Abstract
Background: While it is recognized that peripheral lung structure and ventilation heterogeneity change with age, the effects of age on aerosol deposition in the healthy adult lung is largely unknown.
Methods: A series of aerosol bolus inhalations were repeatedly performed in four healthy subjects over a period of 19 years (years = 0, 9, 15 and 19). For each series, a bolus of 1 μm particles was inhaled at penetration volumes (Vp) ranging from 200 to 1200 mL. Aerosol bolus deposition (DE), dispersion (H), and mode shift (MS) were calculated along with the rate of increase in these parameters with increasing Vp (slope-DE, slope-H, and slope-MS).
Results: Slope-DE significantly increased from 0.040 ± 0.014 (mean ± standard deviation) at year 0 to 0.069 ± 0.007%/mL at year 19 (p = 0.02) with no significant difference in DE at shallow depth (Vp = 200 mL; 14% ± 4% at year 0 vs. 15% ± 7% at year 19, p = 0.25). There was no significant effect of age on either slope-H (0.44 ± 0.05 at year 0 vs. 0.47 ± 0.09 mL/mL at year 19, p = 0.6) or dispersion at shallow depth (192 ± 36 mL at year 0 vs. 220 ± 54 mL at year 19, p = 0.2). Slope-MS became significantly more negative with increasing age (−0.096 ± 0.044 at year 0 vs. −0.171 ± 0.027 mL/mL at year 19, p = 0.001) with no significant difference in MS at shallow depth (12 ± 10 at year 0 vs. 7 ± 15 mL at year 19, p = 0.3).
Conclusions: These data suggest that (1) peripheral deposition increases with aging in the healthy lung, likely as a result of increasing closing volume with age; (2) alterations in the mechanical properties of healthy adult lungs with age occur uniformly; and (3) the significant increase in the magnitude of MS-slope with age is likely due to the concomitant increase in peripheral deposition and possible alterations in flow sequencing.
Keywords: aerosol bolus inhalation, aging, human lung, particle deposition
Introduction
While particle size is the major factor affecting particle deposition in the lung, several other factors influence not only the total deposition of particles but also their regional deposition. These factors include particle characteristics (density, shape, electrostatic charges), physiological factors (breathing pattern, breathing pathway (oral versus nasal), tidal volume, functional residual capacity [FRC]), and lung anatomy (airway and alveolar size, branching angle). Aging affects the structure and function of the adult lung with the major changes manifesting as a reduction in lung elasticity,(1) a decrease in chest wall compliance,(2) and a reduction in respiratory muscle strength.(3) These changes result in airspace dilatation, increased collapsibility of small airways, and a reduction in expiratory volumes, and thus have the potential to affect aerosol deposition.
While the effects of age on lung structure and function are relatively well described, the age dependence on aerosol deposition in the healthy adult lung is largely unknown. In a cross-sectional study,(4) Bennett and colleagues measured the total deposition of 2 μm-diameter particles during spontaneous breathing in 62 healthy adults, 18–80 years of age and found no significant difference in deposition between age groups (18–40 years, 40–60 years, and 60–80 years). Similarly, Rissler and colleagues found no significant age effect on deposition fraction in adults 20–70 years of age.(5) Both studies used spontaneous breathing making it difficult to isolate the effect of lung structure per se on measured deposition. To our knowledge, no longitudinal studies of aerosol deposition in healthy adult lungs have been reported in the literature to date.
Since 1997, our group has performed aerosol bolus inhalation tests in the same four healthy subjects on multiple occasions, using the same breathing pattern and particle size.(6–8) In this study, aerosol bolus inhalation tests were repeated in the same four subjects, bringing the period over which data were collected to 19 years. These data offer a unique opportunity to look at the effect of aging on the fate of inhaled aerosols in a longitudinal fashion. This is the focus of this article.
Methods
Subjects
Four healthy subjects participated in the study. Their relevant anthropometric data are listed in Table 1. Data were collected on various occasions over a period from 1997 to 2016. Except for data collected in 2016 (t = 19 year), all other data have been previously published.(6–8) Studies were all approved by the Human Research Protection Program at the University of California at San Diego at the time of each study. Informed written consent was obtained from each participant.
Table 1.
