Key Points
Question
Which factors are associated with the decision to withdraw life-supporting treatment (LST) in patients with severe traumatic brain injury in the US?
Findings
In this large, multicenter cohort study, race, geographic region, and payment status were significantly associated with the decision to withdraw LST. Associated clinical factors included older age, lower Glasgow Coma Scale score, functionally dependent health status, hematoma, dementia, and disseminated cancer.
Meaning
In addition to clinical factors, there is evidence for socioeconomic variation in the decision to withdraw LST in patients with severe traumatic brain injury.
Abstract
Importance
There are limited data on which factors affect the critical and complex decision to withdraw life-supporting treatment (LST) in patients with severe traumatic brain injury (sTBI).
Objective
To determine demographic and clinical factors associated with the decision to withdraw LST in patients with sTBI.
Design, Setting, and Participants
This retrospective analysis of inpatient data from more than 825 trauma centers across the US in the
American College of Surgeons Trauma Quality Improvement Program database from January 2013 to December 2015 included
adult patients with sTBI and documentation of a decision regarding withdrawal of LST (WLST). Data analysis was conducted in September 2019.
Main Outcomes and Measures
Factors associated with WLST in sTBI.
Results
A total of 37931 patients (9817 women [25.9%]) were included in the multivariable analysis; 7864 (20.7%) had WLST. Black patients (4806 [13.2%]; odds ratio [OR], 0.66; 95% CI, 0.59-0.72; P < .001) and patients of other race (4798 [13.2%]; OR, 0.83; 95% CI, 0.76-0.91; P < .001) were less likely than white patients (26 864 [73.7%]) to have WLST. Patients from hospitals in the Midwest (OR, 1.12; 95% CI, 1.04-1.20; P = .002) or Northeast (OR, 1.23; 95% CI, 1.13-1.34; P < .001) were more likely to have WLST than patients from hospitals in the South. Patients with Medicare (OR, 1.55; 95% CI, 1.43-1.69; P < .001) and self-pay patients (OR, 1.36; 95% CI, 1.25-1.47; P < .001) were more likely to have WLST than patients with private insurance. Older patients and those with lower Glasgow Coma Scale scores, higher Injury Severity Scores, or craniotomy were generally more likely to have WLST. Withdrawal of LST was more likely for patients with functionally dependent health status (OR, 1.30; 95% CI, 1.08-1.58; P = .01), hematoma (OR, 1.19; 95% CI, 1.12-1.27; P < .001), dementia (OR, 1.29; 95% CI, 1.08-1.53; P = .004), and disseminated cancer (OR, 2.82; 95% CI, 2.07-3.82; P < .001) than for patients without these conditions.
Conclusions and Relevance
Withdrawal of LST is common in sTBI and socioeconomic factors are associated with the decision to withdraw LST. These results highlight the many factors that contribute to decision-making in sTBI and demonstrate that in a complex and variable disease process, variation based on race, payment, and region presents as a potential challenge.
This cohort study examines the demographic and clinical factors associated with the decision to withdraw life-supporting treatment in patients with severe traumatic brain injury.
Introduction
Severe traumatic brain injury (sTBI) presents a life-altering event for patients, and physicians must guide families through challenging decisions about which interventions are appropriate and when to discontinue interventions in favor of quality of life. Physicians offer families options for interventions, such as craniotomy, intracranial pressure monitoring, or mechanical ventilation, as lifesaving or life-improving measures. Some families and physicians will choose to remove or withhold further life-sustaining intervention. Physicians may recommend withdrawal of life-sustaining treatment (WLST) when they feel that the patient’s prognosis is very poor and risk of experiencing adversity high. This decision in sTBI is uniquely difficult for several reasons. First, physicians may not agree on a prognosis.1,2,3 Second, patients are typically incapacitated and unable to express their own wishes. Finally, the high acuity and severity of the situation may make it difficult for surrogate decision-makers to provide substituted judgment.4
Understanding when and how WLST occurs in sTBI is necessary to advance the field. Severe TBI is a leading cause of death nationally and internationally5,6 and within-hospital death follows WLST in up to 40% to 70% of patients with critical illness.3,7,8 Withdrawing LST prematurely could lead to lost opportunities for recovery or a self-fulfilling prophecy defined by a clinician’s feeling that a patient’s prognosis is poor because they observe poor outcomes in similar patients who have not had aggressive interventions.9 Alternatively, uniformly sustaining life in sTBI could lead to prolonged adversity, which is deemed a worse outcome by some.10
There is limited evidence on which factors drive the decision to withdraw LST in the setting of sTBI. Medical futility cannot be easily addressed because of the uncertainty about outcomes.11 Despite guidelines for sTBI management,12 there is no clear threshold for WLST; therefore, a combination of factors likely contributes to this decision. In a multicenter study in Canada, Turgeon et al3 found that factors, such as a receipt of a procedure to evacuate a hematoma, age, Glasgow Coma Scale (GCS) score, and computed tomography evidence of brain herniation, were associated with WLST followed by death. They also found in a prior study that the center where a patient presents is associated with the decision to withdraw LST.8 The purpose of this study is to characterize factors associated with WLST in the US.
Methods
Objectives
The primary objective was to identify demographic and clinical factors associated with the decision to withdraw LST in patients with sTBI. We hypothesized that clinical factors used in TBI prognostication, particularly GCS scores, would be most strongly associated with the decision to withdraw LST.
