Abstract
Introduction
Diabetic kidney disease (DKD) is the most prevalent complication in diabetic patients, which contributes to high morbidity and mortality. Urine and plasma metabolomics studies have been demonstrated to provide valuable insights for DKD. However, limited information on spatial distributions of metabolites in kidney tissues have been reported.
Objectives
In this work, we employed an ambient desorption electrospray ionization-mass spectrometry imaging (DESI-MSI) coupled to a novel bioinformatics platform (METASPACE) to characterize the metabolome in a mouse model of DKD.
Methods
DESI-MSI was performed for spatial untargeted metabolomics analysis in kidneys of mouse models (F1 C57BL/6JIns2Akita male mice at 17 weeks of age) of type 1 diabetes (T1D, n = 5) and heathy controls (n = 6).
Results
Multivariate analyses (i.e., PCA and PLS-DA (a 2000 permutation test: P < 0.001)) showed clearly separated clusters for the two groups of mice on the basis of 878 measured m/z’s in kidney cortical tissues. Specifically, mice with T1D had increased relative abundances of pseudouridine, accumulation of free polyunsaturated fatty acids (PUFAs), and decreased relative abundances of cardiolipins in cortical proximal tubules when compared with healthy controls.
Conclusion
Results from the current study support potential key roles of pseudouridine and cardiolipins for maintaining normal RNA structure and normal mitochondrial function, respectively, in cortical proximal tubules with DKD. DESI-MSI technology coupled with METASPACE could serve as powerful new tools to provide insight on fundamental pathways in DKD.
Keywords: Diabetic kidney disease, DESI-MSI, Renal proximal tubule, Lipid metabolism
1. Introduction
Diabetic kidney disease (DKD) is the most prevalent complication in diabetic patients, which contributes to high morbidity and mortality. Urine and plasma metabolomics has identified novel non-invasive biomarkers for DKD and provided valuable insights in our understanding toward the disease (Sharma et al. 2013; Zhang et al. 2015). Particularly, small molecular weight lipids such as fatty acids (FAs), glycerolipids (GLs), glycerophospholipids (GPs), and sphingolipids (SLs) play pivotal roles in regulating normal kidney function and pathogenesis of kidney disease (Zhao et al. 2015). Previous studies reported that DKD and lipid metabolism perturbations are bi-directional related. DKD provokes profound alterations in lipid metabolism and in turn, the associated lipid disorders also contribute to progression of DKD and other cardiovascular complications (Vaziri 2003; Vaziri and Norris 2011). However, knowledge about the localization of different lipid species in kidney tissues of DKD patients/animals are still limited.
Mass spectrometry imaging (MSI) enables simultaneous analysis of multiple molecular components directly from single cells, tissues, organs, or whole animals (Schwamborn and Caprioli 2010). In combination with histological methods, this technique provides information about the spatial distribution of molecules in various biological tissues (Heeren 2015). Matrix-assisted laser desorption/ionization (MALDI)-MSI has been applied to study the spatial distribution of lipids and other small molecules in kidney sections by different investigators (Grove et al. 2014; Jung et al. 2016; Kompauer et al. 2017; Liu et al. 2017; Ruh et al. 2013). Our group previously reported that both type 1 diabetic and high-fat-fed models had increased levels of a sphingomyelin (SM) annotated as SM(d18:1/16:0) in the glomeruli using high spatial resolution (25 μm) MALDI-MSI (Miyamoto et al. 2016), however tubular changes were not reported.
In contrast to conventional MALDI-MSI approaches, which commonly requires analysis in vacuo, desorption electrospray ionization (DESI)-MSI is an ambient ionization technique (Gemperline et al. 2014). Further, another advantage of DESI is its capability for high-throughput analysis as minimal sample preparation is needed, where, for example, coating samples with matrices is not required. DESI-MSI-based spatially resolved metabolomics in different cancer studies have provided new insights into the understanding of tumor-associated metabolic reprogramming and provided a novel approach for functional metabolites based molecular histology (He et al. 2018; Huang et al. 2019; Sun et al. 2019). In addition, DESI-MSI has previously been employed to analyze kidney tissues and other tissue samples (Eberlin et al. 2013, 2014; Wiseman et al. 2008; Zhang et al. 2017) although diabetic changes in mouse kidneys have not previously reported. In diabetes, alterations in the levels of lipids (i.e., fatty acids, phospholipids, and glycolipids) in blood and tissues may contribute to various cellular disorders associated with pathogenesis (Ramanadham et al. 2000; Russo et al. 2013; Weijers 2012). As DKD contributes to overall morbidity and mortality in patients, it is of great importance to investigate how perturbed lipid metabolism at the cellular level contributes to DKD. Thus, new knowledge of spatial localization of lipids within tubules (particularly the mitochondria-rich proximal tubules) and glomeruli would help uncover molecular events that reveal susceptibility and progression in DKD.
The present study is the first report of the application of DESI-MSI to investigate metabolic alterations in renal proximal tubular FAs, GLs, GPs, and SLs in DKD. A new powerful search engine, METASPACE, was used for the metabolite annotation. METASPACE is a false discovery rate (FDR)-controlled metabolite annotation engine at the level of the molecular sum formula (Palmer et al. 2017). In the present report, various subgroups of complex lipids, such as ceramides (Cers), cardiolipins (CLs), phosphatidylethanolamines (PEs), phosphatidylglycerols (PGs), phosphatidylinositols (PIs), and phosphatidylserines (PSs), were identified in renal cortical proximal tubules. 2D mapping of untargeted lipids within renal proximal tubules coupled with statistical analysis of extracted MSI data reveal RNA metabolism, lipid abnormalities and mitochondrial structural components are altered, even with normal histologic appearance. Furthermore, we report a new workflow for identifying lipid biomarkers of DKD using DESI-MSI data sets obtained from proximal tubules. Using a small set of m/z’s with top variable importance in the projection (VIP) scores in the partial least squares-discriminant analysis (PLS-DA) model, it is anticipated that high-throughput DESI-MSI of kidney biopsy tissues could be used to help understand the pathogenesis of DKD.
2. Materials and methods
2.1. Animals, diets, and tissue samples
Five F1 C57BL/6J-Ins2Akita male mice (the mouse model of type 1 diabetes, T1D) at 17-week-old and age-matched C57BL/6J WT mice (n = 6) were screened and sampled in this study. All mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Mice were given standard rodent chow and water ad libitum. All animal studies and experimental procedures were approved by the Institutional Animal Care and Use Committee of University of California, San Diego. Renal cortical tissues from similar regions of the left kidney of each mouse were snap-frozen in liquid nitrogen and stored at a − 80 °C freezer until sectioned. These cortical tissues were affixed to the chuck in a Leica CM1860 cryostat (Leica Biosystems Inc., Buffalo Grove, USA) at − 20 °C with a minimal amount of optimal cutting temperature compound (O.C.T.) (Sakura Finetek, Torrance, CA, USA). To avoid the effect of O.C.T., it was used as a glue and did not touch the regions that were sectioned. Sixteen μm thick sections were cut and thaw mounted on Superfrost Plus Microscope Slides (Fisher Scientific, Hampton, NH, USA). After sectioning, the glass slides were stored in a − 80 °C freezer until MSI. Sample slides were dried for about 15 min before MSI. Sample IDs were blinded and control and diabetic samples were randomly analysed.