Anthropometric Data
| |
|
t = 0 year |
t = 9 years |
t = 15 years |
t = 19 years |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Sex | Age (years) | Height (cm) | Weight (kg) | FVC (%pred) | FEV1/FVC (%pred) | FVC (%pred) | FEV1/FVC (%pred) | FVC (%pred) | FEV1/FVC (%pred) | FVC (%pred) | FEV1/FVC (%pred) |
| S1 | F | 30 | 165 | 62 | 108 | 97 | 98 | 98 | 108 | 96 | 106 | 94 |
| S2 | F | 27 | 163 | 67 | 114 | 86 | 104 | 98 | 99 | 101 | 99 | 101 |
| S3 | M | 46 | 191 | 95 | 121 | 98 | 119 | 98 | — | — | 115 | 101 |
| S4 | M | 40 | 185 | 108 | 104 | 108 | 98 | 112 | 96 | 114 | 90 | 110 |
F, female; FEV1, force expired volume in 1 seconds; FVC, forced vital capacity; M, male; %pred, %predicted; t, time of testing.
Equipment
Aerosol bolus data were collected using similar equipment at all time points(6–8) and is fully described in those publications. Briefly, the system allowed the injection of an aerosol bolus with a half-width of ∼70 mL at a given point in the inhalation by switching computer-controlled pneumatic valves. The measurement of the aerosol concentration was provided by a photometer (model 993000; PARI, GmbH)(9) and flow was measured using a pneumotachograph (Fleisch no. 1; OEM Medical, Richmond, VA) connected to a Validyne differential pressure transducer M-45 (Validyne Engineering, Northridge, CA) through short tubes. The photometer, pneumotachograph, and sliding valves were heated to body temperature to prevent water condensation during testing. A diffusion dryer (dead space ∼10 mL) was positioned between the mouthpiece and the photometer to remove water vapor from the exhaled air, preventing condensation on the lenses of the photometer.
The bolus tube was filled with aerosol composed of monodisperse 1 μm diameter polystyrene particles [CV (coefficient of variation) < 3%; Duke Scientific, Fremont, CA]. The particles were supplied in aqueous suspension and diluted with deionized water before being dispensed through an ultrasonic nebulizer, the Aeroneb™ (Aeroneb Lab; Nektar, San Carlos, CA). Before bolus tube filling, the aerosol was directed through a heated hose and a diffusion dryer to remove water droplets.
A computer equipped with a 12-bit multifunction I/O card (DAQPad 6020E, National Instruments, Austin, TX) was used for data acquisition. Signals from the photometer and the pneumotachograph were sampled at 100 Hz.
Protocol
After a few normal breaths, the subject exhaled to residual volume (RV) to ensure a known lung volume starting point. The test breath consisted of an inspiration from RV to 1 L above FRC at a flow rate of ∼0.5 L/s, immediately followed by an expiration to RV, also at a flow rate of ∼0.5 L/s. A flowmeter provided visual feedback to the subject. During the inspiration, an aerosol bolus of ∼70 mL was introduced at different penetration volumes (Vp) ranging from 200 to 1200 mL. The penetration volume was defined as the volume of air inhaled from the mode of the aerosol bolus to the end of the inhalation. Data were obtained in triplicate at each penetration volume.
Data analysis
For each bolus test, aerosol deposition (DE), dispersion (H), and mode shift (MS) were calculated. Deposition was determined using the following equation
| (1) |
where Nin and Nex are the number of particles in the inspired and expired bolus, respectively, calculated by the integration of the aerosol concentration multiplied by the instantaneous flow rate. The integration was done only for concentrations exceeding 5% of maximal expired aerosol concentration to reduce error due to baseline noise in the signal.(6)
Aerosol dispersion was defined as the change in halfwidth between the inspired and expired bolus and was calculated using the following equation
| (2) |
where Hin and Hex were the halfwidth between the inspired and expired bolus, respectively.(10) The halfwidth was defined as the volume over which particle concentration was higher than half the maximum concentration of the bolus.(6,11)
Mode shift was defined as the difference between the position of the peak of the expired bolus (Mex) and the penetration volume of the inspired bolus (Vp)
| (3) |
A negative value of MS indicates that the position of the mode of the expired bolus had shifted to a smaller lung volume than the location of the inspired bolus, that is, the bolus had moved toward the mouth.