The secondary objective was to characterize differences in outcomes between patients with and without WLST. Main secondary outcome measures included within-hospital death, disposition, length of intensive care unit stay, and number of days on a ventilator.
Institutional Review Board
This study received approval from the Duke University Hospital institutional review board. As this study used deidentified data from a large database, it was determined exempt from informed consent of study participants.
Study Design
We retrospectively analyzed the American College of Surgeons-Trauma Quality Improvement Project (ACS-TQIP) database between 2013 and 2015. We selected this range because the primary outcome measure, WLST, was well documented for these years. The ACS-TQIP database represents more than 700 trauma centers in the US that have elected to participate in standardized trauma reporting.
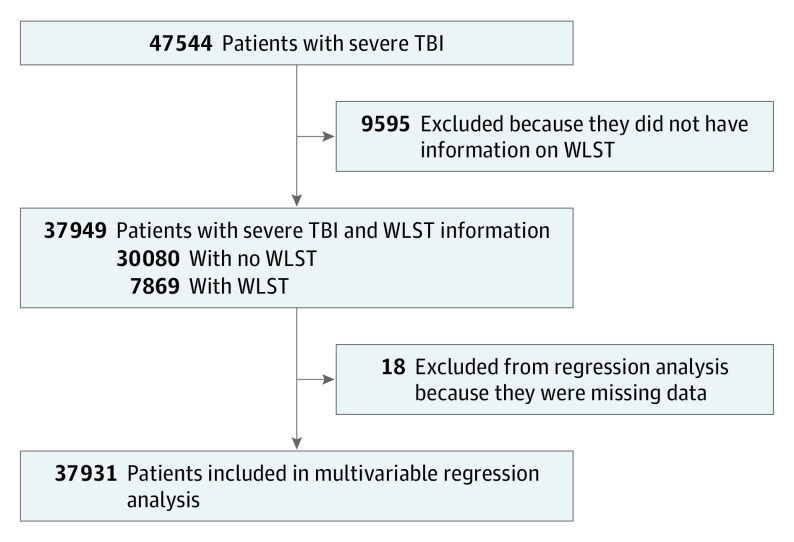
Adult patients with TBI, including skull fracture and intracranial injury, were identified using International Classification of Diseases, Ninth Revision (ICD-9) codes 800 to 804 and 850 to 854, respectively. Blunt and penetrating injury mechanisms were included. The GCS and the head section of the Abbreviated Injury Scale (AIS-Head) criteria were used to select patients with sTBI, which was defined by initial GCS score of 3 to 8 and AIS-Head score of 2 to 5. Patients with GCS score greater than 8 and patients missing the primary outcome field were excluded (Figure 1).
Figure 1. Illustration of Study Design and Number of Individuals Meeting Criteria for Inclusion.
Severe traumatic brain injury (TBI) was defined as an Abbreviated Injury Scale (AIS) score of 2 to 6 and a Glasgow Coma Scale (GCS) score of 3 to 8. The demographic predictors investigated included age group, sex, race, payment status, geographic region, teaching status of the hospital, number of neurosurgeons, and interhospital transfers. The clinical predictors included GCS group, Injury Severity Score (ISS) group, functionally dependent health status, penetrating vs blunt injury, presence or absence of hematoma, craniotomy vs no craniotomy, dementia, cerebrovascular accident (CVA) with residual neurological deficiency, chronic renal failure, and disseminated cancer. The interaction terms included GCS group and ISS group, GCS and age group, and GCS score and craniotomy. WLST indicates withdrawal of life-supporting treatment.
We then separated the patients into 2 groups: those with WLST and those without. The WLST decision needed to be documented in the medical record and included a limit to escalation of treatment for interventions, such as ventilator support, dialysis, medications for blood pressure or cardiac function support, and specific procedures. It was not considered the same as do not resuscitate status.
We analyzed multiple variables for associations with WLST. All pertinent demographic variables available in the database were included: sex, race, ethnicity, payment status, and region. Race was simplified into white, black, and other and ethnicity into Hispanic and non-Hispanic based on the limitations of the database and previously published methods.13,14 Hospital teaching status, interhospital transfer, and number of neurosurgeons were included. Clinical variables were those commonly described in sTBI prognostic models and prior studies,15,16,17,18 including GCS score, hematoma (ICD-9 codes 852 and 853 denote subdural/subarachnoid/extradural and other/unspecified, respectively), craniotomy/craniectomy (ICD-9 codes 01.24 and 01.25, respectively), Injury Severity Score (ISS), lowest systolic blood pressure, alcohol/drug use, time from dispatch of emergency medical services transporting unit to arrival at the emergency department (ED), volume of plasma or platelets transfused within 24 hours of arrival at the ED/hospital, mechanism of injury, palliative care consultation, and penetrating vs blunt injury. The ISS was derived by converting ICD-9 codes to an AIS score using the ICDMAP-90 program (Tri-Analytics Corporation) and then calculating the ISS from the AIS score. Pupillary response and midline shift data were not available in the database during the study period. Variables representing chronic illnesses, such as disseminated cancer, functionally dependent health status, prior cerebrovascular accident, chronic kidney failure, and dementia, were included.
