Abstract
The decrement in plasma glucose concentration with SGLT2 inhibitors (SGLT2i) is blunted by a rise in endogenous glucose production (EGP). We investigated the ability of incretin treatment to offset the EGP increase. Subjects with type 2 diabetes (n = 36) were randomized to 1) canagliflozin (CANA), 2) liraglutide (LIRA), or 3) CANA plus LIRA (CANA/LIRA). EGP was measured with [3-3H]glucose with or without drugs for 360 min. In the pretreatment studies, EGP was comparable and decreased (2.2 ± 0.1 to 1.7 ± 0.2 mg/kg ⋅ min) during a 300- to 360-min period (P < 0.01). The decrement in EGP was attenuated with CANA (2.1 ± 0.1 to 1.9 ± 0.1 mg/kg ⋅ min) and CANA/LIRA (2.2 ± 0.1 to 2.0 ± 0.1 mg/kg ⋅ min), whereas with LIRA it was the same (2.4 ± 0.2 to 1.8 ± 0.2 mg/kg ⋅ min) (all P < 0.05 vs. baseline). After CANA, the fasting plasma insulin concentration decreased (18 ± 2 to 12 ± 2 μU/mL, P < 0.05), while it remained unchanged in LIRA (18 ± 2 vs. 16 ± 2 μU/mL) and CANA/LIRA (17 ± 1 vs. 15 ± 2 μU/mL). Mean plasma glucagon did not change during the pretreatment studies from 0 to 360 min, while it increased with CANA (69 ± 3 to 78 ± 2 pg/mL, P < 0.05), decreased with LIRA (93 ± 6 to 80 ± 6 pg/mL, P < 0.05), and did not change in CANA/LIRA. LIRA prevented the insulin decline and blocked the glucagon rise observed with CANA but did not inhibit the increase in EGP. Factors other than insulin and glucagon contribute to the stimulation of EGP after CANA-induced glucosuria.
Introduction
SGLT2 inhibitors (SGLT2i) block renal glucose–sodium cotransport, producing glucosuria and reducing the plasma glucose concentration in patients with type 2 diabetes (1,2). However, the glucose-lowering efficacy of SGLT2i is partly offset by a rise in endogenous glucose production (EGP) (3,4). After both acute (3) and 4 weeks (4) of SGLT2i administration, plasma glucagon concentration tends to increase, while plasma insulin concentration decreases. These hormonal changes could explain, in part, the rise in EGP. Although other factors, including enhanced renal glucose reabsorption by SGLT1 (5), have been implicated as potential mechanisms responsible for the less-than-expected reduction in plasma glucose concentration after SGLT2 administration, the rise in EGP is an important contributing factor. EGP is under tight hormonal control by insulin and glucagon, and even modest changes in these two hormones can lead to significant changes in EGP (6,7). Further, the plasma glucose concentration itself regulates EGP, and a decrease in plasma glucose concentration, independent of changes in plasma insulin and glucagon, can stimulate EGP (6,8).
Canagliflozin (CANA) promotes urinary glucose loss of ∼70–90 g/day, but the decreases in the HbA1c either in short-term or in longer studies are ∼0.6–0.8% but no greater than1.0% (9,10). In a previous study (3), we demonstrated that a single dose of dapagliflozin reduced the plasma glucose concentration but was accompanied by an elevation in EGP. Further, the increase in EGP was associated with an increase in plasma glucagon concentration and decrease in plasma insulin concentration. We hypothesized that these hormonal changes could, in part, explain the increase in EGP. Glucagon-like peptide 1 (GLP-1) receptor agonists are potent insulin secretagogues and inhibit glucagon secretion (11,12). If the decline in plasma insulin and increase in plasma glucagon contribute to the rise in EGP after SGLT2i, one would predict that simultaneous administration of GLP-1 receptor agonist with SGLT2i would prevent the rise in EGP. Therefore, we administered liraglutide (LIRA) with CANA and quantitated the effect of combination therapy on EGP.
Research Design and Methods
Subjects
Thirty-six subjects with type 2 diabetes were randomized 1:1:1 to receive a single dose (100 mg) of CANA, a single subcutaneous injection (1.2 mg) of LIRA, or CANA plus LIRA (CANA/LIRA). By virtue of the study design, the study was not blinded, although all sample analyses were conducted in blinded fashion. The clinical, laboratory, and anthropometric characteristics were comparable in all three groups (Table 1). Patients were drug naïve (n = 6) or on a stable dose of metformin with or without sulfonylureas (n = 30) (Table 1). Except for diabetes, all subjects were in good general health based on medical history, physical exam, screening blood tests, urinalysis, and electrocardiogram. Weight was stable (±1.5 kg) in all subjects for at least 3 months prior to study, and no subject participated in any excessively heavy exercise programs. Subjects with evidence of proliferative diabetic retinopathy or serum creatinine >1.4 mg/dL (females) or >1.5 mg/dL (males), or with estimated glomerular filtration rate <60 mL/min/1.73 m2, were excluded. The study was approved by the University of Texas Health Science Center at San Antonio (UTHSCSA) Institutional Review Board, and informed written consent was obtained from all participants prior to the study.
Table 1.