Statistical analysis
Data were grouped on the basis of three categorical variables (time, penetration volume, and subject number). A one-way analysis of variance (ANOVA) for correlated samples was performed to test for differences between the chosen categorical variables. Post-ANOVA pair-wise comparisons using Bonferroni adjustment was performed for tests showing significant F-ratios. Significant differences were accepted at the p ≤ 0.05 level. For each subject and for each Vp, one single value for DE, H, and MS was determined at each time point as described below and used in the statistical analysis. Previous studies(7,12,13) have shown that, beyond a penetration volume of 100 mL, these bolus parameters vary linearly with increasing penetration volume. Actual Vp values measured during testing varied from the target penetration volumes specified in the computer software. To compare bolus parameters at target penetration volumes, data points for a given subject and time were obtained for each target penetration volume through linear regression analysis.(7,12)
Results
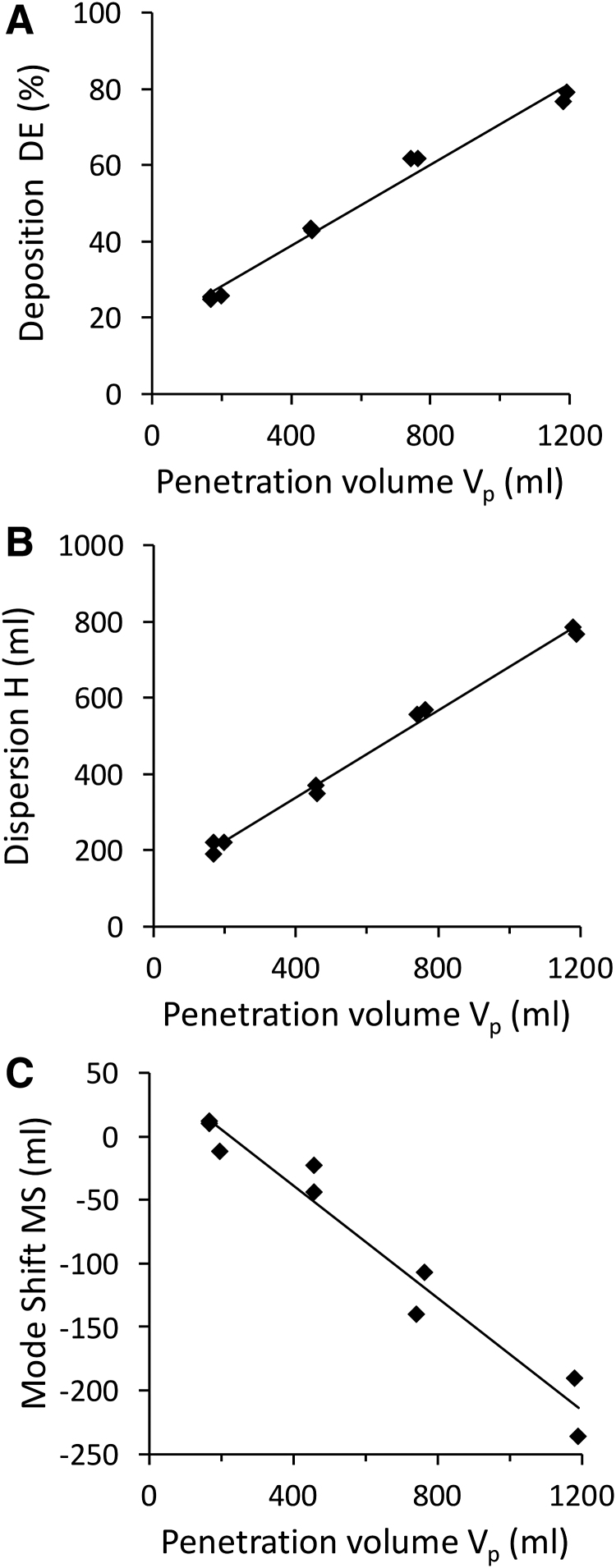
Figure 1 shows raw data obtained at one time point in one subject (S1, 45 years old) for each bolus parameter (i.e., deposition [Fig. 1A], dispersion [Fig. 1B], and mode shift [Fig. 1C]) as a function of penetration volume, illustrating the linear relationship between each bolus parameter and penetration volume. Similar trends were found at each time point for all subjects. There was no effect of age on the fit of the linear regression with bolus data. Regression lines between each bolus parameter and penetration volume were then used to compare datasets obtained at different time points. Regression lines were characterized by their slope (DE-slope, H-slope, and MS-slope for deposition, dispersion, and mode shift, respectively) and by the value of each bolus parameter at shallow depth, defined as Vp = 200 mL (DE200, H200, MS200, respectively).