Data Analysis
Demographic information and clinical characteristics for the 2 groups (WLST and no WLST) were summarized with descriptive statistics. A multivariable logistic regression analysis was used to model the probability of the occurrence of WLST as a function of several variables (Figure 1). We tested all clinically relevant interaction terms and included only the interaction terms with significance (P < .05) in the final models. Variables with missing data for more than 1% of the data set (including race, region, payment, hematoma, ISS group, functionally dependent health status, dementia, cerebrovascular accident, chronic kidney failure, and disseminated cancer) were coded with a dummy variable to represent the missing data in regression analysis. Those with less than 1% missing were included.
To explore the validity of the model, we analyzed model fit. Based on available literature and clinical practice experience, we analyzed interactions between GCS score, ISS, age, and craniotomy, adding these to the model as appropriate. Additionally, we used backward selection modeling techniques. These exploratory analyses were performed to determine whether a better fit, as measured by area under the curve, was attainable. We did not observe a difference in the area under the curve with these techniques. Therefore, we chose a parsimonious version of the model to assess demographic and clinical associations with WLST.
Statistical significance was assessed at P = .05. Analyses were conducted using SAS, version 9.4 (SAS Institute).
Results
Summary of the Patient Population: Demographic and Clinical Variables
A total of 47544 patients met criteria for sTBI. Of those, 9595 (20.2%) did not have data on the primary outcome (WLST) and were excluded, leaving 37949 patients for the descriptive analysis. In total, 37931 patients were included in the multivariable regression analysis based on availability of data elements.
Of the 37931 patients meeting criteria for inclusion in the study (having sTBI between January 2013 and December 2015 and an answer of yes or no to the primary outcome variable [WLST]), 7869 patients (20.7%) had WLST (Figure 1). Of these, 7026 patients with WLST (93.7%) died in the hospital. The median time to WLST was 2 days (quartile 1, 1, quartile 3, 6 days [range, 1-176 days]). Table 1 summarizes the distribution of demographic data between the WLST and no WLST groups. The sex distribution of patients studied was 28 125 men (74.1%) and 9871 women (25.9%). The median age was considerably greater for patients who had WLST than for those who did not (60.0 years [quartile 1, 42.0 years; quartile 3, 76.0 years] vs 40.0 years [quartile 1, 26.0 years; quartile 3, 56.0 years]). The proportion of white (81.6% vs 71.6%) and non-Hispanic (91.6% vs 86.7%) patients was higher for the group with WLST. The percentage of patients with Medicare insurance as payment source was higher for those in the WLST group (37.9% vs 15.1%). However, the proportions of patients with commercial/private insurance (41.2% vs 30.8%) and Medicaid insurance (16.2% vs 9.2%) were higher in the no WLST group.
Table 1. Demographic Information by Withdrawal of Life-supporting Treatment.
| Characteristic | No. (%) | ||
|---|---|---|---|
| No withdrawal of LST | Withdrawal of LST | Total | |
| No. of persons included | 30 080 | 7869 | 37 949 |
| Age, y | |||
| Mean (SD) | 42.9 (18.8) | 57.6 (20.6) | 45.9 (20.1) |
| Median (Q1-Q3) | 40.0 (26.0-56.0) | 60.0 (42.0-76.0) | 44.0 (27.0-61.0) |
| Range | 18.0-89.0 | 18.0-89.0 | 18.0-89.0 |
| Age group, y | |||
| <30 | 9841 (32.7) | 1089 (13.8) | 10 930 (28.8) |
| 30-45 | 7640 (25.4) | 1139 (14.5) | 8779 (23.1) |
| 46-60 | 6719 (22.3) | 1809 (23.0) | 8528 (22.5) |
| 61-75 | 3834 (12.7) | 1848 (23.5) | 5682 (15.0) |
| >75 | 2046 (6.8) | 1984 (25.2) | 4030 (10.6) |
| Sex | |||
| Women | 7412 (24.6) | 2405 (30.6) | 9817 (25.9) |
| Men | 22 662 (75.4) | 5463 (69.4) | 28 125 (74.1) |
| Race | |||
| Black | 4209 (14.6) | 597 (7.9) | 4806 (13.2) |
| White | 20 695 (71.6) | 6169 (81.6) | 26 864 (73.7) |
| Other | 4003 (13.8) | 795 (10.5) | 4798 (13.2) |
| Ethnicity | |||
| Hispanic or Latino | 3580 (13.3) | 593 (8.4) | 4173 (12.3) |
| Not Hispanic or Latino | 23 384 (86.7) | 6506 (91.6) | 29 890 (87.7) |
| Paymenta | |||
| Medicaid | 4611 (16.2) | 698 (9.2) | 5309 (14.7) |
| Medicare | 4315 (15.1) | 2857 (37.9) | 7172 (19.9) |
| Private/commercial insurance | 11 769 (41.2) | 2326 (30.8) | 14 095 (39.1) |
| Self-pay | 5479 (19.2) | 1201 (15.9) | 6680 (18.5) |
| Other | 2370 (8.3) | 465 (6.2) | 2835 (7.9) |
| Teaching status of hospital | |||
| Community | 9978 (33.2) | 2777 (35.3) | 12 755 (33.6) |
| Nonteaching | 1921 (6.4) | 608 (7.7) | 2529 (6.7) |
| University | 18 181 (60.4) | 4484 (57.0) | 22 665 (59.7) |
| No. of neurosurgeons | |||
| 0-2 | 2109 (7.0) | 470 (6.0) | 2579 (6.8) |
| 3-5 | 13 576 (45.1) | 3523 (44.8) | 17 099 (45.1) |
| ≥6 | 14 395 (47.9) | 3876 (49.3) | 18 271 (48.1) |
Abbreviations: LST, life-supporting treatment; Q1, first quartile; Q3, third quartile.