Baseline patient characteristics
| CANA | LIRA | CANA/LIRA | P | |
|---|---|---|---|---|
| n | 12 | 12 | 12 | |
| Age (years) | 53 ± 6 | 53 ± 6 | 51 ± 8 | NS |
| Sex, n male/female | 8/4 | 8/4 | 6/6 | — |
| Weight (kg) | 98.1 ± 11.4 | 96.6 ± 8.4 | 98.8 ± 9.1 | NS |
| BMI (kg/m2) | 33.6 ± 6.5 | 34.8 ± 4.6 | 34.9 ± 4.4 | NS |
| Body fat (%) | 37.8 ± 6.7 | 36.9 ± 7.2 | 37.3 ± 7.7 | NS |
| HbA1c, % (mmol/mol) | 8.2 ± 1.2 (72.7 ± 10.6) | 8.4 ± 1.6 (68.3 ± 13.8) | 8.1 ± 1.8 (65.0 ± 15.3) | NS |
| FPG (mg/dL) | 173 ± 14 | 185 ± 16 | 182 ± 21 | NS |
| Treatment, n# | ||||
| Metformin | 3 | 3 | 2 | — |
| Metformin + sulfonylurea | 27 | 27 | 28 | — |
Data are mean ± SD unless otherwise indicated.
#All other participants were treated with diet alone.
Experimental Design
All studies were performed at the Texas Diabetes Institute Clinical Research Center (CRC) at 6:00 a.m. after a 10-h overnight fast. On the day of screening, subjects received a DEXA scan for quantitation of total body lean and fat mass. Eligible subjects subsequently received two 9-h measurements of EGP with [3-3H]glucose infusion. During one study, EGP was measured without any drugs (“pretreatment study”), and during the second study EGP was measured with a drug or drugs (“treatment study”) as described below. The two studies (pretreatment and treatment studies) were performed on a separate day within a 7- to 14-day period in random fashion. In the interim, subjects were asked to maintain their stable treatment with diet/exercise and either metformin or metformin/sulfonylurea. Subjects were randomized to receive one of the following: 1) a single dose, 100 mg, of CANA; 2) a single subcutaneous injection of 1.2 mg LIRA; or 3) CANA/LIRA. All drugs were given at time t = 0 min, i.e., 180 min after the priming/tracer infusion were begun. After completion of this study, subjects continued on a 4-month treatment period with the medication to which they were randomized. Since the 4-month treatment period is still ongoing, these results will be reported at a later date.
Measurement of EGP
Subjects reported to CRC at 6:00 a.m., a catheter was placed into an antecubital vein, and an individualized prime dose calculated on the basis of the fasting plasma glucose (FPG) values (40 μCi × FPG/100) followed by a continuous (0.4 μCi/min) infusion of [3-3H]glucose was started and continued until 3:00 p.m. At 8:00 a.m., a second catheter was placed retrogradely in a vein on the dorsum of the hand, which was placed in a heated box (70°C) for sampling of arterialized blood. After 3 h of tracer equilibration, arterialized blood samples were drawn at −30, −20, −15, −10,−5, and 0 (time zero = drug administration) minutes. At 9:00 a.m. (time zero), plasma samples were obtained every 20 min for 360 min for determination of plasma glucose, insulin, and glucagon concentrations and [3-3H]glucose radioactivity. At 6:00 a.m., subjects voided and the urine was discarded. Urine was collected from 6:00 a.m. to 9:00 a.m. and from 9:00 a.m. to 3:00 p.m. Urinary volume and glucose concentration were measured to determine urinary glucose excretion (UGE). At 3:00 p.m., subjects received a meal and returned home. At 7- to 14-day intervals, subjects returned to the CRC for a repeat EGP measurement with CANA, LIRA, or CANA/LIRA. Thus, all subjects received measurement of EGP without any drug(s) and served as their own control subjects. Plasma glucose was measured using the glucose-oxidase method (Analox Reagent Instruments, International Point of Care, Toronto, Ontario, Canada); plasma insulin (IBL America, Minneapolis, MN) and C-peptide (MP Biomedicals, Santa Ana, CA) were measured with immunoradiometric assays, and plasma glucagon (MilliporeSigma, Burlington, MA) was measured by radioimmunoassay.
Data Analysis
The primary end point was change from baseline (−30 to 0 min) in EGP during the last hour (300- to 360-min time period) after administration of a single dose of each drug (as monotherapy or in combination) compared with the change in EGP during the pretreatment study. EGP was calculated as previously described (3). Under steady-state postabsorptive conditions, the basal endogenous glucose Ra equals the [3-3H]glucose infusion rate divided by steady-state plasma tritiated glucose specific activity. After drug administration, nonsteady conditions for [3-3H]glucose specific activity prevail and the total body glucose Ra is calculated using the Steele equation. Total glucose Rd is calculated with the same equation, and tissue glucose disposal (tissue Rd) is derived by subtracting the UGE from total Rd.
The change from baseline in EGP after drug administration (treatment study) was compared with the change from baseline in the pretreatment study in each group using a paired t test. Differences between the two studies (EGP measured in the pretreatment and treatment studies) were compared among the three treatment groups with ANCOVA. Post hoc testing was done with the Bonferroni correction.
