review board approved this study and included a waiver of consent because the study involves the secondary analysis of clinical data.
Heart failure admissions were defined as any admission with a primary diagnosis of HF at discharge. For comparison of trends over time, we created 5 multiyear groups (2007-2008, 2009-2010, 2011-2012, 2013-2014, and 2015-2017), and within each group, we randomly selected 1 admission per patient.
Patient characteristics are detailed in the Table and the eTable in the Supplement, including medical record–defined race/ethnicity and sex. Left ventricular ejection fraction (LVEF) data were obtained from natural-language processing of medical records.11 Veterans Affairs pharmacy records were used to determine filled insulin prescriptions, because this medication has been shown to be an independent risk factor for worse HF outcomes.12 Heart failure treatment decisions, such as β-blocker use, were not included, because this is associated with care quality that may drive temporal changes in outcomes.
Table. Select Baseline Patient Characteristicsa.
| Characteristic | Patients, No. (%) | P value | ||||
|---|---|---|---|---|---|---|
| 2007-2008 | 2009-2010 | 2011-2012 | 2013-2014 | 2015-2017 | ||
| Total (N = 304 374) | 52 748 | 56 767) | 57 117 | 59 684 | 78 058 | NA |
| Age, mean (SD), y | 70.0 (11.8) | 70.5 (11.7) | 70.6 (11.7) | 70.9 (11.5) | 71.7 (11.1) | <.001 |
| Sex | ||||||
| Female | 958 (1.82) | 1026 (1.81) | 1128 (1.97) | 1236 (2.07) | 1766 (2.26) | <.001 |
| Male | 51 790 (98.2) | 55 741 (98.2) | 55 989 (98.0) | 58 448 (97.9) | 76 292 (97.7) | |
| Race/ethnicity | ||||||
| Hispanic | 1204 (2.29) | 1337 (2.36) | 1403 (2.47) | 2302 (3.87) | 4265 (5.48) | <.001 |
| Black | 14 334 (27.23) | 15 012 (26.52) | 15 625 (27.46) | 20 775 (34.87) | 23 652 (30.39) | |
| Asian | 317 (0.6) | 356 (0.63) | 450 (0.79) | 541 (0.91) | 572 (0.74) | |
| Native American | 425 (0.81) | 499 (0.88) | 545 (0.96) | 855 (1.44) | 808 (1.04) | |
| Pacific Islander | 329 (0.63) | 331 (0.58) | 390 (0.69) | 467 (0.79) | 615 (0.9) | |
| White | 35 907 (68.2) | 38 914 (68.8) | 38 332 (67.4) | 34 350 (57.8) | 47 702 (61.3) | |
| Declined to state | 92 (0.17) | 115 (0.2) | 109 (0.19) | 93 (0.16) | 171 (0.22) | |
| Other | 26 (0.05) | 41 (0.07) | 42 (0.07) | 31 (0.05) | 38 (0.05) | |
| Vital signs, mean (SD)b | ||||||
| Systolic blood pressure, mm Hg | 135.4 (23.3) | 135.7 (22.3) | 135.8 (22.2) | 136.2 (22.2) | 136.5 (22.1) | <.001 |
| Pulse rate, beats/min | 81.2 (17.4) | 81.1 (17.2) | 81.8 (17.0) | 82.3 (17.1) | 82.6 (16.8) | <.001 |
| Respiratory rate, breaths/min | 20.2 (2.9) | 20.0 (2.8) | 19.8 (2.7) | 19.6 (2.7) | 19.3 (2.6) | <.001 |
| Weight, kg | 95.3 (26.0) | 96.6 (26.6) | 97.1 (26.8) | 97.7 (26.7) | 98.5 (27.2) | <.001 |
| Comorbidities in last 2 y | ||||||
| Ischemic heart disease | 41 655 (79.0) | 45 824 (80.7) | 45 707 (80.0) | 47 383 (79.4) | 61 087 (78.3) | <.001 |
| Diabetes | 31 376 (59.5) | 35 249 (62.1) | 36 016 (63.1) | 37 973 (63.6) | 49 792 (63.8) | <.001 |
| Hypertension | 49 016 (92.9) | 54 240 (95.6) | 54 626 (95.6) | 57 302 (96) | 74 988 (96.1) | <.001 |
| Malignant condition | 8608 (16.3) | 10 558 (18.6) | 10 352 (18.1) | 11 223 (18.8) | 14 632 (18.8) | <.001 |
| Left ventricular ejection fraction | ||||||
| Mean (SD), %c | 41.3 (15.6) | 42.6 (15.5) | 43.2 (15.5) | 43.5 (15.2) | 43.9 (15.0) | <.001 |
| Ejection fraction <40% (n = 47 790) | 10 130 (45.0) | 9338 (40.8) | 8731 (39.7) | 9048 (38.4) | 10 543 (37.9) | |