Payment: other government, not billed (for any reason), and other payments that are not specified are defined as other. No-fault automobile, workers compensation, and Blue Cross/Blue Shield are captured as private/commercial insurance.
Clinical characteristics for patients with sTBI, subdivided by group, are summarized in Table 2. Patients with WLST had increased severity of injury as demonstrated by higher median AIS-Head scores (5 = critical vs 4 = severe) and the proportion of patients in the ISS of greater than 24 group (66.9% vs 50.7%), although the lowest total GCS score and median ISS were similar for both groups. The proportion of patients with hematoma was higher for those in the WLST group (78.8% vs 71.1%). The lowest median systolic blood pressure was similar between the WLST and no WLST groups (75.0 mm Hg vs 74.0 mm Hg). Patients in the WLST group had higher proportions of functionally dependent health status (3.1% vs 1.3%), dementia (4.2% vs 1.4%), chronic kidney failure (1.8% vs 0.7%), and other comorbidities than those who continued with LST. The most frequent mechanism of injury for patients with WLST was a fall (46.5%), while the most frequent mechanism of injury for those who continued to have treatment was a transportation-associated injury (56.6%), and patients who did not have WLST had a higher proportion of penetrating injury (9.1% vs 4.9%).
Table 2. Clinical Characteristics by Withdrawal of Life-supporting Treatment.
| Characteristic | No withdrawal of LST | Withdrawal of LST | Total |
|---|---|---|---|
| No. of persons included | 30 080 | 7869 | 37 949 |
| Highest AIS-Head score | |||
| Mean (SD) | 4.0 (0.7) | 4.5 (0.6) | 4.1 (0.7) |
| Median (Q1-Q3) | 4.0 (4.0-4.0) | 5.0 (4.0-5.0) | 4.0 (4.0-5.0) |
| Range | 2.0-5.0 | 2.0-5.0 | 2.0-5.0 |
| ISS | |||
| Mean (SD) | 26.1 (12.1) | 28.2 (12.3) | 26.5 (12.1) |
| Median (Q1-Q3) | 25.0 (17.0-30.0) | 26.0 (17.0-34.0) | 25.0 (17.0-33.0) |
| Range | 4.0-75.0 | 4.0-75.0 | 4.0-75.0 |
| ISS group, No. (%) | |||
| <12 | 1264 (4.8) | 79 (1.1) | 1343 (4.1) |
| 12-24 | 11 612 (44.5) | 2238 (32.0) | 13 850 (41.8) |
| >24 | 13 241 (50.7) | 4678 (66.9) | 17 919 (54.1) |
| Lowest total GCS score | |||
| Mean (SD) | 4.0 (1.7) | 3.6 (1.3) | 3.9 (1.6) |
| Median (Q1-Q3) | 3.0 (3.0-5.0) | 3.0 (3.0-3.0) | 3.0 (3.0-4.0) |
| Range | 3.0-8.0 | 3.0-8.0 | 3.0-8.0 |
| GCS score group, No. (%) | |||
| 3-4 | 22 003 (73.1) | 6649 (84.5) | 28 652 (75.5) |
| 5-6 | 3499 (11.6) | 697 (8.9) | 4196 (11.1) |
| 7-8 | 4578 (15.2) | 523 (6.6) | 5101 (13.4) |
| Mechanism of injury, No. (%) | |||
| Assault | 364 (1.2) | 80 (1.0) | 444 (1.2) |
| Fall | 8196 (27.4) | 3632 (46.5) | 11 828 (31.4) |
| Transportation | 16 910 (56.6) | 2894 (37.0) | 19 804 (52.5) |
| Other | 4402 (14.7) | 1212 (15.5) | 5614 (14.9) |
| Interhospital transfer, No. (%) | 8322 (27.7) | 2533 (32.2) | 10 855 (28.6) |
| Functionally dependent health status, No. (%) | 361 (1.3) | 224 (3.1) | 585 (1.7) |
| Hematoma, No. (%) | 21 380 (71.1) | 6197 (78.8) | 27 577 (72.7) |
| Lowest systolic blood pressure, mm Hga | |||
| Mean (SD) | 67.9 (45.5) | 74.1 (44.6) | 69.5 (45.3) |
| Median (Q1-Q3) | 74.0 (40.0-98.0) | 75.0 (51.0-101.0) | 74.0 (45.0-98.0) |
| (Range) | (0.0-238.0) | (0.0-220.0) | (0.0-238.0) |
| Penetrating injury, No. (%) | 2725 (9.1) | 388 (4.9) | 3113 (8.2) |
| Dementia, No. (%) | 381 (1.4) | 306 (4.2) | 687 (1.9) |
| Cerebrovascular accident residual neurological deficiency, No. (%) | 281 (1.0) | 166 (2.3) | 447 (1.3) |
| Chronic renal failure, No. (%) | 209 (0.7) | 130 (1.8) | 339 (1.0) |
| Disseminated cancer, No. (%) | 91 (0.3) | 116 (1.6) | 207 (0.6) |
Abbreviations: AIS-Head, head section of the Abbreviated Injury Scale; GCS, Glasgow Coma Scale; ISS, Injury Severity Score; LST, life-supporting treatment; Q1, first quartile; Q3, third quartile.