Similar comparisons were made for the change in plasma insulin and glucagon concentrations during each study. All values are presented as mean ± SEM, except for patient characteristics that are presented as mean ± SD. A P value <0.05 was considered statistically significant.
Results
During the pretreatment study, UGE was similar in all three groups during the 3-h equilibration period (∼17 mg/min) and during the subsequent 6-h study period (∼8 mg/min). In the CANA treatment study, UGE was mean ± SEM 11 ± 8 mg/min during the 3-h equilibration period and increased to 88.5 ± 13.3 mg/min (P < 0.001) during the 6 h after CANA administration. In the LIRA treatment study, UGE was 10 ± 6 mg/min in the equilibration period and decreased to 6 ± 5 mg/min after LIRA injection. In CANA/LIRA treatment study, UGE was 7 ± 4 mg/min during the equilibration period and increased to 111 ± 33 mg/min (P < 0.001).
The mean ± SEM FPG concentration (−30 to 0 min) during the baseline (pretreatment) study was 165 ± 8 mg/dL in CANA, 167 ± 9 mg/dL in LIRA, and 175 ± 7 mg/dL in CANA/LIRA. During the 300- to 360-min time period, the mean plasma glucose decreased similarly to 144 ± 6, 137 ± 9, and 127 ± 6 mg/dL, respectively (all P < 0.05 vs. fasting). Thus, the decrement in FPG was 21 ± 11, 30 ± 8, and 48 ± 12 mg/dL, respectively, in CANA, LIRA, and CANA/LIRA groups during the pretreatment studies (P = not significant [NS]) (Table 2). During the treatment study with drug administration, the mean plasma glucose concentration decreased from 156 ± 10 to 125 ± 9 mg/dL during the 300- to 360-min time period in the CANA group. After LIRA, the mean plasma glucose decreased from 147 ± 7 to 112 ± 6 mg/dL and after CANA/LIRA the mean plasma glucose decreased from 186 ± 12 to 122 ± 8 mg/dL (all P < 0.05 for predrug vs. postdrug). Thus, the decrement in FPG after drug administration was 31 ± 6, 35 ± 9, and 65 ± 11 mg/dL in CANA, LIRA, and CANA/LIRA groups during treatment studies, respectively (P < 0.05 CANA/LIRA vs. CANA or LIRA).
Table 2.
Decrement in FPG concentration and decrement in EGP during control study performed without drug and treatment study performed with CANA, LIRA, and CANA/LIRA
| Parameters | CANA | LIRA | CANA/LIRA |
|---|---|---|---|
| ΔFPG (mg/dL) without drug | −21 ± 11 | −30 ± 8 | −48 ± 12 |
| ΔFPG (mg/dL) with drug | −31 ± 6 | −35 ± 9 | −65 ± 11* |
| ΔEGP (mg/kg ⋅ min) without drug | −0.5 ± 0.1 | −0.6 ± 0.2 | −0.6 ± 0.1 |
| ΔEGP (mg/kg ⋅ min) with drug | −0.2 ± 0.1† | −0.8 ± 0.2 | −0.2 ± 0.1† |
Data are mean ± SEM.
*P < 0.05 vs. CANA and vs. LIRA;
†P < 0.05 vs. baseline study without drug.
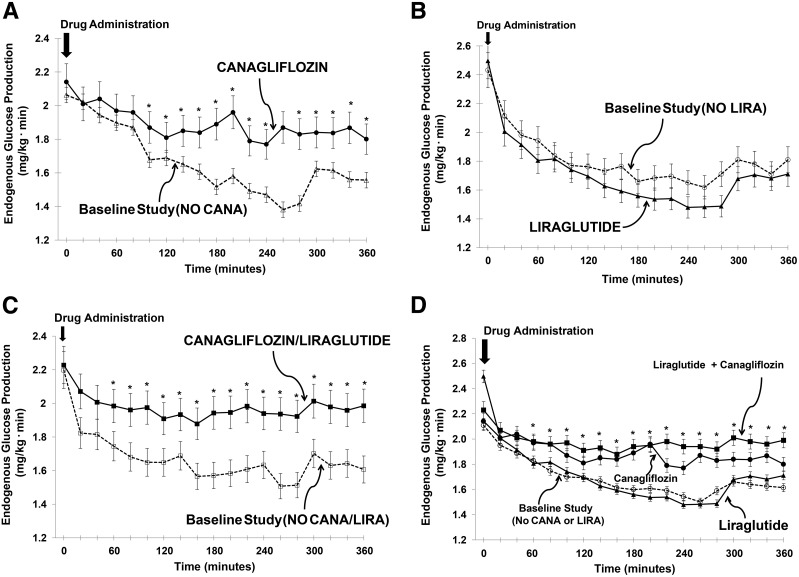
During the pretreatment study, mean ± SEM EGP decreased similarly, by 0.5 ± 0.1, 0.6 ± 0.2, and 0.6 ± 0.1 mg/kg ⋅ min, during the 300- to 600-min time period in CANA, LIRA, and CANA/LIRA groups, respectively (P < 0.01 in all groups vs. fasting EGP, and P = NS between groups). During the repeat measurement of EGP (treatment study), the decrease in EGP in subjects receiving CANA was significantly less than in the pretreatment study (from 2.1 ± 0.1 to 1.9 ± 0.1 mg/kg ⋅ min; Δ = −0.2 ± 0.1 mg/kg ⋅ min; P < 0.05 vs. pretreatment study) (Figs. 1A and 2A and Table 2). In contrast, after a single injection of LIRA, the decrement in EGP (−0.8 ± 0.2 mg/kg ⋅ min) was comparable with that seen during the pretreatment study (from 2.4 ± 0.2 to 1.8 ± 0.2 mg/kg ⋅ min) (Figs. 1B and 2A). After combined CANA/LIRA treatment, the decrement in EGP (−0.2 ± 0.2 mg/kg ⋅ min) was less than the decrement in the pretreatment study (P < 0.05) and similar to that in subjects receiving CANA alone (−0.2 ± 0.1 mg/kg ⋅ min) (Figs. 1C and 2A and Table 2). The decrease in plasma glucose concentration strongly correlated with the decrease in EGP in all treatment studies, including in subjects receiving CANA alone (r = 0.87, P < 0.01), LIRA alone (r = 0.91, P < 0.01), and CANA/LIRA (r = 0.57, P < 0.05).