FIG. 1.
Aerosol deposition (A), dispersion (B) and mode shift (C) in subject S1 at one time point. Raw data for each individual bolus test are shown along with linear regression line from which data at targeted penetration volume were calculated (see Data Analysis section for details).
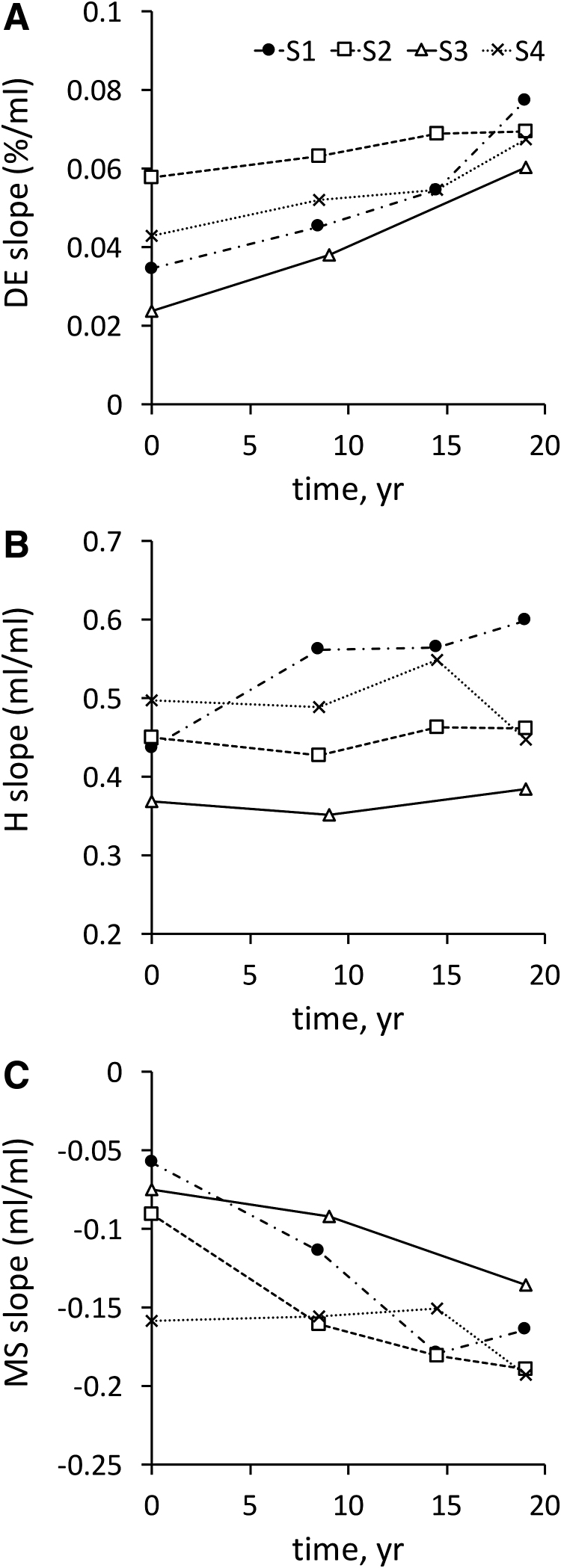
Aerosol bolus data were collected at times 0, 9, and 19 years for all subjects and also at t = 15 years for three of the four subjects. Figure 2 shows the individual regression slope for each aerosol bolus parameter (DE-slope, H-slope, and MS-slope) as a function of time.
FIG. 2.
Individual slope of the regression lines between aerosol bolus parameters and penetration volume as a function of time. (A) DE-slope. (B) H-slope. (C) MS-slope. DE, deposition; H, dispersion; MS, mode shift.