Association of Race, Geography, Insurance, and Hospital Status With WLST
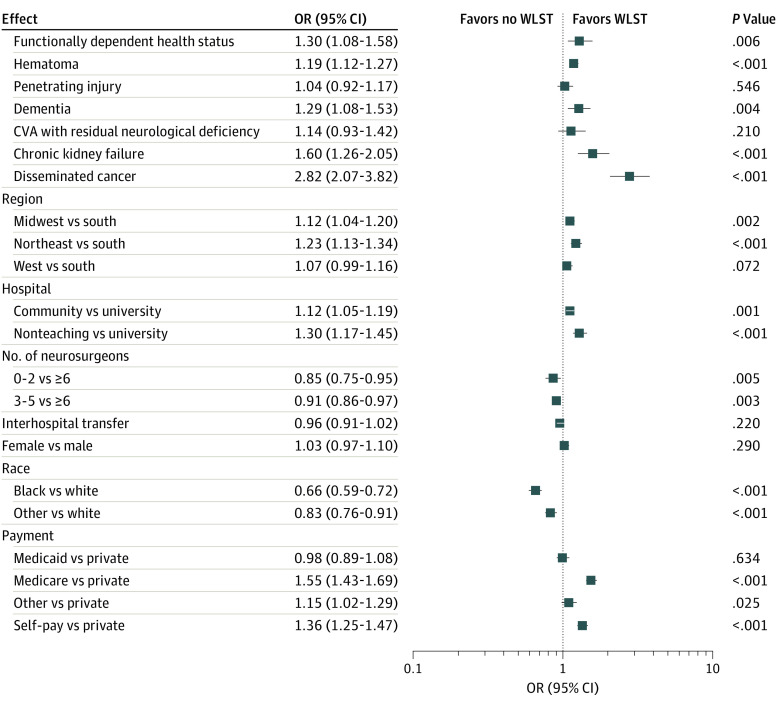
Of the demographic variables, race, geographic region, and payment were significantly associated with WLST after controlling for other covariates (Figure 2). Black patients were less likely than white patients to have WLST (OR, 0.66; 95% CI, 0.59-0.72; P < .001). Similarly, patients of other race were less likely than white patients to have WLST (OR, 0.83; 95% CI, 0.76-0.91; P < .001). Patients from hospitals in the Midwest (OR, 1.12; 95% CI, 1.04-1.20; P = .002) or Northeast (OR, 1.23; 95% CI, 1.13-1.34; P < .001) were more likely to have WLST than patients from hospitals in the South. Compared with patients with private insurance, the odds ratio of having WLST was 1.55 (95% CI, 1.43-1.69; P < .001) for patients with Medicare and 1.36 (95% CI, 1.25-1.47; P < .001) for self-pay patients.
Figure 2. Odds Ratio Estimates for Withdrawal of Life-supporting Treatment (WLST).
Odds ratio estimates for the unknown levels vs the reference levels are omitted. Error bars indicate 95% CIs. CVA indicates cerebrovascular accident.
The number of neurosurgeons at the institution was significantly associated with the decision to withdraw LST, particularly when a hospital had few neurosurgeons. The odds ratio of WLST in institutions with 0 to 2 neurosurgeons compared with 6 or more was 0.85 (95% CI, 0.75-0.95; P = .01). Hospital teaching status was also significantly associated after controlling for other factors. The odds ratio of having WLST for patients from a community hospital to those from a university hospital was 1.12 (95% CI, 1.05-1.19; P = .001) and the odds ratio for patients from a non–teaching hospital compared with those from a university was 1.30 (95% CI, 1.17-1.45; P < .001).
Association of Markers of Severity of Injury and Patients With Greater Illness Severity With WLST
Several clinical variables were significantly associated with WLST after controlling for other factors. Patients classified as functionally dependent were more likely to have WLST than those who were functionally independent (OR, 1.30; 95% CI, 1.08-1.58; P = .01) when other covariates were held constant. Patients with hematoma were more likely to have WLST than those without (OR, 1.19; 95% CI, 1.12-1.27; P < .001). Patients who had dementia (OR, 1.29; 95% CI, 1.08-1.53; P = .004), chronic kidney failure (OR, 1.60; 95% CI, 1.26-2.05; P < .001), and disseminated cancer (OR, 2.82; 95% CI, 2.07-3.82; P < .001) were more likely to have WLST than patients without these conditions.
Probability of WLST by Key Interacting Variables
eTable 1 in the Supplement summarizes the significance of interactions between GCS score, ISS, craniotomy, and age. These interactions were significant and therefore included in the logistic regression model. Patients with lower ISS were generally more likely to have WLST. The odds of having WLST were generally higher for patients who had craniotomy than for those who did not. Older patients were more likely to have WLST, especially when GCS was higher. In general, the associations of ISS, craniotomy, and age with WLST were strengthened at higher GCS scores.