2.2. DESI-MS imaging
A 2D Omni Spray (Prosolia Inc., Indianapolis, IN, USA) coupled to a Q Exactive mass spectrometer (Thermo Scientific, San Jose, CA, USA) was used for tissue profiling and imaging with a spatial resolution of 200 μm. DESI-MSI was performed in the negative ion mode from m/z 100 to 1500, using the Q Exactive mass spectrometer which allows for high mass accuracy (< 5 ppm mass error) and high mass resolution (70,000 resolving power at m/z 200) measurements (Feider et al. 2016). Mass spectra were acquired in centroid mode. The histologically compatible solvent system dimethylformamide: acetonitrile 1:1 (v:v) was used for analysis at a flow rate of 1.2 μL/min with 5 kV applied to the solvent (Eberlin et al. 2011).
2.3. Lipid identification
Lipid species were identified using high mass accuracy/resolution measurements by Q Exactive Hybrid Quadrupole-Orbitrap Fourier Transform mass spectrometer (Thermo Scientific, San Jose, CA, USA). Metabolite annotations from MSI were conducted using METASPACE (http://metaspace2020.eu) and further validated by collision induced dissociation or higher-energy collisional dissociation tandem MS analysis (Palmer et al. 2017). Fragmentation patterns were compared with literatures and used in conjunction with data from Human Metabolome Database (HMDB, http://www.hmdb.ca) and METLIN (https://metlin.scripps.edu) for identification (Smith et al. 2005; Wishart et al. 2013).
2.4. Tissue staining and histopathology evaluation
The same tissue sections analyzed by DESI-MSI were stained using standard hematoxylin and eosin (H&E) staining protocol (Fischer et al. 2008). All the H&E stained slides were scanned using an Aperio CS2 image capture device (Leica Biosystems Inc., Buffalo Grove, USA) with a × 40 magnification. Whole-slides images were viewed and analyzed using the Aperio ImageScope software (Leica Biosystems Inc., Buffalo Grove, USA) by an experienced renal pathologist.
2.5. 2D imaging data analysis
RAW files were converted into images using ProteoWizard Toolkit software and imzMLConverter 1.3.0 (Chambers et al. 2012; Race et al. 2012). Then the data were uploaded into the source imaging software packages BioMap (Novartis, Basel, Switzerland) or MSiReader for visualization (Robichaud et al. 2013). Regions of interest (ROIs) or pixels (n = 15) for proximal tubules were randomly selected and drawn on the reference ion image (i.e., m/z 885.550, PI (38:4)) with the H&E-stained tissue section as a background (Supplementary Fig. 1). To exclude pixels that contained glomeruli, the localization of a phosphatidic acid (PA (36:1)) was used as a contrast ion as described previously (Grove et al. 2014). All the selected 15 ROIs from each tissue section only contained proximal tubules and had no distribution of PA (36:1).
2.6. Statistical analysis
Raw ion intensity data of ROIs were extracted by MSiReader and then normalized by total ion current (TIC) (Robichaud et al. 2013). MetaboAnalyst 3.0 was employed for statistical analyses (Xia et al. 2015). Briefly, mass tolerance (m/z) was set at 0.025 and data normalization (i.e., log transformation and auto-scaling) was done prior to statistical analysis to create a Gaussian distribution. Univariate analysis (i.e., Wilcoxon Mann–Whitney test) was used to compare m/z’s in cortical proximal tubules between two groups of mice. Principal component analysis (PCA), partial least squares-discriminant analysis (PLS-DA), and hierarchical clustering analysis (e.g., heatmap) were performed via MetaboAnalyst. In the PLS-DA model, a variable importance in the projection (VIP) plot was used to rank m/z’s based on their importance in discriminating two groups of mice (Xia et al. 2015).
3. Results
3.1. Molecular characterization of lipids in renal cortical tissues from mice
In total, 161–208 metabolites were annotated in all kiney corical tissues using METASPACE at 10% FDR (annotation results are available at: https://metaspace2020.eu/datas ets?src=DESI&q=guans hi). A representative summed mass spectra (negative ion mode) of normal renal proximal tubules in the range from m/z 100 to 1000 is shown in Fig. 1a. As shown in the figure, the most abundant feature is PI (18:0/20:4) at m/z 885.551, which is commonly detected in many tissues. In the mass range from m/z 100 to 400, small metabolites (e.g., glutamic acid (m/z 146.047), glu-conic acid (m/z 195.050), pseudouridine (m/z 279.039)) and FAs (e.g., FA (16:0), FA (18:2), FA (20:4), and FA (22:6)) with various lengths and unsaturation levels of carbon chains were detected. In the mass range from m/z 400 to 1000, most features were annotated as ceramides (e.g., Cer (d42:2)), CLs (e.g., CL (72:7)), PEs (e.g., PE (38:4)), PGs (e.g., PG (34:1)), PIs (e.g., PI (38:3)), and PSs (e.g., PS (38:4)), using high mass accuracy measurements and tandem MS analyses.
Fig. 1.