Figure 1.
EGP during the baseline study and after drug administration. The mean ± SEM rate of EGP (mg/kg ⋅ min) from 0 to 360 min in the baseline study (dotted line and open symbols) and after drug administration (solid line and solid symbols) is depicted. A single dose of CANA (A) significantly attenuated the decrease in EGP by ∼50%. When subjects received a single injection of LIRA, there was no change in EGP (B). After the administration of CANA/LIRA, the decline in EGP was attenuated by ∼50% (C), i.e., similar to results with CANA alone (A). Comparable results were obtained when the data in each of the baseline studies without drug administration were averaged and compared with drug treatment (D). *P < 0.05, CANA and CANA/LIRA vs. baseline study and vs. LIRA.
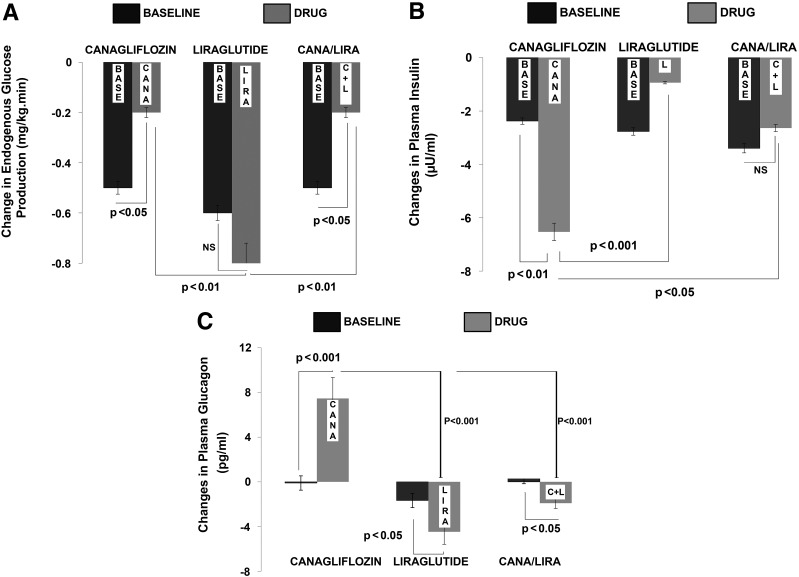
Figure 2.
Mean ± SEM changes in EGP (A) and in plasma insulin (B) and glucagon (C) concentrations during the 300- to 360-min time period in the baseline (BASE) study and after each drug administration. After a single dose of CANA, there was a significant ∼50% reduction in EGP (A, left) compared with the baseline study. A similar reduction was observed when CANA and LIRA were administered in combination (A, right). After a single injection of LIRA, there was no significant change in EGP (A, center). The reduction in EGP after CANA was similar to that seen after the combination treatment with CANA/LIRA injection, but both were statistically different (P < 0.01) compared with the change seen with LIRA injection alone. After a single dose of CANA, there was a significant reduction in plasma insulin concentration compared with the baseline study (B, left). After a single injection of LIRA, there was a small nonsignificant attenuation in the decrease in plasma insulin concentration (P = NS) from baseline (B, middle). When LIRA was administered with CANA, there was no change in plasma insulin concentration (B, right). The reduction in plasma insulin after CANA was significantly greater than that with LIRA alone (P < 0.001) and that after combination treatment with CANA/LIRA (P < 0.05). After CANA administration, the elevation in plasma glucagon concentration was significantly greater than during the baseline study (C, left). In contrast, with both LIRA alone (C, middle) and CANA/LIRA (C, left), plasma glucagon decreased slightly. The increase in plasma glucagon after CANA was significantly greater and went in a direction opposite that observed after the LIRA injection (P < 0.001) and also after the combination treatment with CANA/LIRA (P < 0.001). C+L, combination treatment with CANA/LIRA injection.