| No./total No. | 10 130/22 527 | 9338/22 896 | 8731/22 004 | 9048/23 570 | 10 543/27 797 | |
| Ejection fraction ≥40% (n = 71 004) | 12 397 (55.0) | 13 558 (59.2) | 13 273 (60.3) | 14 522 (61.6) | 17 254 (62.1) | |
| No./total No. | 12 397/22 527 | 13 558/22 896 | 13 273/22 004 | 14 522/23 570 | 17 254/27 797 | |
| B-type natriuretic peptide level in last 6 mo, quintile, No. (%)d,e | ||||||
| 1 | 5678 (15.5) | 7096 (15.5) | 7807 (16.7) | 8483 (17.2) | 12 090 (18.3) | <.001 |
| 2 | 6049 (16.6) | 8426 (18.4) | 8685 (18.6) | 8942 (18.2) | 12 625 (19.1) | |
| 3 | 7054 (19.3) | 9018 (19.7) | 9192 (19.7) | 9910 (20.1) | 13 117 (19.9) | |
| 4 | 7835 (21.5) | 10 206 (22.3) | 10 003 (21.4) | 10 518 (21.37) | 13 926 (21.1) | |
| 5 | 9914 (27.1) | 10 993 (24.0) | 11 074 (23.7) | 11 360 (23.1) | 14 228 (21.6) | |
| Missing | 16 218 (30.1) | 11 028 (19.4) | 10 356 (18.1) | 7460 (23.5) | 10 471 (17.5) | |
Abbreviation: NA, not applicable.
SI conversion factors: To convert B-type natriuretic peptide to nanograms per liter, multiply by 1.0; white blood cells to cells × 109 per liter, multiply by 0.001.
A select subset of baseline characteristics is reported (additional baseline characteristics, eTable in the Supplement). The P values were determined by analysis of variance for continuous variables and χ2 test for categorical variables for homogeneity of baseline characteristics across the years of the study period. Missing covariate data were imputed using the most common category for categorical and the mean for continuous variables.
The vital sign value from the inpatient admission that was obtained closest to the date of admission. Nonphysiologic vital sign values were classified as missing.
Incomplete coverage of study population with echocardiogram (61% missing data).
B-type natriuretic peptide values: quintile 1, 0 to 310 pg/mL; quintile 2, 311 to 636 pg/mL; quintile 3, 637 to 1140 pg/mL; quintile 4, 1141 to 2212 pg/m; quintile 5, more than 2212 pg/mL; N-terminal-pro-B-type natriuretic peptide values: quintile 1, 0 to 1491 pg/mL; quintile 2, 1492 to 3385 pg/mL; quintile 3, 3386 to 6494 pg/mL; quintile 4, 6495 to 13566 pg/mL; quintile 5, more than 13566 pg/mL.
The laboratory value most recently recorded prior to or on the day of the index admission was used. Nonphysiologic laboratory values were classified as missing, as well as white blood cell count greater than 50 000 cells per microliter to limit the influence of extreme observations.
We determined all-cause 30-day and 1-year readmission rates and the mortality rate following discharge. Readmissions were excluded if they were not paid for by the VAHCS (approximately 10%-15%). Our group has previously described the VAHCS’ documentation of death.13
All statistical analysis was performed with SAS software version 9.0 (SAS Institute Inc). Patient baseline characteristics were evaluated for stability over the study period using analysis of variance for continuous variables and χ2 test for categorical variables. The Mantel-Haenszel χ2 test was used to evaluate trends for unadjusted outcomes. Separate logistic regression models with dummy variables for multiyear groups were built for 30-day mortality and readmission. All variables shown in the Table and the eTable in the Supplement were included, except LVEF, which was only used in subgroup analysis (LVEF ≥40% and <40%) because of missing data. A 2-sided P value was considered significant at less than .05. Data were analyzed from October 2018 to March 2020.