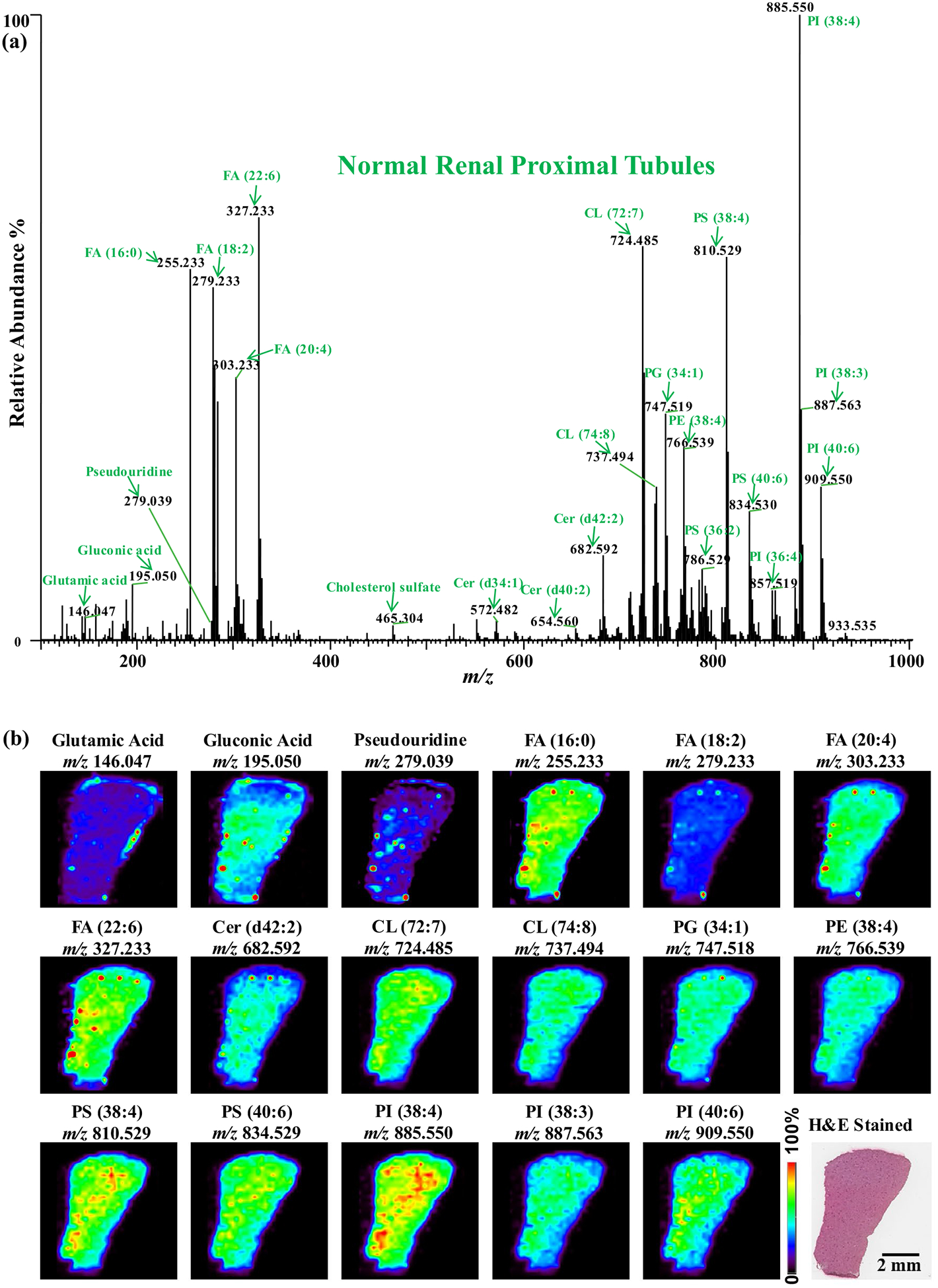
DESI-MS imaging of normal renal cortical tissue from mice. a A representative summed mass spectra (negative ion mode) of normal renal proximal tubules in the range from m/z 100 to 1000. The lipids show high abundances in normal renal proximal tubules with selected molecular ions highlighted in green. b Selected DESI-MS ion images of the normal renal cortical tissue including an optical image of the H&E-stained tissue section
Seventeen representative ion images obtained from a normal renal cortical tissue section are shown in Fig. 1b. All the ions (e.g., lipids) were homogenously distributed in normal renal cortex. The same tissue section which were analyzed by DESI-MSI was H&E-stained and overlapped with ion images. The distribution of lipids was homogenous within all selected ROIs (i.e., renal proximal tubules) in both healthy and diabetic groups.
A total of 878 m/z’s annotated in proximal tubules were used for statistical analyses. Results from the univariate analysis showed that 549 m/z’s in cortical proximal tubules were significantly different (P < 0.05) between healthy and diabetic mice (Fig. 2). Annotations of ions (i.e., m/z’s) from METASPACE revealed a wide distribution of various lipid species including FAs, GLs, GPs, and SLs in cortical proximal tubules. Representative lipids detected in DESI-MSI analyses of renal cortical tissues are shown in Table 1. Of interest is that there is widespread alteration of metabolites in normal appearing proximal tubules from diabetic kidneys.
Fig. 2.

Important features (m/z’s) selected by t test/Wilcoxon rank-sum test with threshold 0.05. The light purple solid circles represent features above the threshold. Note the P values are transformed by − log 10, so that the more significant features (with smaller P values) will be plotted higher on the graph. Seven representative metabolites are highlighted. Intensity values were normalized by TIC. Green: CON; Red: diabetic
Table 1.
Representative lipids detected in DESI-MSI analyses of renal cortical tissues from healthy and diabetic mice in negative ion mode
| m/z | Lipid classa | Tentative annotationb | Proposed formula |
|---|---|---|---|
| 135.030 | – | Threonic acid | C4H8O5 |
| 277.216 | FA | FA (18:3) | C18H30O2 |
| 279.039 | – | Pseudouridine | C9H12N2O6 |
| 279.233 | FA | FA (18:2) | C18H32O2 |
| 301.217 | FA | FA (20:5) | C20H30O2 |
| 337.311 | FA | FA (22:1) | C22H42O2 |
| 646.614 | Cer | Cer (d42:2) | C42H81NO3 |
| 714.505 | PE | PE (24:2) | C39H74NO8P |
| 716.523 | PE | PE(34:1) | C39H76NO8P |
| 721.502 | PG | PG (32:0) | C38H75O1()P |
| 722.513 | PE | PE (36:4) | C41H74NO7P |
| 747.518 | PG | PG(34:1) | C40H77O10P |
| 750.543 | PE | PE (38:5) | C43H78NO7P |
| 762.508 | PE | PE (38:6) | C43H74NO8P |
| 766.539 | PE | PE (38:4) | C43H78NO8P |
| 782.498 | PS | PS (36:4) | C42H74NO10P |
| 790.539 | PE | PE (40:6) | C45H78NO8P |
| 793.502 | PG | PG (38:6) | C44H75O10P |
| 810.529 | PS | PS (38:4) | C44H78NO10P |
| 834.529 | PS | PS (40:6) | C46H76NO10P |
| 857.519 | PI | PI (36:4) | C45H79O13P |
| 885.550 | PI | PI (38:4) | C47H83O13P |
| 909.550 | PI | PI (40:6) | C49H83O13P |
| 1449.981 | CL | CL (72:7) | C81H144O17P2 |
| 1473.981 | CL | CL (74:9) | C83H144O17P2 |
| 1475.996 | CL | CL (74:8) | C83H146O17P2 |
Structures were assigned based on accurate mass and database information from HMDB and METLIN
FA fatty acid, Cer ceramide, PE phosphatidylethanolamine, PG phosphatidylglycerol, PS phosphatidylserine, PI phosphatidylinositol, CL cardiolipin
(X:Y) denotes total number of carbons (X) and double bonds (Y) in the fatty acid chains
3.2. Altered abundances of lipids in renal cortical proximal tubules of diabetic mice
Lipids were widely distributed in renal cortex of diabetic mice. However, significant alterations were observed in diabetic renal cortex when compared with healthy mice. Figure 3 displays 30 representative 2D ion images of a kidney cortical section from each group of mice (i.e., one healthy mouse and one diabetic mouse). Particularly, diabetic mice presented relatively higher abundances of threonic acid (m/z 135.030), pseudouridine (m/z 279.039), hexose (m/z 215.033), glycerylphosphorylethanolamine (GPE) (m/z 214.049), and numerous features in unsaturated FAs (e.g., FA (18:3), FA (18:2), FA (20:5), and FA (22:1)) in cortical tissues (Fig. 3). However, a series of other lipid species detected in the range from m/z 700 to 1000 had lower relative abundance in renal cortical tissues of diabetic mice (Fig. 3). For example, PEs (e.g., PE (34:2), PE (36:4), PE (38:5), and PE (38:6)), PGs (e.g., PG (32:0), PG (34:1), and PG (38:6)), PIs (e.g., PI (36:4), PI (38:4), and PI (40:6)), and PSs (e.g., PS (36:4), PS (38:4), and PS (40:6)), were present at a lower relative abundance in renal cortical tissues of diabetic mice compared with healthy ones. Moreover, molecular distributions of other lipid species including Cers (e.g., Cer (d42:2)) and CLs (e.g., CL (72:7), CL (74:9), and CL (74:8)) also showed significantly reduced relative intensities in diabetic renal tissues compared with healthy kidneys.