Tissue Rd changed similarly from baseline (−30 to 0 min) to steady state (300–360 min) in the CANA pretreatment study (mean ± SEM 2.1 ± 0.1 to 1.6 ± 0.1 mg/kg ⋅ min), in the LIRA pretreatment study (2.5 ± 0.2 to 1.9 ± 0.2 mg/kg ⋅ min), and in the CANA/LIRA pretreatment study (2.2 ± 0.2 to 1.8 ± 0.2 mg/kg ⋅ min). Similarly, in the CANA treatment study, tissue Rd decreased from 2.1 ± 0.1 to 1.6 ± 0.1 mg/kg ⋅ min, in the LIRA treatment study from 2.4 ± 0.2 to 1.8 ± 0.2 mg/kg ⋅ min, and in the CANA/LIRA treatment study from 2.2 ± 0.2 to 1.6 ± 0.2 mg/kg ⋅ min (P = NS between groups; P < 0.05 all baseline vs. steady state).
During the pretreatment studies, the mean ± SEM plasma insulin concentration tended to decrease similarly in all three groups (decrement = −2.0 ± 0.4 μU/mL in CANA, −3.0 ± 0.5 μU/mL in LIRA, and −3.0 ± 0.6 μU/mL in CANA/LIRA; all P = NS), with a mean absolute decrease from 19 ± 1.1 to 17 ± 2.0 μU/mL. After CANA treatment, plasma insulin decreased from 18 ± 2 to 11 ± 2 μU/mL (P < 0.01 vs. pretreatment study), whereas it did not change significantly after LIRA treatment (from 17 ± 2 to 16 ± 2 μU/mL) (Fig. 2B). When CANA was given in combination with LIRA (CANA/LIRA treatment study), plasma insulin also did not change significantly (16 ± 1 to 14 ± 2 μU/mL). Thus, after CANA treatment the decrement in plasma insulin concentration (−7.0 ± 0.2 μU/mL) was greater than during the pretreatment study (P < 0.01) and greater than with LIRA treatment alone (−1.0 ± 0.6 μU/mL) and with CANA/LIRA treatment (−3.0 ± 0.6 μU/mL) (both P < 0.05) (Fig. 2B).
During the pretreatment studies, the mean ± SEM plasma C-peptide concentration decreased similarly in all three groups from 4.7 ± 0.3 ng/mL during the −30 to 0 min time period to 3.9 ± 0.3 ng/mL during 300–360 min (P < 0.05). After CANA treatment, plasma C-peptide decreased from 4.2 ± 0.4 to 3.1 ± 0.5 ng/mL (P < 0.05), whereas after LIRA treatment it did not change (4.8 ± 0.3 to 4.7 ± 0.6 ng/mL, P < 0.05 vs. CANA). After CANA/LIRA treatment, plasma C-peptide decreased slightly but not significantly (4.6 ± 0.3 to 3.8 ± 0.4 ng/mL). The decrement in plasma C-peptide during the pretreatment study (−0.7 ± 0.2 ng/mL) was ablated by LIRA treatment (increase of 0.1 ± 0.3 ng/mL, P < 0.01), whereas it was unchanged after CANA treatment (−0.7 ± 0.2 ng/mL) and CANA/LIRA treatment (−0.9 ± 0.1 ng/mL).
During the pretreatment studies, the mean ± SEM plasma glucagon concentration did not change in any of the three groups (73 ± 5 to 72 ± 5 pg/mL). After CANA treatment, plasma glucagon increased from 69 ± 3 pg/mL (−30 to 0 min) to 78 ± 2 pg/mL (300–360 min) (P < 0.05). In contrast, after LIRA treatment plasma glucagon decreased from 93 ± 6 to 80 ± 6 pg/mL at 200 min and then returned to the fasting value (89 ± 7 pg/mL). After CANA/LIRA, plasma glucagon did not change (69 ± 5 vs. 67 ± 9 pg/mL). The change in plasma glucagon after CANA (increase of 8.0 ± 1.0 pg/mL) was significantly greater (P < 0.001) than the change (−0.1 ± 0.3 pg/mL) during the pretreatment study (Fig. 2C). In contrast, the change in plasma glucagon levels after LIRA treatment (−5.0 ± 1.0 pg/mL from 300 to 360 min) was significantly less (P < 0.05) than during the pretreatment study (−2.0 ± 1.0 pg/mL). After CANA/LIRA treatment, the change in plasma glucagon levels (−2.0 ± 0.7 pg/mL) also was smaller than that seen during the pretreatment study (increase of 0.5 ± 0.6 pg/mL) (Fig. 2C).
The mean ± SEM ratio of plasma glucagon (pg ⋅ mL−1) to insulin (μU ⋅ mL−1) during the pretreatment study increased nonsignificantly from 3.8 ± 0.5 to 4.3 ± 0.4. After CANA treatment, the plasma glucagon-to-insulin ratio increased significantly from 3.9 ± 0.6 to 6.8 ± 0.5 (P < 0.05 vs. fasting and vs. pretreatment study). After LIRA treatment (5.4 ± 0.4 vs. 5.5 ± 0.6) and CANA/LIRA treatment (4.2 ± 0.4 to 4.7 ± 0.5), the glucagon-to-insulin ratio did not change.