Results
There were 304 374 HF admissions included from 164 566 patients from 2007 to 2017. Of all admissions, 298 260 (98.0%) were in male patients, and 195 205 (64.4%) were in white patients. The mean (SD) age was 70.8 (11.5) years. Select baseline characteristics are presented in the Table (eTable in the Supplement for additional baseline characteristics). Despite increases in age, diabetes, hypertension, malignant conditions, and other comorbidities during the study period, there were significant improvements in clinical markers, such as mean (SD) weight (2007-2008: 95.3 [26.0] kg vs 2015-2017: 98.5 [27.2] kg; P < .001), mean (SD) systolic blood pressure (2007-2008: 135.4 [23.3] mm Hg vs 2015-2017: 136.5 [22.1] mm Hg; P < .001), and B-type natriuretic peptide (quintile 1 [0-310 pg/mL], 2007-2008: 5678 of 52 748 patients [15.5%]; 2015-2017: 12 090 of 78 058 patients [18.3%]; quintile 5 [>2212 pg/mL], 2007-2008: 9914 of 52 748 patients [27.1%]; 2015-2017: 14 228 of 78 058 patients [21.6%]; P < .001; to convert B-type natriuretic peptide to nanograms per liter, multiply by 1.0).
The overall unadjusted rates of 30-day readmission, 30-day mortality, 1-year readmission, and 1-year mortality were 21.7% (n = 65 687), 5.6% (n = 16 931), 61.4% (n = 171 263), and 25.0% (n = 76 144), respectively. The 30-day and 1-year readmission rates decreased over the study period by 2% (from 11 792 of 52 748 patients to 15 578 of 76 662 patients) and 1.1% (from 32 341 of 52 748 patients to 31 659 of 52 544 patients), respectively (P < .001 for trend for both). Thirty-day mortality decreased by 0.5% (from 3060 of 52 748 patients to 4159 of 78 058 patients) while 1-year mortality increased by 1.3% (from 12 775 of 52 748 patients to 19 910 of 78 058 patients; P < .001 for trend for both).
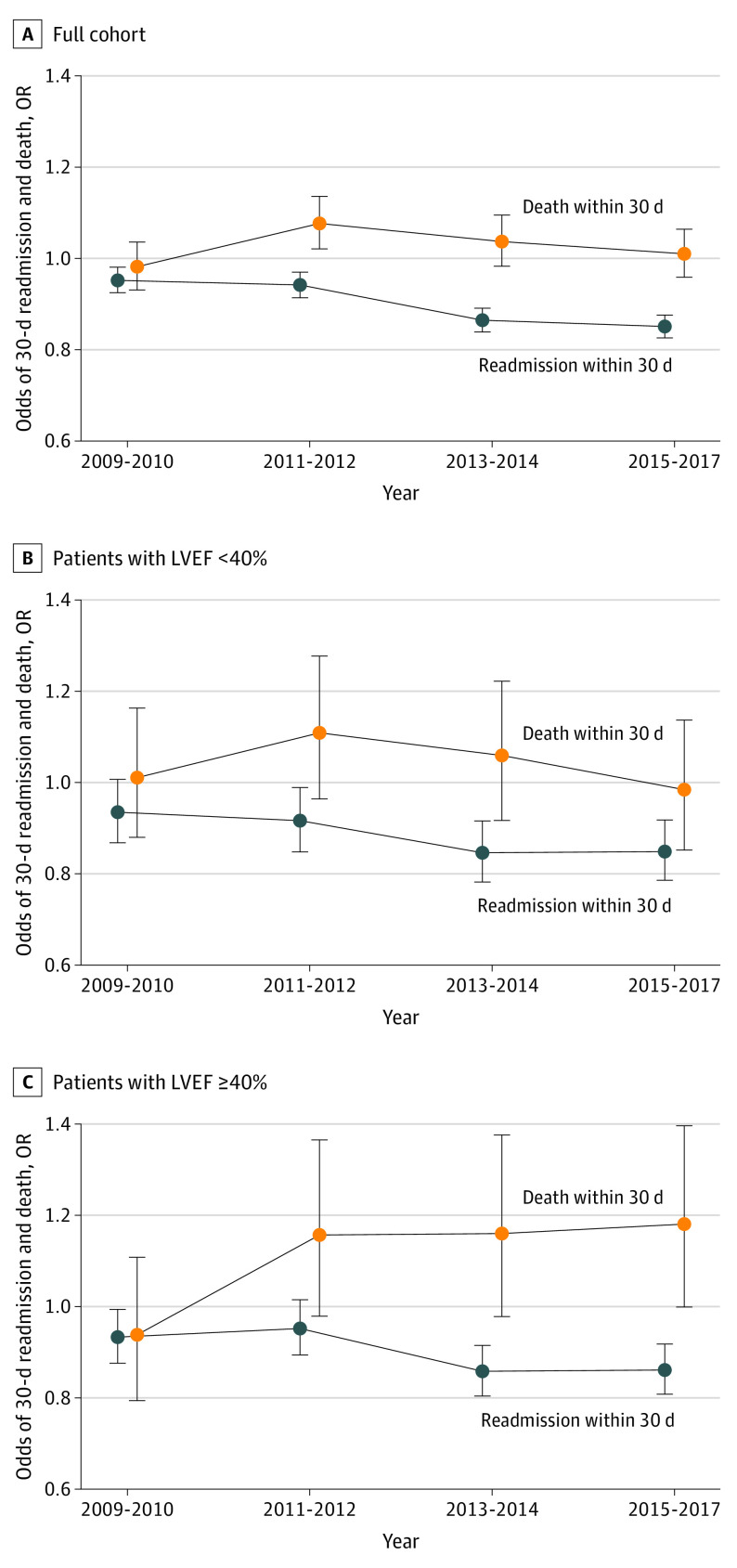