Fig. 3.

Comparison of 30 representative DESI-MSI ion images of normal (CON) and diabetic renal cortical tissues from mice. Several molecular ions including small metabolites (e.g., threonic acid, glycerylphosphorylethanolamine (GPE), hexose, and pseudouridine), fatty acids (FAs), ceramides (Cers), phosphatidylethanolamines (PEs), phosphatidylglycerols (PGs), phosphatidylinositols (PIs), and phosphatidylserines (PSs) were significantly altered in diabetic renal cortical tissues. Optical images of H&E-stained renal tissue sections from normal and diabetic mice is also shown
Alterations of lipid profiles observed in renal proximal tubules of diabetic mice were further evaluated by comparisons of mass spectra for two interesting m/z regions including m/z 250 to 350 (Fig. 4), and m/z 700 to 900 (Supplementary Fig. 2). Comparisons of the lipid perturbations were performed between the similar ROIs selected from cortical proximal tubules in two groups of mice. As shown in Fig. 4, relative abundances of most detected features including pseudouridine and FAs with different lengths of carbon chain and unsaturation levels were greater in proximal tubules of diabetic mice (P < 0.01). Interestingly, alterations in fatty acid unsaturation levels in the length of 18 carbon chain of FA were observed (Fig. 4). In particular, diabetic mice had relatively greater abundance of FA (18:3) and FA (18:2), whereas lower abundances of FA (18:1) and FA (18:0) in proximal tubules in comparison with normal mice. Results from mass spectra also indicated that changes in the m/z range from 700 to 900 were consistent with findings from ion images (Supplementary Fig. 2).
Fig. 4.

Representative DESI-MSI mass spectra range from m/z 250 to 350 extracted from a normal and b diabetic renal proximal tubules
3.3. Multivariate analysis of lipid profiles in renal proximal tubules
Multivariate analysis showed that when lipid profiles in proximal tubules from diabetic mice were compared with those from normal mice, both score plots of the unsupervised PCA (Supplementary Fig. 3A) and supervised PLS-DA (Fig. 5a) exhibited two clearly separated clusters. A permutation test (P < 0.001, Supplementary Fig. 3B) was performed for the PLS-DA model and it showed that the observed separation was not by chance. Moreover, hierarchical clustering analysis (e.g., heatmap; Fig. 5b) also showed well separated clusters for the two mouse groups on the basis of 878 measured m/z’s in renal proximal tubules. Specifically, T1D mice had increased relative abundances of features in the m/z range from 180 to 300 (e.g., pseudouridine and FAs) in cortical proximal tubules relative to healthy controls. However, lower relative abundances of lipids in the m/z range from 700 to 900 including PEs (e.g. PE (38:6), m/z 762.508), PSs (e.g. PS (40:7), m/z 832.513), and PIs (e.g. PI (38:5), m/z 883.534) were observed in cortical proximal tubules of T1D mice (Fig. 5b).
Fig. 5.
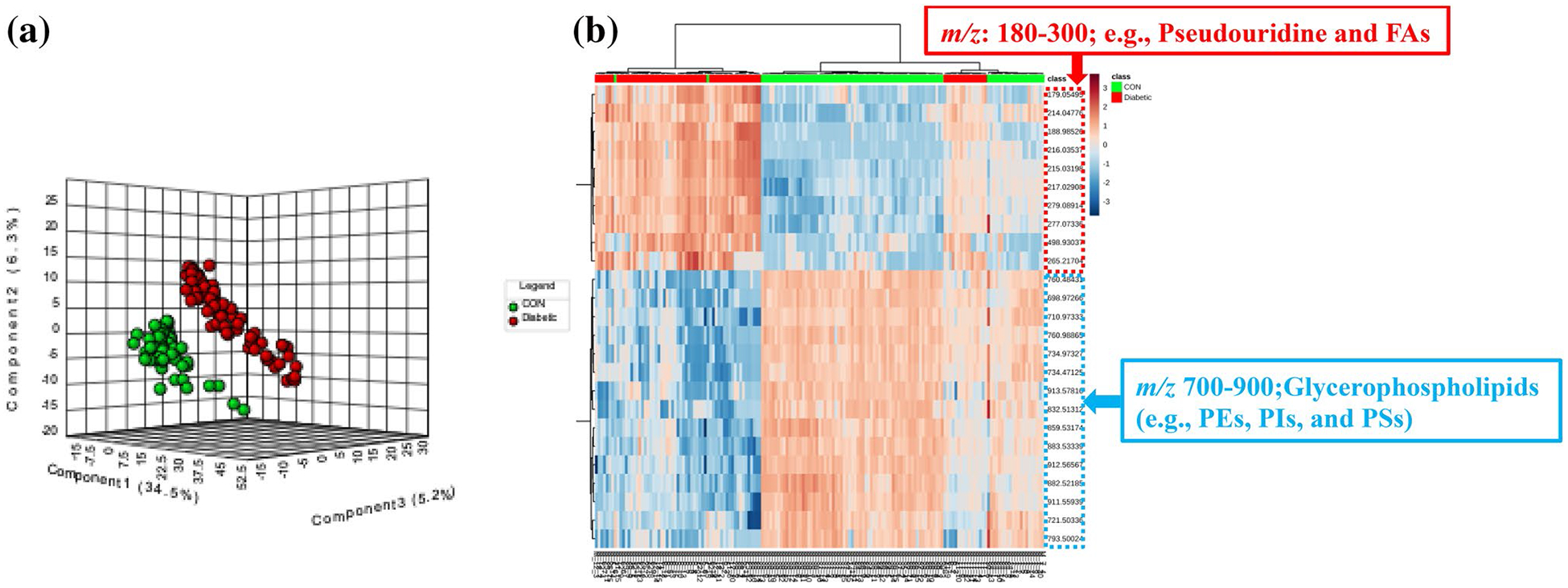
a The score plot of partial least squares-discriminant analysis (PLS-DA) of 90 ROIs (15 × 6) from control mice and 75 ROIs (15 × 5) from T1D mice showed two separated clusters for the two mouse groups for 878 measured m/z’s in renal proximal tubules. b Heatmap displayed top 25 m/z’s ranked by variable importance in projection (VIP) score in the PLS-DA model to retain the most contrasting patterns. Diabetic mice had increased relative abundances of pseudouridine and FAs (m/z 200–300) in renal proximal tubules; however, relative abundances of glycerophospholipids (e.g. PEs, PSs, and PIs; m/z 700–900) were lower in diabetic proximal tubules
4. Discussion
In this study, we employed an ambient DESI-MSI approach to characterize the metabolome in cortical tissues of a mouse model of DKD coupled to a novel bioinformatics platform (METASPACE). As a high-throughput automated bioinformatics tool, METASPACE could help annotate hundreds of metabolites in high resolution MSI data in < 10 min, simultaneously. Significant alterations of lipid profiles were detected in renal cortical proximal tubules of mice with T1D compared with controls. In particular, TID mice had increased relative abundance of selected small metabolites (e.g., pseudouridine and hexose) and polyunsaturated fatty acids (PUFAs), and decreased relative abundance of phospholipids, particularly cardiolipins, in renal cortical proximal tubules.