During the pretreatment studies, UGE amounted to ∼7.5 mg/min and over the same time period mean ± SEM EGP was 157.0 ± 21.0 mg/min. With CANA treatment, UGE increased to 88.5 ± 13.3 mg/min and during the same time period EGP was 180.0 ± 14.7 mg/min. Thus, the EGP increment of 22.8 ± 7.8 mg/min compared with the pretreatment study after CANA offset the increment in UGE brought about with CANA by ∼28% (22.8 of 81.1 mg/min, where 81.1 mg/min is the difference in UGE between 88.5 mg/min with CANA minus 7.5 mg/min in the pretreatment study). With LIRA treatment, EGP during 0–360 min was 167.5 ± 18.6 mg/min, while UGE was 15.6 mg/min. With CANA/LIRA treatment, EGP was 193.3 ± 13.6 mg/min and UGE was 111.2 ± 12.3 mg/min during 0–360 min.
Discussion
The results of the current study confirm observations from prior studies and provide several novel observations. Consistent with previous studies (4,5), CANA offset the decrement in EGP by 0.3 mg/kg ⋅ min, which blunted the decline in plasma glucose concentration. Similar to dapagliflozin and empagliflozin (4,5), the attenuated decrement in EGP caused by CANA is evident after a single administration of the drug. Also consistent with previous observations (4,5), the blunted decline in EGP was associated with a significant increase in plasma glucagon concentration and decline in plasma insulin concentration. The novel observation, and in contrast to our hypothesis, was that LIRA failed to inhibit the CANA-induced rise in EGP despite blocking the elevation in plasma glucagon concentration and preventing the decline in plasma insulin concentration. Even though EGP in subjects receiving CANA was comparable with that in subjects receiving CANA/LIRA, there was a greater decrease in plasma glucose concentration when CANA/LIRA was given in combination compared with CANA alone or LIRA alone (mean ± SEM decrement in plasma glucose 31 ± 6, 35 ± 9, and 65 ± 11 mg/dL in CANA, LIRA, and CANA/LIRA groups, respectively). This can be explained, in part, by a higher starting plasma glucose (186 ± 12 mg/dL in CANA/LIRA vs. 156 ± 10 mg/dL with CANA and 147 ± 7 mg/dL with LIRA) and, in part, by a greater increase in UGE in CANA/LIRA from 0 to 360 min (111.2 ± 12.3 mg/min) versus CANA (88.5 ± 13.3 mg/min) and LIRA (5.6 ± 4.7 mg/min). These results indicate that factors other than glucagon and insulin must be responsible for the acute effect (within 40 min) on EGP (3) that persists at 4 weeks after administration of SGLT2i (4). Our results also help to explain why combining a GLP-1 receptor agonist with a SGLT2i might not produce an additive effect to reduce HbA1c (13), especially at HbA1c levels >8.0% (14).
Of note, the decrement in plasma insulin concentration during the pretreatment study was exaggerated after a single dose of CANA, and this decline was significantly attenuated when CANA and LIRA were administered in combination. The changes in plasma C-peptide concentration closely paralleled the changes in plasma insulin concentration, thus supporting that the decrement in plasma insulin concentration was secondary to a decrease in pancreatic insulin secretion. With regard to glucagon, the plasma concentration increased from 69 to 78 pg/mL after CANA and declined from 93 to 80 pg/mL after the administration of CANA/LIRA in combination, yet during the same time period, EGP rose by an identical amount in CANA and CANA/LIRA. Despite completely negating the rise in glucagon-to-insulin ratio after CANA, LIRA failed to attenuate the effect on EGP observed with CANA. Further support that glucagon is not involved in the CANA-induced rise in EGP is evident from the time courses of decline in EGP and change in plasma glucagon concentration (data not shown). Thus, the blunted decline in EGP observed with CANA and CANA/LIRA is evident within 40 min and becomes statistically significant by 120 min; yet, the plasma glucagon concentration is significantly lower during the 0- to 120-min time period. Also, in absolute terms the rise in plasma glucagon after CANA is quite modest.
What then is the signal that is elicited by the kidney after the induction of glucosuria with an SGLT2i that prompts the liver to produce glucose, i.e., the renal-hepatic axis? This signal must be rapidly generated, since administration of empagliflozin to subjects with normal glucose tolerance fails to alter the FPG concentration despite the rapid onset of glucosuria within 30 min (15). SGLT2 transporters are present only in the kidney (16) and perhaps in the α-cell (17), although the latter idea is controversial (18). Therefore, the initiating event must be related to excessive glucose loss in the urine, and intermediary messenger(s) originating in the kidney are likely to take part in this signaling process. Although this study was not designed to identify potential contributors to the metabolic adjustment to acute glucosuria, several factors including neural or hormonal changes or changes in circulating substrate(s) must be considered. Because of the rapidity of increase in EGP after CANA administration, the sympathoadrenergic system is likely to be involved. The fact that subjects had no complaints suggestive of a catecholamine surge and that neither the pulse rate nor blood pressure increased during the study (data not shown) argue against a role for epinephrine release by the adrenal medulla. Nonetheless, a role for norepinephrine, other neuro-hormonal mediators, or a neurogenic connection between the kidney and liver via the portal system or indirectly via the brain cannot be excluded. With respect to the first idea, in animals, as well as in subjects with and without diabetes, elevated renal vein norepinephrine and dopamine levels have been demonstrated during a hypoglycemic insulin clamp compared with a euglycemic insulin clamp (19,20). It also is possible that glucosuria triggers the release of a “renal signal” into the circulation, perhaps by “sensing” along the nephron of urinary glucose/calorie loss. Although the existence and the nature of such a mediator(s) are speculative, the signal could arise within renal tubules in response to a change in cellular glucose trafficking. Regardless of its origin, any “renal signal” in response to SGLT2i-induced glucosuria must be transmitted systemically either via neurogenic pathways or by a circulating humoral factor.