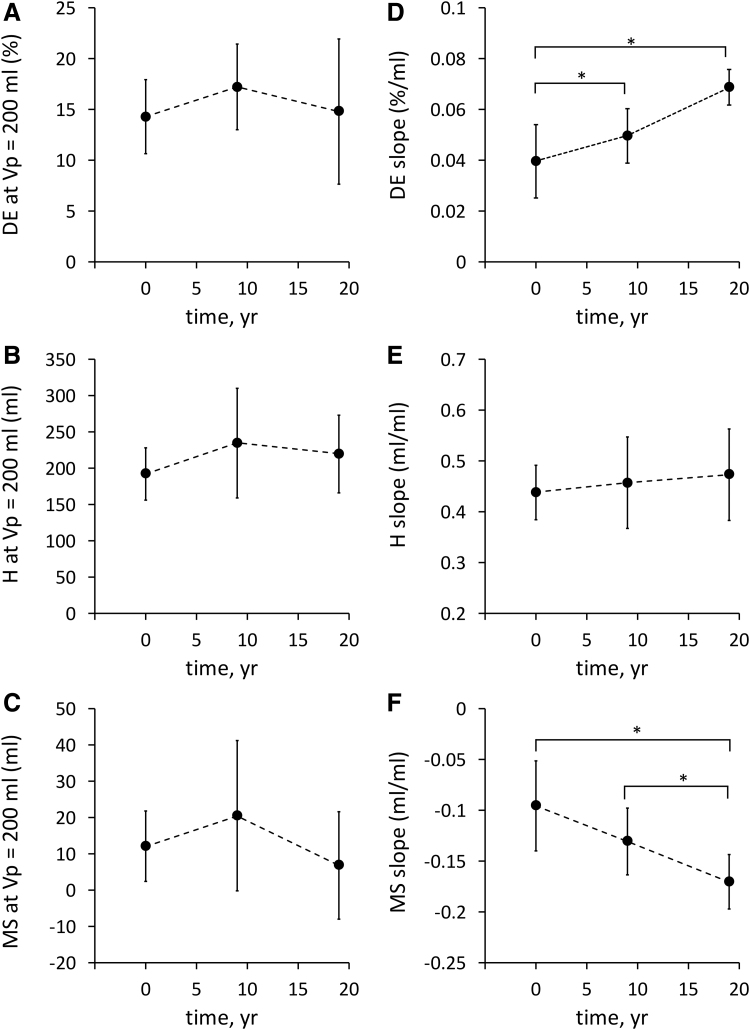
Figure 3 displays data averaged over the four subjects (mean ± standard deviation for data collected at times 0, 9, and 19 years. Figure 3A–C shows the averaged value for each bolus parameter at shallow depth (DE200, H200, and MS200, respectively). Figure 3D–F shows the averaged slope of the regression lines (DE-slope, H-slope, and MS-slope, respectively). Slope-DE significantly increased from 0.040 ± 0.014 at year 0 to 0.050 ± 0.011 at year 9 and to 0.069 ± 0.007%/mL at year 19 (p = 0.02, Fig. 3D) with no significant difference in DE at shallow depth (DE200: 14 ± 4 at year 0 vs. 17 ± 4 at year 9 vs. 15% ± 7% at year 19, p = 0.25, Fig. 3A). There was no significant effect of age on either slope-H (0.44 ± 0.05 at year 0 vs. 0.46 ± 0.09 at year 9 vs. 0.47 ± 0.09 mL/mL at year 19, p = 0.6, Fig. 3E) or dispersion at shallow depth (H200: 192 ± 36 at year 0 vs. 234 ± 76 at year 9 vs. 220 ± 54 mL at year 19, p = 0.2, Fig. 3B). Finally, MS-slope was significantly more negative with time (−0.096 ± 0.044 at year 0 vs. −0.131 ± 0.033 at year 9 vs. −0.171 ± 0.027 mL/mL at year 19, p = 0.001, Fig. 3F) with no significant variation in mode shift at shallow depth (MS200: 12 ± 10 at year 0 vs. 20 ± 21 at year 9 vs. 7 ± 15 mL at year 19, p = 0.3, Fig. 3F).
FIG. 3.