Multivariable analysis demonstrated a decrease in the risk-adjusted odds ratio (aOR) of 30-day readmission but not 30-day mortality (Figure). Relative to 2007 to 2008, the aOR of 30-day readmission was 0.85 (95% CI, 0.83-0.88) and the aOR of 30-day mortality was 1.01 (95% CI, 0.96-1.06) from 2015 to 2017. Similar trends were found for readmissions from days 31 through 60 (2015-2017: aOR, 0.86 [95% CI, 0.84-0.89] vs 2007-2008). Stratification by LVEF confirmed that the aOR of 30-day readmission rates decreased for both those with LVEF of 40% or greater and those with LVEF less than 40% without a significant change in 30-day mortality (Figure). When VA region was examined, there were significant readmission reductions in the Northeast (2015-2017: aOR, 0.88 [95% CI, 0.82-0.94] vs 2007-2008), South (2015-2017: aOR, 0.83 [95% CI, 0.79-0.87] vs 2007-2008), and Midwest (2015-2017: aOR, 0.81 [95% CI, 0.76-0.86] vs 2007-2008), while the West had no significant readmission reduction. The interaction between region and period was significant for 30-day readmission in multivariate adjusted analyses (eg, interaction β coefficients for 2009 to 2010: Midwest, 0.011; South, 0.0048; for 2015 to 2017: Midwest, −0.019; South, −0.0063; global interaction P < .001), but not 30-day mortality.
Figure. Risk-Adjusted Odds Ratios (ORs) for 30-Day Readmission and Death After Heart Failure Hospitalization.
A, Risk-adjusted ORs for readmission and death in the full cohort (relative to 2007-2008). The adjusted odds of readmission declined throughout the study period, while adjusted odds of mortality initially increased but then returned to baseline levels. Despite lack of financial penalties, the Veterans Affairs Health Care System achieved steady reductions in adjusted odds of readmission and avoided concurrent increases in mortality. Risk-adjusted ORs of 30-day readmission and death in patients with left ventricular ejection fraction (LVEF) less than 40% (n = 47 790) (B) and LVEF of 40% or greater (n = 71 004) (C). There were 8496 readmissions and 1565 deaths at 30 days among patients with LVEF less than 40%. There were 10 340 readmissions and 2258 deaths at 30 days among patients with LVEF of 40% or more. There were similar trends in readmission reduction for both LVEF strata, indicating that readmission reduction interventions may have similar effectiveness in these populations, while there were no significant changes in mortality regardless of strata.