4.1. Increased pseudouridine and PUFAs in renal cortical proximal tubules from T1D mice
Pseudouridine, the C-glycosidic derivative of uridine, is a modified nucleoside found in RNA. Strong correlation between elevated serum pseudouridine concentrations and reduced kidney function has been previously reported (Sekula et al. 2016). Moreover, pseudouridine was recognized as a new biomarker with better performance in chronic kidney disease stratification than creatinine, but the exact role of pesudouridine in kidney diseases remains unclear (Hocher and Adamski 2017). As the C-glycosidic derivative of uridine, pseudouridine is a modified and the most abundant nucleoside found in various RNA species (tRNA, rRNA, snRNA, and snoRNA). The unusual conversion of uridine residues to pseudouridine might be related with its potential biological roles in RNA metabolism (Charette and Gray 2000). It was demonstrated that pseudouridine excretion is impaired in renal failure, and its retention is attributed to increased tubular reabsorption (Dzurik et al. 1992). Consistent with this, we found T1D mice had increased levels of pseudouridine in cortical proximal tubules, which could be the site of reabsorption or production. Pseudouridine retention in proximal tubules may lead to dysfunction and impaired kidney function.
Fatty acids are classified into saturated FAs and unsaturated FAs with lengths of 4–28 carbon chains and 0–6 double bonds. Significant alterations of FA metabolism have been demonstrated in different chronic kidney disease (CKD) models (Chen et al. 2017; Kang et al. 2015; Liu et al. 2017). Specifically, fatty acid (e.g., long-chain fatty acids, LCFAs) β-oxidation (FAO) is impaired in animals with CKD (Chen et al. 2017; Kang et al. 2015). Impaired β-oxidation of FAs may result from mitochondrial dysfunction in diabetes, which further contribute to the development of renal disease (Dugan et al. 2013; Sharma et al. 2013). Fatty acids, especially LCFAs, cannot penetrate mitochondrial membranes, and require carnitine-dependent transport via carnitine palmitoyltransferase-1 (CPT-1), which is the rate-limiting enzyme in FAO (McGarry and Brown 1997). Transcriptomics analyses of both human CKD samples and mouse models of tubulointerstitial fibrosis revealed decreased expression of key rate limiting enzymes (e.g., CPT and ACOX) of FAO (Kang et al. 2015).
By the DESI-MSI analysis of renal cortical tissues, we observed relative higher abundance of LCFAs (e.g., FA (18:3), FA (18:2), and FA (20:4)) in renal cortical proximal tubules of T1D mice. Our findings are consistent with previous studies that LCFAs β-oxidation is inhibited in CKD or DKD and we add new information on the spatial metabolomics level to confirm the accumulation of LCFAs in cortical proximal tubules in this mouse model of T1D. The observed elevated levels of LCFAs in diabetic kidneys presumably contribute to the decreased energy metabolism through the FOA pathway, which could affect kidney function and expedite the development of renal diseases.
Another significant finding for FAs is that higher degrees of FA unsaturation were observed for several PUFAs with different length of carbon chains (e.g., C18:3, C18:2, C20:5, C20:4, C22:6, and C22:5) in cortical proximal tubules from T1D mice. However, monounsaturated fatty acids (MUFAs, e.g., C18:1 and C22:1) and saturated FAs (e.g., C18:0) were less abundant in diabetic mice. Evidence for the presence of enhanced oxidative stress in CKD has been demonstrated by different investigators (Oberg et al. 2004; Shah et al. 2007; Vaziri 2003). Alterations of unsaturated lipids (e.g. FAs) provide a direct evaluation of oxidative stress in pathological kidneys with different severities of CKD (Chen et al. 2017). The kidney is a highly susceptible to damage caused by reactive oxygen species (ROS), due to the abundance of long-chain PUFAs (Ozbek 2012). Increased levels of long-chain PUFAs in cortical proximal tubules of T1D mice suggest that diabetic mice experience higher susceptibility to peroxidation, which further causes kidney damage and impair its function. Due to the instability of ROS and their short half-life, it is very difficult to measure oxidative stress in clinical examination. Findings from the current study shed new insight on direct measurement of oxidative stress in kidney tissues using MSI technique.
4.2. Decreased phospholipids in renal cortical proximal tubules from T1D mice
Cardiolipin (CL) is a unique phospholipid, which is almost exclusively localized at the inner mitochondrial membrane (Zhang et al. 2016). It plays a pivotal role in energy metabolism, mainly by maintaining stability for the individual mitochondrial enzymes and enzyme complexes involved in the respiratory chain (Houtkooper and Vaz 2008). Alterations of CL have been reported in various diseases such as diabetes and heart failure (Chicco and Sparagna 2007). In a recent MALDI-MSI study of kidney from the non-alcoholic steatohepatitis (NASH) mouse model, it was reported that the levels of CLs were lower in the kidney cortex of NASH mice and it might be associated with the pathogenesis of CKD (Hayasaka et al. 2016). Decreased CL species in the renal cortex of NASH mice might be explained by CL oxidation (oxidative stress in prevalent in CKD), which triggers the mitochondrial switch from ATP production to apoptosis in the kidney (Hayasaka et al. 2016). Diabetic mice in our study also showed reduced relative abundance of CLs (i.e., CL (72:7), CL (74:9), and CL (74:8)) in renal cortical proximal tubules, suggesting the presence of mitochondrial dysfunction and subsequent CKD. To the best of our knowledge, this is the first report of reduced CLs in the diabetic kidney using DESI-MSI.