Bolus parameters at shallow depth (i.e., at Vp = 200 mL; [A] DE200, [B] H200, [C] MS200) and slope of the regression lines between aerosol bolus parameters and penetration volume ([D] DE-slope, [E] H-slope, [F] MS-slope). Data are averaged over the four subjects (mean ± standard deviation) and displayed as a function of time. *p < 0.05. Note that because data from one subject (S3) were not available at year 15, only the three time points for which n = 4 are shown. Vp, penetration volume.
Discussion
We have collected aerosol bolus data in four healthy subjects at regular time intervals over a 19-year period and determined the effect of aging on several aerosol bolus parameters. This study offers a number of findings. First, while there was no major difference in deposition at shallow depth (Fig. 3A), there was an increase in peripheral deposition with aging (Fig. 3D), which we speculate is a result of increasing closing volume with age. Second, neither dispersion at shallow depth (Fig. 3B) nor the increase in dispersion as penetration depth increased (Fig. 3E) was affected by age, suggesting that alterations in the mechanical properties of healthy adult lungs with age occur uniformly. Third, the magnitude of the mode shift increased with age for a bolus that penetrates deep into the lung (Fig. 3F) likely because of the concomitant increase in peripheral deposition and alterations in flow sequencing. These findings are discussed in detail below.
Aerosol deposition
While numerous studies have been performed to measure the deposition of inhaled particles under different conditions and for a wide range of particle size,(14–17) very few have looked at the effect of age on deposition in the human adult lung.(4,5) Two cross-sectional studies in subjects 18–80(4) and 20–70 years of age(5) found no significant effect of age on aerosol deposition during spontaneous breathing. However, when subjects were asked to breathe in a fixed pattern with a tidal volume of 360 mL and a breathing period of 3.4 seconds, Bennett and colleagues found significantly lower deposition in the 60–80-year age group than in the 18–40-year age group.(4) This is in contrast to our data that showed an increase in peripheral deposition with aging (Fig. 3D). Differences in experimental protocols likely explain these conflicting results. The study by Bennett and colleagues(4) used tidal breathing of 2 μm-diameter particles over a 30-second period while the current study used 1 μm-diameter aerosol bolus inhalations with a tightly controlled breathing maneuver from RV to FRC +1 L and back to RV. The loss of elastic recoil with aging causes an increase in mean airspace size, which could explain the decrease in deposition observed by Bennett and colleagues.(4) However, the reduction in lung elastic recoil with increasing age also results in airway closure occurring at higher lung volumes(18) than in the younger lung. This would tend to trap aerosols in the distal lung toward the end of a deep exhalation, and thus increase peripheral deposition. Such effect should be minimal during tidal breathing as closing volume is less than the expiratory reserve volume for healthy subjects, at least for those younger than about 65 years.(18) On the other hand, in lung volume maneuvers beginning at RV, closing volume is likely to affect the deposition of aerosol boluses inhaled deep in the lung periphery and this effect is expected to increase with age as closing volume increases with age. Based on data from Leblanc and colleagues,(18) closing volume increases by an average of 570 mL over a 19-year period. As breathing volume and flow rates were kept constant between study sessions, the increase in closing volume with age is likely the main mechanism responsible for the increase in peripheral deposition we measured.
Aerosol bolus dispersion
While there was an increase in peripheral deposition with age, our data showed no effect of age on either slope-H (Fig. 3E) or dispersion at shallow depth (Fig. 3B). Aerosol bolus dispersion has been shown to be sensitive to both convective ventilatory inhomogeneities in the lung(11,19,20) and to structural change in the airspaces.(13,21–23) In particular, regional variations in airway resistance and in the compliance of subtended acinar units create heterogeneities in regional time constants, which affect the synchrony of regional ventilation during expiration, which leads to an increase in the width of the expired bolus. The absence of change in slope-H over time suggests that any variation in resistance and compliance of lung units occurs relatively uniformly throughout the lung during healthy aging.