Discussion
During the past decade, 30-day readmission rates for patients at VAHCS admitted for HF decreased, while 30-day mortality rates remained unchanged. These trends are consistent across HF with reduced LVEF (HFrEF) and HF with preserved LVEF (HFpEF).
During the past decade, concurrent with CMS efforts to improve value of care through readmission reduction, the VAHCS pursued efforts including improving adherence to recommended care processes, improving care transitions and public reporting, and incorporating readmission rates into the overall hospital star rating.9 We sought to evaluate trends in HF outcomes during this time, especially given concerns about HRRP’s mortality outcomes and evidence showing HF readmission is a poor quality marker in VAHCS.13 While many of these interventions are not unique to VAHCS, the VAHCS, as the largest integrated health care system in the US, has differed in its implementation in 2 key aspects: (1) the VAHCS has the ability to broadly and consistently implement coordinated interventions, and (2) VAHCS hospitals were not penalized financially. The VAHCS experienced unadjusted readmission reductions similar to those observed in patients in CMS fee-for-service (FFS) categories in Inpatient Prospective Payment System hospitals (VAHCS: 2007-2008, 22.4%; 2013-2014, 21.1%; CMS FFS: 2005-2007, 21.8%; 2012-2015, 20.9%).3 These trends persisted in risk-adjusted models accounting for claims and clinical factors, such as vital signs and laboratory data, which allowed for robust adjustment for trends in illness severity.
Additionally, the similar trends observed for patients with HFrEF and HFpEF indicate readmission reduction interventions may have similar success in targeting these populations. While therapeutic options for patients with HFpEF are limited, the slow course of this disease and the importance of diuretic titration may lend itself well to outpatient monitoring schemes. Additionally, it has been shown that the proportion of readmissions with a noncardiovascular primary diagnosis, especially among those with HFpEF, is high,14 indicating that programs focused on outpatient monitoring may allow early identification of new, noncardiac issues.
The stable 30-day mortality in the VAHCS contrasts with the conflicting evidence in the CMS FFS population, with many analyses reporting increasing 30-day mortality.3,4,5,6,7,8 Wadhera et al15 recently demonstrated in CMS FFS beneficiaries that there was an inverse probability weighting adjusted mortality rate of 8.3% from 2005 to 2007 and a rate of 9.5% from 2012 to 2015. Critics of the heavy HRRP penalties argue that readmission financial penalties led to patients being inappropriately managed as outpatients or not admitted from the emergency department when appropriate and disadvantaged hospitals with low local socioeconomic status.15 While we could not account for secular trends in HF severity that might differ between VA and non-VA populations or additional policy changes unique to these systems, the lack of increase in mortality in the VAHCS despite robust risk adjustment is potentially consistent with these hypotheses. Possible contributions of the VAHCS programs to these trends may be attributable to the VAHCS’ ability to track patients across centers through its single electronic medical record, extensive programs for outpatient monitoring, free care to much of its population, and the absence of the complex incentive structure that financial penalties may drive. The similar mortality outcomes between HFrEF and HFpEF suggest non-therapy–based systems of care may have driven similar changes as therapeutic options for HFpEF are limited. Finally, we did note an increase in the unadjusted 1-year mortality rate after HF readmission. While markers of illness severity, such as vital signs, improved throughout the study period, patients appeared to have more comorbidities over time, which may affect long-term noncardiac and cardiac mortality.
Worsening HF illness severity that is not completely adjusted for has been proposed as a contributor to worsening post-HF hospitalization mortality rates in the CMS FFS population.6,15 In the VAHCS population, the rates of several important comorbidities such as diabetes, hypertension, and malignant conditions increased during the study period; however, concurrently, weight, blood pressure, and LVEF also increased, indicating that adjustment for clinical factors may actually capture an overall decline in HF illness severity. Use of clinical markers as available in future studies may allow for more robust adjustment for general trends in illness severity and mitigate the influence of drift in illness severity coding over time. Regardless, it remains that one-fifth of patients in this cohort were readmitted and 1 in 20 died within 30 days of HF admission, highlighting the ongoing need for effective and well-evaluated efforts to improve quality and value of care for patients with HF.