Although studies in experimental animals have demonstrated an increase in glucose output from the liver after glucosuria caused by phlorizin administration (21), it is possible that an increase in renal gluconeogenesis, which is known to take place in the proximal tubules (22–27), contributes to the rise in EGP after administration of the SGLT2i. The ability of the kidney to produce glucose is well-documented, and, unlike in the liver, renal gluconeogenesis is insensitive to changes in plasma glucagon concentration (26). Studies in animals (27,28) and humans (22,29,30) have shown that during prolonged fasting and in response to acidosis and hypoglycemia, renal glucose production is augmented. We previously demonstrated that, after glucose ingestion, glucose uptake by the liver is five- to sixfold greater than when the glucose is administered intravenously (31,32). In follow-up studies, Cherrington (6) demonstrated that the signal for enhanced hepatic glucose uptake after glucose ingestion results from the marked increase in glucose gradient from the portal vein to the hepatic artery. It is possible that a similar mechanism exists in the kidney. Inhibition of glucose absorption in the proximal tubule would be expected to result in a decrease in the renal vein glucose concentration. This would lead to a widening of the arteriovenous glucose concentration difference and, like in the liver but in the opposite direction, provide the signal for the kidney to enhance its production of glucose. However, from the quantitative standpoint it is unlikely that the kidney alone can explain the marked increase in EGP (29). When administered to normal subjects, SGLT2i produce ∼50–60 g/day glucosuria (28); yet, the FPG concentration remains unchanged (15,33). It is unlikely that the kidneys can produce such a large amount of glucose via gluconeogenesis. Moreover, the kidney contains only a small amount of glycogen (22).
A previous report indicated that a direct stimulatory action of SGLT2i on the pancreatic α-cells could be responsible for the increase in plasma glucagon concentration after exposure to SGLT2i (17). Presently, it is unclear whether a similar effect occurs in vivo in human subjects (18). However, if this were to occur in humans, obliteration of the rise in plasma glucagon concentration when LIRA was coadministered with CANA in the current study indicates that any direct stimulatory effect on glucagon release by the pancreatic α-cells was blocked by the GLP-1 receptor agonist.
There are some limitations to the current study. First, the period of observation was short, only 360 min, and changes that might have occurred beyond this time period in EGP, as well as in plasma glucagon and insulin concentrations, were not evaluated. Second, the extent to which the acute effect of SGLT2 inhibition on EGP and circulating hormone levels is representative of the long-term response to chronic glucosuria induced by SGLT2i in patients with type 2 diabetes remains unknown. On the other hand, our findings clearly demonstrate that the immediate stimulation of EGP is not offset by preventing the decline in plasma insulin and rise in plasma glucagon concentration.
In summary, the rise in EGP after induction of glucosuria with an SGLT2i is preserved, despite prevention of the decrease in plasma insulin and blockade of the elevation in plasma glucagon achieved with LIRA. These results strongly suggest that factors other than pancreatic hormones mediate the increase in EGP in response to SGLT2i-induced glucosuria. The rapid increase in EGP after inhibition of renal tubular glucose absorption suggests that neuro-hormonal–mediated mechanisms are involved. Whether the increase in EGP is sustained over a long period of time, and the extent to which the increase in EGP affects blood glucose control, remains to be determined.
Article Information
Acknowledgments. The authors give special thanks to the nurse coordinators, Horst Khan and Sara Olivarri, affiliated with the nursing staff of the UTHSCSA for help and support with patient recruitment and management.
Funding. This study was partly supported by the Texas Diabetes Institute of the University Health System (technicians and laboratory equipment and supplies) and the Division of Diabetes, UTHSCSA (nursing staff, faculty time and effort, and additional laboratory technicians, equipment, materials, and supplies).