Phospholipids (e.g., Cers, PEs, and PIs) are closely associated to the pathogenesis and progression of DKD and appear to be potential biomarkers for kidney diseases (Yang et al. 2007; Zhao 2013; Zhao et al. 2015). For instance, a decreasing trend of PI biomarker, PI (40:6), in the plasma was identified for type II DKD (Zhu et al. 2011). A number of studies have demonstrated the abnormal lipid metabolism in the pathogenesis of DKD, however, the changing directions of different phospholipid classes (e.g., PEs, PGs, PIs, and PSs) are diverse in plasma/serum, urine, or renal extracts (Yang et al. 2013; Zhao et al. 2015; Zhu et al. 2011). Several investigators reported that accumulation of lipids in kidney played a pivotal role in the pathobiology of DKD (Herman-Edelstein et al. 2014; Wang et al. 2005; Zhao et al. 2015). However, most accumulated lipid species or lipotoxicity in DKD are more related with free unsaturated FAs because of supressed FAO and oxidized phospholipids due to oxidative stress (Bobulescu 2010; Jimi et al. 1999; Stadler et al. 2015). In a MALDI-MSI study conducted by (Grove et al. 2014), the authors demonstrated that levels of two lysophospholipids including lyso-PCs and lysophosphatidic acids (LPAs) were remarkably elevated in glomeruli of diabetic mice. Moreover, levels of glucose modified PE species (i.e., Amadori-PEs) were greater in renal cortex of type 2 DKD mice. The mechanism of these alterations and how lyso-PCs and Amadori-PEs are involved in the pathogenesis of DKD are still unknown.
In the current DESI-MSI study, we measured various glycerolipid species with distinct polar heads (e.g., choline, ethanolamine, glycerol, inositol, and serine) and different chain lengths of two FAs. Unlike the previously reported lipid accumulation theory in diabetic kidney, we found that relative abundance of glycerolipids were decreased in cortical proximal tubules of T1D mice. Indeed, in a rat model of celiptium-induced renal toxicity and lipid peroxidation, a 15% decline in total phospholipids and especially, a 50% decrease in PEs were detected in the renal cortex (Dadoun and Raguenezviotte 1990). In contract, free FAs were increased in renal cortex of celiptium-treated rats. The authors hypothesized that elevated free FAs and reduced total phospholipids in rats with nephrotoxicity might originate from excessive breakdown of phospholipids. Since oxidative stress has been identified as a critical factor that contributes to the initiation and progression of DKD (Jha et al. 2016; Stanton 2011), we speculate that glycerolipids (e.g., PEs, PIs, and PSs) are the major lipid species that are more vulnerable to be oxidized in renal proximal tubules of DKD. Unfortunately, the current study did not measure oxidized-glycerolipid species. Further research is warranted to confirm this hypothesis.
Although we have shown significant changes of small metabolites and lipids, the current study still has some limitations. Some lipids can suppress the signals of other lipids and other endogenous compounds. Especially, those lower abundant lipids/metabolites can be suppressed by lipids with higher abundances and ionization efficiencies in DESI (Wu et al. 2013). One of the solutions to increase the coverage of metabolite species is to enhance the sensitivity of supressed analytes by switching the polarity of ion detection or by adduct formation (Ifa et al. 2008). The addition of reagents to the solvent can also improve the sensitivity and ionization efficiency of particular lipid/metabolite species (i.e., targeted species) (Wu et al. 2013). Limits of detection may be improved by using the MS/MS, MSn, or MRM scan modes, but these methods require a priori knowledge of the metabolites/lipids of interest (Wiseman et al. 2008). Another limitation of the current study is the relatively low spatial resolution. There are multiple parameters affecting the spatial resolution including the emitter incident angle, spray tip-surface distance, solvent composition, nebulizing gas flow rate, step-size, diameter of the mass spectrometer entry orifice, scanning mode, imaging scan rate, and the mass spectrometer data acquisition rate. Detailed information about how to improve spatial resolution of DESI-MSI has been discussed previously (Campbell et al. 2012).
5. Conclusions
In summary, this study demonstrates that DESI-MSI can be successfully used to distinguish cortical proximal tubules in healthy and diabetic mice based on altered relative abundance of lipid profiles. Through comparison of ion images, representative mass spectra, and univariate and multivariate statistical analyses, significant perturbations of lipid species were detected in proximal tubules of T1D mice. In particular, results from the current study further support a role for pseudouridine in DKD and the data suggests that pseudouridine accumulation might originate from cortical proximal tubules. Our findings show that diabetic mice experienced accumulation of free PUFAs and decreased glycerolipids in renal proximal tubules. Increased free PUFAs could arise from suppressed FAO and/or enhanced glycerolipid breakdown. Moreover, glycerolipids could be the principal lipid species that are involved in excessive peroxidation with DKD. Reduced cardiolipins likely indicate mitochondrial dysfunction in proximal tubules of diabetic kidneys. DESI-MSI technology coupled with the new annotation engine (i.e., METASPACE) will serve as powerful tools to provide insight on fundamental pathways in DKD.
Supplementary Material
Acknowledgements
K.S. and G.Z. were supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant (5R24DK082841-08 to KS). L.S.E. was supported by the National Cancer Institute of the National Institutes of Health under Award R00CA190783.
Footnotes
Conflict of interest The authors declare no conflict of interest.
Ethical approval All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent This article does not contain any studies with human participants.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11306-020-1637-8) contains supplementary material, which is available to authorized users.