Limitations
In addition to limitations discussed above, this was an observational study, which meant we had no ability to evaluate causality in the association between VAHCS efforts to reduce readmissions and the outcomes of interest. Additionally, it is possible that there are unobserved drivers of a concurrent national trend toward decreased readmissions that we could not completely address.
Conclusions
During a similar period as recent studies of the outcomes of HRRP on the CMS FFS population, the VAHCS experienced similar 30-day readmission rate reductions, despite lack of a financial penalty. Additionally, in this analysis, 30-day mortality rates did not clearly increase, as has been suggested for the CMS FFS population.
eTable. Additional baseline patient characteristics.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146-e603. doi: 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi: 10.1056/NEJMsa0803563 [DOI] [PubMed] [Google Scholar]
- 3.Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C, Yeh RW. Association of the Hospital Readmissions Reduction Program with mortality among Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA. 2018;320(24):2542-2552. doi: 10.1001/jama.2018.19232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Medicare Payment Advisory Commission (MedPAC) Report to the congress: Medicare and the health care delivery system. Published June 2018. Accessed May 14, 2020. http://medpac.gov/docs/default-source/reports/jun18_medpacreporttocongress_sec.pdf
- 5.Bergethon KE, Ju C, DeVore AD, et al. . Trends in 30-day readmission rates for patients hospitalized with heart failure: findings from the Get With the Guidelines–Heart Failure Registry. Circ Heart Fail. 2016;9(6):e002594. doi: 10.1161/CIRCHEARTFAILURE.115.002594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A, Allen LA, Bhatt DL, et al. . Association of the Hospital Readmissions Reduction Program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol. 2018;3(1):44-53. doi: 10.1001/jamacardio.2017.4265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dharmarajan K, Wang Y, Lin Z, et al. . Association of changing hospital readmission rates with mortality rates after hospital discharge. JAMA. 2017;318(3):270-278. doi: 10.1001/jama.2017.8444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blecker S, Herrin J, Li L, Yu H, Grady JN, Horwitz LI. Trends in hospital readmission of Medicare-covered patients with heart failure. J Am Coll Cardiol. 2019;73(9):1004-1012. doi: 10.1016/j.jacc.2018.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Department of Veterans Affairs End of year hospital star rating (FY2018). Published 2019. Accessed August 28, 2019. https://www.va.gov/QUALITYOFCARE/measure-up/End_of_Year_Hospital_star_Rating_FY2018.asp
- 10.American College of Cardiology Quality improvement for institutions: hospital to home. Accessed August 28, 2019. https://cvquality.acc.org/initiatives/hospital-to-home
- 11.Patterson OV, Freiberg MS, Skanderson M, J Fodeh S, Brandt CA, DuVall SL. Unlocking echocardiogram measurements for heart disease research through natural language processing. BMC Cardiovasc Disord. 2017;17(1):151. doi: 10.1186/s12872-017-0580-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cosmi F, Shen L, Magnoli M, et al. . Treatment with insulin is associated with worse outcome in patients with chronic heart failure and diabetes. Eur J Heart Fail. 2018;20(5):888-895. doi: 10.1002/ejhf.1146 [DOI] [PubMed] [Google Scholar]
- 13.Patel J, Sandhu A, Parizo J, Moayedi Y, Fonarow GC, Heidenreich PA. Validity of performance and outcome measures for heart failure. Circ Heart Fail. 2018;11(9):e005035. doi: 10.1161/CIRCHEARTFAILURE.118.005035 [DOI] [PubMed] [Google Scholar]
- 14.Goyal P, Loop M, Chen L, et al. . Causes and temporal patterns of 30-day readmission among older adults hospitalized with heart failure with preserved or reduced ejection fraction. J Am Heart Assoc. 2018;7(9):e007785. doi: 10.1161/JAHA.117.007785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadhera RK, Joynt Maddox KE, Kazi DS, Shen C, Yeh RW. Hospital revisits within 30 days after discharge for medical conditions targeted by the Hospital Readmissions Reduction Program in the United States: national retrospective analysis. BMJ. 2019;366:l4563. doi: 10.1136/bmj.l4563 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Additional baseline patient characteristics.