Duality of Interest. This study was partly supported by Janssen Pharmaceuticals (investigator-initiated grant to R.A.D.). R.A.D. also receives grant support from AstraZeneca; is a member of the advisory boards of AstraZeneca, Janssen Pharmaceuticals, Lexicon, the Boehringer Ingelheim and Lilly Diabetes Alliance, and Novo Nordisk; and is a member of the speakers’ bureaus of Novo Nordisk, Merck, and AstraZeneca. E.C. receives grant support from AstraZeneca and Janssen Pharmaceuticals; is a member of the advisory boards of VeroScience, the Boehringer Ingelheim and Lilly Diabetes Alliance, and Sanofi; and is a member of the speakers’ bureaus of AstraZeneca, Janssen Pharmaceuticals, and the Boehringer Ingelheim and Lilly Diabetes Alliance. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. R.M., H.A.-J., A.M.A., J.A., C.T., and E.C. conducted the studies and analyzed and interpreted data. R.A.D. and E.C. designed the study; reviewed, analyzed, and interpreted data; and wrote the manuscript. M.A.-G. reviewed and critiqued the manuscript. R.A.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol 2017;13:11–26 [DOI] [PubMed] [Google Scholar]
- 2.Cersosimo E. Renal glucose handling and the kidney as a target for anti-diabetic medication. Curr Trends Endocrinol 2014;7:81–94 [Google Scholar]
- 3.Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdul-Ghani MA, DeFronzo RA, Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30-50% of filtered glucose load in humans. Diabetes 2013;62:3324–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherrington AD. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes 1999;48:1198–1214 [DOI] [PubMed] [Google Scholar]
- 7.Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab 2011;14:9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Andres R, Bedsoe TA, Boden G, Faloona GA, Tobin JD. A test of the hypothesis that the rate of fall in glucose concentration triggers counterregulatory hormonal responses in man. Diabetes 1977;26:445–452 [DOI] [PubMed] [Google Scholar]
- 9.Sha S, Polidori D, Heise T, et al. Effect of the sodium glucose co-transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2014;16:1087–1095 [DOI] [PubMed] [Google Scholar]
- 10.Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care 2013;36:2508–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bunck MC, Cornér A, Eliasson B, et al. Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care 2011;34:2041–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandoval DA, D’Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev 2015;95:513–548 [DOI] [PubMed] [Google Scholar]
- 13.Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2016;4:1004–1016 [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab 2017;19:1353–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Jobori H, Daniele G, Cersosimo E, et al. Empagliflozin and kinetics of renal glucose transport in healthy individuals and individuals with type 2 diabetes. Diabetes 2017;66:1999–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011;91:733–794 [DOI] [PubMed] [Google Scholar]
- 17.Bonner C, Kerr-Conte J, Gmyr V, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 2015;21:512–517 [DOI] [PubMed] [Google Scholar]
- 18.Wang MY, Yu X, Lee Y, et al. Dapagliflozin suppresses glucagon signaling in rodent models of diabetes. Proc Natl Acad Sci U S A 2017;114:6611–6616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gustavson SM, Chu CA, Nishizawa M, et al. Effects of hyperglycemia, glucagon, and epinephrine on renal glucose release in the conscious dog. Metabolism 2004;53:933–941 [DOI] [PubMed] [Google Scholar]
- 20.Cersosimo E, Zaitseva IN, Ajmal M. Effects of beta-adrenergic blockade on hepatic and renal glucose production during hypoglycemia in conscious dogs. Am J Physiol 1998;275:E792–E797 [DOI] [PubMed] [Google Scholar]
- 21.Kolodny EH, Kline R, Altszuler N. Effect of phlorizin on hepatic glucose output. Am J Physiol 1962;202:149–154 [DOI] [PubMed] [Google Scholar]
- 22.Cahill GF., Jr Starvation in man. N Engl J Med 1970;282:668–675 [DOI] [PubMed] [Google Scholar]
- 23.Krebs HA, Hems R, Gascoyne T. Renal gluconeogenesis. IV. Gluconeogenesis from substrate combinations. Acta Biol Med Ger 1963;11:607–615 [PubMed] [Google Scholar]
- 24.Cersosimo E, Judd RL, Miles JM. Insulin regulation of renal glucose metabolism in conscious dogs. J Clin Invest 1994;93:2584–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stumvoll M, Chintalapudi U, Perriello G, Welle S, Gutierrez O, Gerich J. Uptake and release of glucose by the human kidney. Postabsorptive rates and responses to epinephrine. J Clin Invest 1995;96:2528–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stumvoll M, Meyer C, Kreider M, Perriello G, Gerich J. Effects of glucagon on renal and hepatic glutamine gluconeogenesis in normal postabsorptive humans. Metabolism 1998;47:1227–1232 [DOI] [PubMed] [Google Scholar]
- 27.Cersosimo E, Molina PE, Abumrad NN. Renal lactate metabolism and gluconeogenesis during insulin-induced hypoglycemia. Diabetes 1998;47:1101–1106 [DOI] [PubMed] [Google Scholar]
- 28.Eid A, Bodin S, Ferrier B, et al. Intrinsic gluconeogenesis is enhanced in renal proximal tubules of Zucker diabetic fatty rats. J Am Soc Nephrol 2006;17:398–405 [DOI] [PubMed] [Google Scholar]
- 29.Owen OE, Felig P, Morgan AP, Wahren J, Cahill GF Jr. Liver and kidney metabolism during prolonged starvation. J Clin Invest 1969;48:574–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cersosimo E, Garlick P, Ferretti J. Abnormal glucose handling by the kidney in response to hypoglycemia in type 1 diabetes. Diabetes 2001;50:2087–2093 [DOI] [PubMed] [Google Scholar]
- 31.DeFronzo RA, Ferrannini E, Hendler R, Wahren J, Felig P. Influence of hyperinsulinemia, hyperglycemia, and the route of glucose administration on splanchnic glucose exchange. Proc Natl Acad Sci U S A 1978;75:5173–5177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrannini E, Wahren J, Felig P, DeFronzo RA. The role of fractional glucose extraction in the regulation of splanchnic glucose metabolism in normal and diabetic man. Metabolism 1980;29:28–35 [DOI] [PubMed] [Google Scholar]
- 33.Sha S, Devineni D, Ghosh A, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab 2011;13:669–672 [DOI] [PubMed] [Google Scholar]