References
- Bobulescu IA (2010). Renal lipid metabolism and lipotoxicity. Current Opinion in Nephrology and Hypertension, 19(4), 393–402. 10.1097/MNH.0b013e32833aa4ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DI, Ferreira CR, Eberlin LS, & Cooks RG (2012). Improved spatial resolution in the imaging of biological tissue using desorption electrospray ionization. Analytical and Bioanalytical Chemistry, 404(2), 389–398. 10.1007/s00216-012-6173-6. [DOI] [PubMed] [Google Scholar]
- Chambers MC, Maclean B, Burke R, Amodei D, Ruderman DL, Neumann S, et al. (2012). A cross-platform toolkit for mass spectrometry and proteomics. Nature Biotechnology, 30(10), 918–920. 10.1038/nbt.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charette M, & Gray MW (2000). Pseudouridine in RNA: What, where, how, and why. IUBMB Life, 49(5), 341–351. 10.1080/152165400410182. [DOI] [PubMed] [Google Scholar]
- Chen DQ, Chen H, Chen L, Vaziri ND, Wang M, Li XR, et al. (2017). The link between phenotype and fatty acid metabolism in advanced chronic kidney disease. Nephrology, Dialysis, Transplantation, 32(7), 1154–1166. 10.1093/ndt/gfw415. [DOI] [PubMed] [Google Scholar]
- Chicco AJ, & Sparagna GC (2007). Role of cardiolipin alterations in mitochondrial dysfunction and disease. American Journal of Physiology-Cell Physiology, 292(1), C33–C44. 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- Dadoun C, & Raguenezviotte G (1990). Celiptium-induced nephrotoxicity and lipid-peroxidation in rat renal cortex. Cancer Chemotherapy and Pharmacology, 27(3), 178–186. 10.1007/Bf00685710. [DOI] [PubMed] [Google Scholar]
- Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE, et al. (2013). AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. The Journal of Cinical Investigation, 123(11), 4888–4899. 10.1172/JCI66218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzurik R, Lajdova I, Spustova V, & Opatrny K (1992). Pseudouridine excretion in healthy subjects and its accumulation in renal failure. Nephron, 61(1), 64–67. 10.1159/000186836. [DOI] [PubMed] [Google Scholar]
- Eberlin LS, Ferreira CR, Dill AL, Ifa DR, Cheng L, & Cooks RG (2011). Nondestructive, histologically compatible tissue imaging by desorption electrospray ionization mass spectrometry. ChemBioChem, 12(14), 2129–2132. 10.1002/cbic.201100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberlin LS, Norton I, Orringer D, Dunn IF, Liu XH, Ide JL, et al. (2013). Ambient mass spectrometry for the intra-operative molecular diagnosis of human brain tumors. Proceedings of the National academy of Sciences of the United States of America, 110(5), 1611–1616. 10.1073/pnas.1215687110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberlin LS, Tibshirani RJ, Zhang JL, Longacre TA, Berry GJ, Bingham DB, et al. (2014). Molecular assessment of surgical-resection margins of gastric cancer by mass-spectrometric imaging. Proceedings of the National academy of Sciences of the United States of America, 111(7), 2436–2441. 10.1073/pnas.1400274111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feider CL, Elizondo N, & Eberlin LS (2016). Ambient ionization and FAIMS mass spectrometry for enhanced imaging of multiply charged molecular ions in biological tissues. Analytical Chemistry, 88(23), 11533–11541. 10.1021/acs.analchem.6b02798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AH, Jacobson KA, Rose J, & Zeller R (2008). Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harbor Protocols. 10.1101/pdb.prot4986. [DOI] [PubMed] [Google Scholar]
- Gemperline E, Chen BM, & Li LJ (2014). Challenges and recent advances in mass spectrometric imaging of neurotransmitters. Bioanalysis, 6(4), 525–540. 10.4155/bio.13.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove KJ, Voziyan PA, Spraggins JM, Wang SW, Paueksakon P, Harris RC, et al. (2014). Diabetic nephropathy induces alterations in the glomerular and tubule lipid profiles. Journal of Lipid Research, 55(7), 1375–1385. 10.1194/jlr.M049189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka T, Fuda H, Hui SP, & Chiba H (2016). Imaging mass spectrometry reveals a decrease of cardiolipin in the kidney of NASH model mice. Analytical Sciences, 32(4), 473–476. 10.2116/analsci.32.473. [DOI] [PubMed] [Google Scholar]
- He J, Sun C, Li T, Luo Z, Huang L, Song X, et al. (2018). A sensitive and wide coverage ambient mass spectrometry imaging method for functional metabolites based molecular histology. Advanced Science, 5(11), 1800250 10.1002/advs.201800250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeren RMA (2015). Getting the picture: The coming of age of imaging MS. International Journal of Mass Spectrometry, 377, 672–680. 10.1016/j.ijms.2014.04.021. [DOI] [Google Scholar]
- Herman-Edelstein M, Scherzer P, Tobar A, Levi M, & Gafter U (2014). Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. The Journal of Lipid Research, 55(3), 561–572. 10.1194/jlr.P040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocher B, & Adamski J (2017). Metabolomics for clinical use and research in chronic kidney disease. Nature Reviews Nephrology, 13(5), 269–284. 10.1038/nrneph.2017.30. [DOI] [PubMed] [Google Scholar]
- Houtkooper RH, & Vaz FM (2008). Cardiolipin, the heart of mitochondrial metabolism. Cellular and Molecular Life Sciences, 65(16), 2493–2506. 10.1007/s00018-008-8030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Mao X, Sun C, Luo Z, Song X, Li X, et al. (2019). A graphical data processing pipeline for mass spectrometry imaging-based spatially resolved metabolomics on tumor heterogeneity. Analytica Chimica Acta, 1077, 183–190. 10.1016/j.aca.2019.05.068. [DOI] [PubMed] [Google Scholar]
- Ifa DR, Manicke NE, Dill AL, & Cooks RG (2008). Latent fingerprint chemical imaging by mass spectrometry. Science, 321(5890), 805 10.1126/science.1157199. [DOI] [PubMed] [Google Scholar]
- Jha JC, Banal C, Chow BSM, Cooper ME, & Jandeleit-Dahm K (2016). Diabetes and kidney disease: Role of oxidative stress. Antioxidants & Redox Signaling, 25(12), 657–684. 10.1089/ars.2016.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimi S, Uesugi N, Saku K, Itabe H, Zhang B, Arakawa K, et al. (1999). Possible induction of renal dysfunction in patients with lecithin:cholesterol acyltransferase deficiency by oxidized phosphatidylcholine in glomeruli. Arteriosclerosis, Thrombosis, and Vascular Biology, 19(3), 794–801. 10.1161/01.atv.19.3.794. [DOI] [PubMed] [Google Scholar]
- Jung JW, Lee MS, Choi HJ, Jung S, Lee YJ, Hwang GS, et al. (2016). Mass spectrometric imaging of metabolites in kidney tissues from rats treated with furosemide. American Journal of Physiology-Renal Physiology, 310(11), F1317 10.1152/ajprenal.00524.2015. [DOI] [PubMed] [Google Scholar]
- Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, et al. (2015). Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nature Medicine, 21(1), 37–46. 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompauer M, Heiles S, & Spengler B (2017). Atmospheric pressure MALDI mass spectrometry imaging of tissues and cells at 1.4-mum lateral resolution. Nature Methods, 14(1), 90–96. 10.1038/nmeth.4071. [DOI] [PubMed] [Google Scholar]