A second variable of interest was payment group. There was concern that age was a major contributor to the difference between the WLST and no WLST groups when considering payment status. This is because of specific, known criteria to obtain Medicare insurance. Of patients with Medicare (7172 [18.9%]), 5542 (77.3%) were 65 years or older. eTable 2 in the Supplement breaks down the proportion of patients with WLST by those younger than 65 years and 65 years or older for each payment type and shows a similar distribution in each age group, with WLST being higher in the older group across payment statuses.
Variation of Length of Stay and Discharge Disposition With Decision to Withdraw LST
Length of stay (LOS) and discharge disposition data are summarized in Table 3. Patients with WLST had considerably lower LOS (median, 3.0 days [quartile 1, 1.0 days; quartile 3, 7.0 days] vs 10.0 days [quartile 1, 3.5 days; quartile 3, 21.0 days]), total intensive care unit LOS (median, 3.0 [quartile 1, 1.0 days; quartile 3, 14.0 days] vs 6.0 days [quartile 1, 3.0 days; quartile 3, 7.0 days]), and total ventilator days (median: 2.0 [quartile 1, 1.0 days; quartile 3, 6.0 days] vs 4.0 days [quartile 1, 2.0 days; quartile 3, 11.0 days]) compared with patients with no WLST. Most patients who withdrew LST died in the hospital (7026 [93.7%]), while patients who continued with treatment had multiple dispositions: transferred to other hospitals (13 562 [47.1%]), discharged to home (8572 [29.8%]), and deceased (5961 [18.3%]). A total of 359 patients (4.8%) in the WLST group were discharged to hospice compared with 395 patients (1.4%) in the no WLST group.
Table 3. Length of Stay and Disposition by Withdrawal of Life-supporting Treatment.
| Characteristic | No withdrawal of LST | Withdrawal of LST | Total |
|---|---|---|---|
| No. of persons included | 30 080 | 7869 | 37 949 |
| Total LOS | |||
| No. with data | 30 040 | 7868 | 37 908 |
| Mean (SD), d | 15.4 (17.4) | 5.3 (7.3) | 13.3 (16.4) |
| Median (Q1-Q3), d | 10.0 (3.5-21.0) | 3.0 (1.0-7.0) | 8.0 (2.0-19.0) |
| (Range), d | (1.0-357.0) | (1.0-179.0) | (1.0-357.0) |
| Total ICU LOS | |||
| No. with data | 27 542 | 7909 | 34 751 |
| Mean (SD), d | 9.7 (10.0) | 5.2 (6.3) | 8.8 (9.5) |
| Median (Q1-Q3), d | 6.0 (3.0-14.0) | 3.0 (1.0-7.0) | 5.0 (2.0-13.0) |
| (Range), d | (1.0-178.0) | (1.0-180.0) | (1.0-180.0) |
| Total ventilator days | |||
| No. with data | 25 960 | 7327 | 33 987 |
| Mean (SD), d | 7.5 (9.0) | 4.8 (6.0) | 6.9 (8.5) |
| Median (Q1-Q3), d | 4.0 (2.0-11.0) | 2.0 (1.0-6.0) | 3.0 (2.0-10.0) |
| Range, d | 1.0-207.0 | 1.0-180.0 | 1.0-207.0 |
| Discharge disposition, No. (%) | |||
| Deceased/expired | 5961 (18.3) | 7026 (93.7) | 12 987 (33.9) |
| Discharged/transferred to home | 8572 (29.8) | 28 (0.4) | 8600 (23.7) |
| Discharged/transferred to hospital | 13 562 (47.1) | 74 (1.0) | 13 636 (37.6) |
| Discharged/transferred to hospice | 395 (1.4) | 359 (4.8) | 754 (2.1) |
| Other | 987 (3.4) | 10 (0.1) | 997 (2.7) |
Abbreviations: ICU, intensive care unit; LOS, length of stay; LST, life-supporting treatment; Q1, first quartile; Q3, third quartile.
Discussion
In this large, multisite cohort of patients with sTBI, one-third of patients died during their presenting admission. Most of these patients died after WLST. The decision to withdraw LST for patients with TBI is a challenging one; our findings highlight clinical and demographic factors that may be involved in these complex choices. Our study has 3 major findings. First, expected clinical markers of severity of injury and overall patient health status are associated with WLST. Second, demographic variables, such as race, payment status, and hospital type, have associations with WLST. Finally, LOS, within-hospital death, and discharge disposition differ between patients with and without WLST.
While clinicians and surrogate decision-makers likely value prognostic information as they make decisions about WLST,19 existing data suggest that prognostication can be inaccurate.3,20 Further, clinicians’ and surrogates’ predictions of outcome are often discordant because of differences in understanding and beliefs.21 Our findings of multiple clinical factors associated with WLST point out the complexity of the decision.
Clinical practice and prior data suggest that increased severity of injury, advanced age, and comorbidities should have strong associations with WLST.20 This study confirmed that factors, such as older age, lower GCS scores, and higher overall injury severity, are associated with increased likelihood of WLST. These findings highlight several additional clinical factors, such as the presence of hematoma, certain comorbidities, and functionally dependent health status, that may serve as proxies for poor prognosis. Having a craniotomy was additionally associated with an increased probability of having WLST. This opposes previously observed results22 and challenges the commonly held view of therapeutic momentum.23 We found this trend to be similar despite GCS scores.