Multiple breath washout (MBW) tests have been extensively used to study the distribution of ventilation in the lung with the ability to discern between ventilation inhomogeneities arising from the proximal and peripheral region of the lung through the MBW indices of Scond and Sacin, respectively. In a cross-sectional MBW study looking at the effect of age on ventilation heterogeneity in subjects 20–80 years of age, Verbanck and colleagues showed an age-squared dependence on diffusive ventilatory inhomogeneities (Sacin) and a smaller age effect on convective ventilatory inhomogeneities (Scond).(24) However, unlike gases, when particles are used, diffusive processes are very much smaller. Thus, any changes in aerosol bolus dispersion primarily reflects convective ventilatory inhomogeneities. Over a 19-year period, Scond is expected to increase by ∼20%(24) in contrast to our data that showed a nonsignificant increase in slope-H (∼9%, Fig. 3E) over the same time period. One further factor in these differences is the increase in peripheral deposition with age (Fig. 3D), which serves to erode the tail of the exhaled bolus and thus reduce its spread, minimizing the increase in aerosol dispersion.(20)
Mode shift
Our data showed no significant effect of age on mode shift at shallow depth (Fig. 3C). Yet, at larger penetration volumes, the mode of the expired boluses was shifted to smaller volumes, (toward the mouth) in an age-dependent manner (Fig. 3F). This shift might be explained by changes in flow sequencing and/or by an increase in peripheral particle deposition. A change in flow sequencing could manifest as a central shift of the mode of the bolus without necessarily increasing bolus dispersion. This is because sequencing does not automatically imply a change in the relative filling of different regions of the lung but, rather, a reordering of the emptying sequence of those regions.(25) The small increase in Scond with age(24) supports a contribution of flow sequencing to the age-dependent increase in the magnitude of the mode shift. The increase in slope-MS may also be explained by the increase in peripheral deposition within increasing age. Indeed, particles that penetrate deeper into the lung deposit more, eroding the distal tail of the bolus and, therefore, shifting the mode of the expired bolus proximally.
In conclusion, we collected the first ever longitudinal dataset of aerosol bolus inhalations over a 19-year period. Although unique, this dataset only includes a small number of healthy subjects and it is thus unknown if our results can be generalized to a larger group of subjects. Nevertheless, in the four subjects we investigated, data showed that both aerosol bolus deposition and mode shift were affected by age. Aging caused peripheral deposition to increase likely because of an increase in closing volume. Increased peripheral deposition along with increase in flow sequencing likely explain the mouthward shift of the peak of the expired bolus, however aerosol bolus dispersion was unaltered.
Acknowledgments
This work benefited substantially from the technical contribution of Trevor Cooper, Janelle Fine, and Jeff Struthers. Data from earlier years were collected under support from NIEHS and NASA under various grants as indicated in the referenced publications from which the data are drawn.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
The authors declare they have no competing financial interests.
Funding Information
This work was partially supported by the National Institute of Environmental Health Sciences (NIEHS) of the National Institutes of Health under Award Number U01ES028669.
Reviewed by:
Kirby Zeman
Gerhard Scheuch
References
- 1. Knudson RJ, Dumont FC, Kennedy TC, and Knudson DE: Effect of aging alone on mechanical properties of the normal adult human lung. J Appl Physiol. 1977;43:1054–1062 [DOI] [PubMed] [Google Scholar]
- 2. Rizzato G, and Marazzini L: Thoracoabdominal mechanics in elderly men. J Appl Physiol. 1970;28:457–460 [DOI] [PubMed] [Google Scholar]
- 3. Harik-Khan RI, Wise RA, and Fozard JL: Determinants of maximal inspiratory pressure: The baltimore longitudinal study of aging. Am J Respir Crit Care Med. 1998;158:1459–1464 [DOI] [PubMed] [Google Scholar]