- Liu HH, Li W, He Q, Xue JJ, Wang JY, Xiong CQ, et al. (2017). Mass spectrometry imaging of kidney tissue sections of rat subjected to unilateral ureteral obstruction. Scientific Reports. 10.1038/Srep41954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry JD, & Brown NF (1997). The mitochondrial carnitine palmitoyltransferase system—From concept to molecular analysis. European Journal of Biochemistry, 244(1), 1–14. 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Hsu CC, Hamm G, Darshi M, Diamond-Stanic M, Decleves AE, et al. (2016). Mass spectrometry imaging reveals elevated glomerular ATP/AMP in diabetes/obesity and identifies sphingomyelin as a possible mediator. EBioMedicine, 7, 121–134. 10.1016/j.ebiom.2016.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, et al. (2004). Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney International, 65(3), 1009–1016. 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- Ozbek E (2012). Induction of oxidative stress in kidney. International Journal of Nephrology, 2012, 465897 10.1155/2012/465897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A, Phapale P, Chernyavsky I, Lavigne R, Fay D, Tarasov A, et al. (2017). FDR-controlled metabolite annotation for high-resolution imaging mass spectrometry. Nature Methods, 14(1), 57–60. 10.1038/Nmeth.4072. [DOI] [PubMed] [Google Scholar]
- Race AM, Styles IB, & Bunch J (2012). Inclusive sharing of mass spectrometry imaging data requires a converter for all. Journal of Proteomics, 75(16), 5111–5112. 10.1016/j.jprot.2012.05.035. [DOI] [PubMed] [Google Scholar]
- Ramanadham S, Hsu F, Zhang S, Bohrer A, Ma Z, & Turk J (2000). Electrospray ionization mass spectrometric analyses of phospholipids from INS-1 insulinoma cells: Comparison to pancreatic islets and effects of fatty acid supplementation on phospholipid composition and insulin secretion. Biochimica et Biophysica Acta, 1484(2–3), 251–266. 10.1016/s1388-1981(00)00022-6. [DOI] [PubMed] [Google Scholar]
- Robichaud G, Garrard KP, Barry JA, & Muddiman DC (2013). MSiReader: An open-source interface to view and analyze high resolving power MS imaging files on matlab platform. Journal of American Society for Mass Spectrometry, 24(5), 718–721. 10.1007/s13361-013-0607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruh H, Salonikios T, Fuchser J, Schwartz M, Sticht C, Hochheim C, et al. (2013). MALDI imaging MS reveals candidate lipid markers of polycystic kidney disease. Journal of Lipid Research, 54(10), 2785–2794. 10.1194/jlr.M040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SB, Ross JS, & Cowart LA (2013). Sphingolipids in obesity, type 2 diabetes, and metabolic disease. Handbook of Exprimental Pharmacology, 216, 373–401. 10.1007/978-3-7091-1511-4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwamborn K, & Caprioli RM (2010). MALDI imaging mass spectrometry—Painting molecular pictures. Molecular Oncology, 4(6), 529–538. 10.1016/j.molonc.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekula P, Goek ON, Quaye L, Barrios C, Levey AS, Romisch-Marg W, et al. (2016). A metabolome-wide association study of kidney function and disease in the general population. Journal of the American Society of Nephrology, 27(4), 1175–1188. 10.1681/Asn.2014111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SV, Baliga R, Rajapurkar M, & Fonseca VA (2007). Oxidants in chronic kidney disease. Journal of the American Society of Nephrology, 18(1), 16–28. 10.1681/Asn.2006050500. [DOI] [PubMed] [Google Scholar]
- Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, et al. (2013). Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. Journal of the American Society of Nephrology, 24(11), 1901–1912. 10.1681/ASN.2013020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, O’Maille G, Want EJ, Qin C, Trauger SA, Brandon TR, et al. (2005). METLIN—A metabolite mass spectral database. Therapeutic Drug Monitoring, 27(6), 747–751. 10.1097/01.ftd.0000179845.53213.39. [DOI] [PubMed] [Google Scholar]
- Stadler K, Goldberg IJ, & Susztak K (2015). The evolving understanding of the contribution of lipid metabolism to diabetic kidney disease. Current Diabetes Reports, 15(7), 40 10.1007/s11892-015-0611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton RC (2011). Oxidative stress and diabetic kidney disease. Current Diabetes Reports, 11(4), 330–336. 10.1007/s11892-011-0196-9. [DOI] [PubMed] [Google Scholar]
- Sun C, Li T, Song X, Huang L, Zang Q, Xu J, et al. (2019). Spatially resolved metabolomics to discover tumor-associated metabolic alterations. Proceedings of the National academy of Sciences of the United States of America, 116(1), 52–57. 10.1073/pnas.1808950116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri ND (2003). Molecular mechanisms of lipid disorders in nephrotic syndrome. Kidney International, 63(5), 1964–1976. 10.1046/j.1523-1755.2003.00941.x. [DOI] [PubMed] [Google Scholar]
- Vaziri ND, & Norris K (2011). Lipid disorders and their relevance to outcomes in chronic kidney disease. Blood Purification, 31(1–3), 189–196. 10.1159/000321845. [DOI] [PubMed] [Google Scholar]
- Wang Z, Jiang T, Li J, Proctor G, McManaman JL, Lucia S, et al. (2005). Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes, 54(8), 2328–2335. 10.2337/diabetes.54.8.2328. [DOI] [PubMed] [Google Scholar]
- Weijers RN (2012). Lipid composition of cell membranes and its relevance in type 2 diabetes mellitus. Current Diabetes Review, 8(5), 390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman JM, Ifa DR, Zhu Y, Kissinger CB, Manicke NE, Kissinger PT, et al. (2008). Desorption electrospray ionization mass spectrometry: Imaging drugs and metabolites in tissues. Proceedings of the National academy of Sciences of the United States of America, 105(47), 18120–18125. 10.1073/pnas.0801066105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu YF, et al. (2013). HMDB 3.0—The human metabolome database in 2013. Nucleic Acids Research, 41(D1), D801–D807. 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Dill AL, Eberlin LS, Cooks RG, & Ifa DR (2013). Mass spectrometry imaging under ambient conditions. Mass Spectrometry Reviews, 32(3), 218–243. 10.1002/mas.21360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia JG, Sinelnikov IV, Han B, & Wishart DS (2015). MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Research, 43(W1), W251–W257. 10.1093/nar/gkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WL, Bai Q, Li DD, Ta-La A, Wang S, Zhao RS, et al. (2013). Changes of urinary phospholipids in the chronic kidney disease patients. Biomarkers, 18(7), 601–606. 10.3109/1354750X.2013.837100. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang J, Qin L, Shou Z, Zhao J, Wang H, et al. (2007). Rapamycin prevents early steps of the development of diabetic nephropathy in rats. American Journal of Nephrology, 27(5), 495–502. 10.1159/000106782. [DOI] [PubMed] [Google Scholar]
- Zhang JL, Feider CL, Nagi C, Yu WD, Carter SA, Suliburk J, et al. (2017). Detection of metastatic breast and thyroid cancer in lymph nodes by desorption electrospray ionization mass spectrometry imaging. Journal of the American Society for Mass Spectrometry, 28(6), 1166–1174. 10.1007/s13361-016-1570-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yu W, Ryu SW, Lin J, Buentello G, Tibshirani R, et al. (2016). Cardiolipins are biomarkers of mitochondria-rich thyroid oncocytic tumors. Cancer Research, 76(22), 6588–6597. 10.1158/0008-5472.CAN-16-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YM, Zhang SW, & Wang GX (2015). Metabolomic biomarkers in diabetic kidney diseases—A systematic review. Journal of Diabetes and Its Complications, 29(8), 1345–1351. 10.1016/j.jdiacomp.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Zhao YY (2013). Metabolomics in chronic kidney disease. Clinica Chimica Acta, 422, 59–69. 10.1016/j.cca.2013.03.033. [DOI] [PubMed] [Google Scholar]
- Zhao YY, Vaziri ND, & Lin RC (2015). Lipidomics: New insight into kidney disease. Advances in Clinical Chemistry, 68, 153–175. 10.1016/bs.acc.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Zhu C, Liang QL, Hu P, Wang YM, & Luo GA (2011). Phospholipidomic identification of potential plasma biomarkers associated with type 2 diabetes mellitus and diabetic nephropathy. Talanta, 85(4), 1711–1720. 10.1016/j.talanta.2011.05.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