The role of race, geography, and payment status on the decision to withdraw LST in sTBI is complex. As demonstrated in other disease processes,22 black patients were less likely to have WLST than white patients, even when injury severity was similar. In other clinical contexts, such as cardiac disease, black patients are less likely to undergo intervention, but in this study, black patients were less likely to decline intervention and opt for WLST.24 Taken together, these findings support a growing body of literature about different contexts of WLST conversations in black patients, including a lack of trust in health care clinicians and different rates of advance directives.25 There were also regional differences in WLST. Southern patients were least likely to have WLST. While ACS-TQIP uses an opt-in process and is thus not nationally representative, this finding is consistent with other work suggesting that treatment center location can affect decisions in sTBI.3
Medicare and self-pay patients were more likely to have WLST than those with private insurance. In emergent settings, payment status is not intended to affect treatment decisions. The families of self-pay patients may be considering rising health care costs in their decision to pursue WLST or other unmeasured socioeconomic factors may be contributing. The reasons underlying this finding in Medicare patients are unclear, but possibilities include Medicare patients being more prepared for such events with prior discussions and advance directives, although these data were collected before the 2015 change in Medicare reimbursements for advance care planning.26
The LOS and discharge data associated with WLST demonstrate the importance of these decisions. Patients with WLST accounted for most within-hospital deaths. These results may reflect the overall sicker status of patients with WLST. The time to WLST was brief, as was the LOS in this group compared with those without LST. Withdrawal of LST often immediately preceded in-hospital death, consistent with previous studies.3,7,8 Current recommendations suggest avoiding early decisions about WLST because of inaccurate prognostication.8,27 This study shows a trend of early WLST that warrants further investigation to determine whether the new guidelines will affect this practice.
Limitations
Our study has several limitations. Inherent to the use of large databases is the inability to analyze subjective factors associated with outcomes. Other unmeasured covariates, including individual physician and certain demographic factors, were not considered in our model. Specifically, demographic variables, such as education, income, and zip code, were unavailable. Key prognostic indicators, such as pupillary response and midline shift,15,16 were also unavailable during the study period. We only had access to inpatient data, so interpretations of results, especially regarding mortality, are confined to the present admission. Future investigations using different methods should be conducted to further elucidate the clinical, socioeconomic, and subjective factors associated with WLST in sTBI.
Conclusions
We have identified several variables associated with WLST in patients with sTBI. Patients with greater severity of injury and illness have more frequent WLST. Race, region, and insurance status are also associated with the decision to withdraw LST. These results highlight potential challenges in decision-making in sTBI.
eTable 1. Odds ratio estimates for interaction effects for withdrawal of life-supporting treatment
eTable 2. Withdrawal of life-supporting treatment by age <65 and ≥65 years old for each payment type
References
- 1.Williamson TL, Abdelgabir J, Barks MC, Zakare R, Ubel PA. The impact of prognostic estimates on surgical decision making in the setting of severe traumatic brain injury: a survey of neurosurgeons. Published online March 2, 2020. PLOS One. doi: 10.1371/journal.pone.0228947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Côte N, Turgeon AF, Lauzier F, et al. Factors associated with the withdrawal of life-sustaining therapies in patients with severe traumatic brain injury: a multicenter cohort study. Neurocrit Care. 2013;18(1):154-160. doi: 10.1007/s12028-012-9787-9 [DOI] [PubMed] [Google Scholar]
- 3.Turgeon AF, Lauzier F, Burns KEA, et al. ; Canadian Critical Care Trials Group . Determination of neurologic prognosis and clinical decision making in adult patients with severe traumatic brain injury: a survey of Canadian intensivists, neurosurgeons, and neurologists. Crit Care Med. 2013;41(4):1086-1093. doi: 10.1097/CCM.0b013e318275d046 [DOI] [PubMed] [Google Scholar]
- 4.Anderson WGAR, Arnold RM, Angus DC, Bryce CL. Posttraumatic stress and complicated grief in family members of patients in the intensive care unit. J Gen Intern Med. 2008;23(11):1871-1876. doi: 10.1007/s11606-008-0770-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finkelstein E, Corso PS, Miller TR. The Incidence and Economic Burden of Injuries in the United States. Oxford University Press; 2006. doi: 10.1093/acprof:oso/9780195179484.001.0001 [DOI] [Google Scholar]
- 6.National Scientific Advisory Committee Identifying priorities for research and capacity development in injury as a multi-institute strategic initiative within the Canadian Institutes of Health Research: listening for direction on injury: final report 2004. Accessed September 1, 2019. https://injuryprevention.bmj.com/content/10/6/334