- 4. Bennett WD, Zeman KL, and Kim C: Variability of fine particle deposition in healthy adults: Effert of age and gender. Am J Respir Crit Care Med. 1996;153:1641–1647 [DOI] [PubMed] [Google Scholar]
- 5. Rissler J, Gudmundsson A, Nicklasson H, Swietlicki E, Wollmer P, and Londahl J: Deposition efficiency of inhaled particles (15–5000 nm) related to breathing pattern and lung function: An experimental study in healthy children and adults. Part Fibre Toxicol. 2017;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Darquenne C, West JB, and Prisk GK: Deposition and dispersion of 1 μm aerosol boluses in the human lung: Effect of micro- and hypergravity. J Appl Physiol. 1998;85:1252–1259 [DOI] [PubMed] [Google Scholar]
- 7. Darquenne C, and Prisk GK: Deposition of inhaled particles in the human lung is more peripheral in lunar than in normal gravity. Eur J Appl Physiol. 2008;103:687–695 [DOI] [PubMed] [Google Scholar]
- 8. Darquenne C, and Prisk GK: Particulate deposition in the human lung under lunar habitat conditions. Aviat Space Environ Med. 2013;84:190–195 [DOI] [PubMed] [Google Scholar]
- 9. Westenberger S, Gebhart J, Jaser S, Knoch M, and Kostler R: A novel device for the generation and recording of aerosol micro-pulses in lung diagnostic. J Aerosol Sci. 1992;23:S449–S452 [Google Scholar]
- 10. Heyder J, Blanchard JD, Feldman HA, and Brain JD: Convective mixing in human respiratory tract: Estimates with aerosol boli. J Appl Physiol. 1988;64:1273–1278 [DOI] [PubMed] [Google Scholar]
- 11. Darquenne C, West JB, and Prisk GK: Dispersion of 0.5–2 μm aerosol in μG and hypergravity as a probe of convective inhomogeneity in the lung. J Appl Physiol. 1999;86:1402–1409 [DOI] [PubMed] [Google Scholar]
- 12. Peterson JB, Prisk GK, and Darquenne C: Aerosol deposition in the human lung periphery is increased by reduced-density gas breathing. J Aerosol Med Pulm Drug Deliv. 2008;21:159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blanchard JD: Aerosol bolus dispersion and aerosol-derived airway morphometry: Assessment of lung pathology and response to therapy, part 1. J Aerosol Med. 1996;9:183–205 [DOI] [PubMed] [Google Scholar]
- 14. Heyder J, Armbruster L, Gebhart J, Grein E, and Stahlhofen W: Total deposition of aerosol particles in the human respiratory tract for nose and mouth breathing. J Aerosol Sci. 1975;6:311–328 [Google Scholar]
- 15. Heyder J, Gebhart J, Rudolf G, Schiller CF, and Stahlhofen W: Deposition of particles in the human respiratory tract in the size range 0.005–15μm. J Aerosol Sci. 1986;17:811–825 [Google Scholar]
- 16. Foord N, Black A, and Walsh M: Regional deposition of 2.5–7.5 Êm diameter inhaled particles in healthy male non-smokers. J Aerosol Sci. 1978;9 343–357 [Google Scholar]
- 17. Stahlhofen W, Rudolf G, and James AC: Intercomparison of experimental regional aerosol deposition data. J Aerosol Med. 1989;2 285–308 [Google Scholar]
- 18. Leblanc P, Ruff F, and Milic-Emili J: Effect of age and body position on “airway closure” in man. J Appl Physiol. 1970;28:448–451 [DOI] [PubMed] [Google Scholar]
- 19. Darquenne C, and Prisk GK: Aerosols in the study of convective acinar mixing. Resp Physiol Neurobiol. 2005;148 207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosenthal FS: The effect on nonuniform ventilation on the dispersion of inspired aerosol boluses—A modeling study. J Aerosol Med. 1993;6:177–197 [Google Scholar]
- 21. Hardy KG, Gann LP, Tennal KB, Walls R, Hiller FC, and Anderson PJ: Sensitivity of aerosol bolus behavior to metacholine-induced bronchoconstriction. Chest. 1998;114:404–410 [DOI] [PubMed] [Google Scholar]
- 22. Anderson PJ, and Dolovich MB: Aerosols as diagnostic tools. J Aerosol Med. 1994;7:77–88 [DOI] [PubMed] [Google Scholar]
- 23. Anderson PJ, Hardy KG, Gann LP, Cole R, and Hiller FC: Detection of small airway dysfunction in asymptomatic smokers using aerosol bolus behavior. Am J Respir Crit Care Med. 1994;150:995–1001 [DOI] [PubMed] [Google Scholar]
- 24. Verbanck S, Van Muylem A, Schuermans D, Bautmans I, Thompson B, and Vincken W: Transfer factor, lung volumes, resistance and ventilation distribution in healthy adults. Eur Respir J. 2016;47:166–176 [DOI] [PubMed] [Google Scholar]
- 25. Mills CN, Darquenne C, and Prisk GK: Mode shift of an inhaled aerosol bolus is correlated with flow sequencing in the human lung. J Appl Physiol. 2002;92:1232–1238 [DOI] [PubMed] [Google Scholar]