- 7.Rocker GM, Cook DJ, Shemie SD. Brief review: practice variation in end of life care in the ICU: implications for patients with severe brain injury. Can J Anaesth. 2006;53(8):814-819. doi: 10.1007/BF03022799 [DOI] [PubMed] [Google Scholar]
- 8.Turgeon AF, Lauzier F, Simard JF, et al. ; Canadian Critical Care Trials Group . Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ. 2011;183(14):1581-1588. doi: 10.1503/cmaj.101786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin M, Bonomo J, Hemphill JC III. Intersection of prognosis and palliation in neurocritical care. Curr Opin Crit Care. 2017;23(2):134-139. doi: 10.1097/MCC.0000000000000396 [DOI] [PubMed] [Google Scholar]
- 10.Livingston DH, Tripp T, Biggs C, Lavery RF. A fate worse than death? Long-term outcome of trauma patients admitted to the surgical intensive care unit. J Trauma. 2009;67(2):341-348. doi: 10.1097/TA.0b013e3181a5cc34 [DOI] [PubMed] [Google Scholar]
- 11.Honeybul S, Gillett GR, Ho KM. Uncertainty, conflict and consent: revisiting the futility debate in neurotrauma. Acta Neurochir (Wien). 2016;158(7):1251-1257. doi: 10.1007/s00701-016-2818-0 [DOI] [PubMed] [Google Scholar]
- 12.Carney N, Totten AM. Guidelines for the Management of Severe Traumatic Brain Injury. 4th ed Neurosurg; 2016. [Google Scholar]
- 13.Feeney JM, Santone E, DiFiori M, Kis L, Jayaraman V, Montgomery SC. Compared to warfarin, direct oral anticoagulants are associated with lower mortality in patients with blunt traumatic intracranial hemorrhage: A TQIP study. J Trauma Acute Care Surg. 2016;81(5):843-848. doi: 10.1097/TA.0000000000001245 [DOI] [PubMed] [Google Scholar]
- 14.McCredie VA, Alali AS, Xiong W, et al. Timing of withdrawal of life-sustaining therapies in severe traumatic brain injury: Impact on overall mortality. J Trauma Acute Care Surg. 2016;80(3):484-491. doi: 10.1097/TA.0000000000000922 [DOI] [PubMed] [Google Scholar]
- 15.Murray GD, Butcher I, McHugh GS, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):329-337. doi: 10.1089/neu.2006.0035 [DOI] [PubMed] [Google Scholar]
- 16.Steyerberg EW, Mushkudiani N, Perel P, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5(8):e165. doi: 10.1371/journal.pmed.0050165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorji N, Zador Z, Poon S. A configurational analysis of risk patterns for predicting the outcome after traumatic brain injury. AMIA Annu Symp Proc. 2018;2017:780-789. [PMC free article] [PubMed] [Google Scholar]
- 18.Perel P, Arango M, Clayton T, et al. ; MRC CRASH Trial Collaborators . Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008;336(7641):425-429. doi: 10.1136/bmj.39461.643438.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinn T, Moskowitz J, Khan MW, et al. What families need and physicians deliver: contrasting communication preferences between surrogate decision-makers and physicians during outcome prognostication in critically ill TBI patients. Neurocrit Care. 2017;27(2):154-162. doi: 10.1007/s12028-017-0427-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turgeon AF, Dorrance K, Archambault P, et al. ; Canadian Traumatic Brain Injury Research Consortium and the Canadian Critical Care Trials Group . Factors influencing decisions by critical care physicians to withdraw life-sustaining treatments in critically ill adult patients with severe traumatic brain injury. CMAJ. 2019;191(24):E652-E663. doi: 10.1503/cmaj.190154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White DB, Ernecoff N, Buddadhumaruk P, et al. Prevalence of and factors related to discordance about prognosis between physicians and surrogate decision makers of critically ill patients. JAMA. 2016;315(19):2086-2094. doi: 10.1001/jama.2016.5351 [DOI] [PubMed] [Google Scholar]
- 22.Diringer MN, Edwards DF, Aiyagari V, Hollingsworth H. Factors associated with withdrawal of mechanical ventilation in a neurology/neurosurgery intensive care unit. Crit Care Med. 2001;29(9):1792-1797. doi: 10.1097/00003246-200109000-00023 [DOI] [PubMed] [Google Scholar]
- 23.Rodrigo C, Amarasuriya M, Wickramasinghe S, Constantine GR. Therapeutic momentum: a concept opposite to therapeutic inertia. Int J Clin Pract. 2013;67(1):97-98. doi: 10.1111/ijcp.12043 [DOI] [PubMed] [Google Scholar]
- 24.Fiscella K, Sanders MR. Racial and ethnic disparities in the quality of health care. Annu Rev Public Health. 2016;37(1):375-394. doi: 10.1146/annurev-publhealth-032315-021439 [DOI] [PubMed] [Google Scholar]
- 25.Orlovic M, Smith K, Mossialos E. Racial and ethnic differences in end-of-life care in the United States: evidence from the Health and Retirement Study (HRS). SSM Popul Health. 2018;7:100331-100331. doi: 10.1016/j.ssmph.2018.100331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeitoun N. New Medicare rule will reimburse physicians for advance care planning. Hospitalist. 2015;2015(11):1 Accessed September 1, 2019. https://www.the-hospitalist.org/hospitalist/article/122030/health-policy/new-medicare-rule-will-reimburse-physicians-advance-care [Google Scholar]
- 27.Souter MJ, Blissitt PA, Blosser S, et al. Recommendations for the critical care management of devastating brain injury: prognostication, psychosocial, and ethical management: a position statement for healthcare professionals from the Neurocritical Care Society. Neurocrit Care. 2015;23(1):4-13. doi: 10.1007/s12028-015-0137-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Odds ratio estimates for interaction effects for withdrawal of life-supporting treatment
eTable 2. Withdrawal of life-supporting treatment by age <65 and ≥65 years old for each payment type