Abstract
Scope:
Orange juice contains flavanones including hesperidin and narirutin, albeit at lower concentrations as compared to orange fruit. Therefore, we compared bioavailability and colonic catabolism of flavanones from orange juice to a 2.4-fold higher dose from fresh oranges.
Methods and results:
Following a randomized two-way cross-over design, 12 healthy subjects consumed a test meal comprising either fresh oranges or pasteurized orange juice, delivering 1774 and 751 μmol of total Citrus flavanones, respectively. Deglucuronidated and desulfated hesperetin, naringenin, and the flavanone catabolites 3-(3′-hydroxy-4′-methoxyphenyl)propionic acid, 3-(3′-hydroxyphenyl)hydracrylic acid, 4-hydroxyhippuric acid, and hippuric acid were quantitated in 24-h urine by UHPLC-MS/MS. Differences in urinary hesperetin excretion were found to be nonsignificant (p = 0.5209) both after consumption of orange fruit (21.6 ± 8.0 μmol) and juice (18.3 ± 7.2 μmol). By analogy, postprandial flavanone catabolite excretions were highly similar between treatments. Excretion of 3-(3′-hydroxy-4′-methoxyphenyl)propionic acid was inversely related to that of hesperetin, illustrating the catabolite/precursor relationship.
Conclusion:
Despite 2.4-fold higher doses, excretion of flavanones from ingested fresh orange fruit did not differ from that following orange juice consumption, possibly due to a saturation of absorption or their entrapment in the fiber-rich matrix of the fruit.
Keywords: Bioavailability, Flavonoids, Hesperetin, Metabolism, Naringenin
1. Introduction
In 2015, a total of 48 million tons of orange fruit (Citrus sinensis L. Osbeck) was produced for fresh consumption and juice production [1]. Orange juice is the most popular fruit juice in the European Union and the United States, reaching annual per capita consumptions of approximately 5 – 13 L [2, 3]. In addition to high concentrations of vitamin C, the provitamin A carotenoids β-carotene and β-cryptoxanthin) as well as the macular pigments lutein and zeaxanthin, both oranges and orange juice represent rich dietary sources of the flavanones hesperidin and narirutin [4–6]. Being almost exclusively present in Citrus fruits, hesperidin and narirutin constitute more than 90% of the flavonoids in sweet oranges (C. sinensis) and represent a significant portion of daily human flavonoid intake [7, 8]. Hesperidin and narirutin intake in Finland has been estimated to 28.3 and 8.3 mg/day, respectively, representing 67% of total daily flavonoid intake [9]. Similarly, Citrus fruits and juices were described to be among the top five most important dietary flavonoid sources of U.S. adults [10].
Numerous health benefits have previously been related to the consumption of oranges and orange juice, often attributed to their high flavanone contents. Epidemiological studies have indicated regular consumption of Citrus fruits to be cardioprotective [11] and associated with lower risk of stroke [11, 12] and asthma [12]. Prospective studies have related long-term orange juice consumption with improved vascular function [13], reduction of total cholesterol levels [14], and the prevention of increased endotoxin levels after meals high in fat and carbohydrate [15]. However, a clear-cut relationship of these effects and the responsible bioactive compounds has not yet been ascertained. In order to exert any potential health benefit, flavanones and their colonic catabolites need to be bioavailable in humans. Intestinal absorption and metabolism of dietary flavanone glycosides is a complicated process and has gained much attention in recent research [16–19]. Recent studies have provided evidence that a maximum of 30% of an ingested dose of hesperidin might be absorbed in the small intestine [18, 20]. However, the exact mechanisms of absorption and metabolism in the enterocyte remain to be elucidated. The majority of an ingested hesperidin dose is believed to reach the colon, particularly because the terminal ruti-nose moiety of hesperidin and narirutin prevents deglycosylation by brush border β-glucosidases (e.g., lactase phloridzin hydrolase) or direct absorption in the small intestine via sodium–glucose linked transporters such as SGLT1 [21]. Once having reached the colon, hesperidin and narirutin are hydrolyzed by the colonic microbiota, primarily yielding their corresponding aglycones hesperetin and naringenin, which are then absorbed by colonocytes. After conjugation with glucuronic acid or sulfate, they are released into the human blood stream [21, 22]. Nonetheless, reported urinary flavanone recoveries are poor, that is, only 4.1–5.4% and 2.1–12.5% of total hesperidin and narirutin intake has been estimated to be bioavailable, respectively [16,17,21,23]. Since a substantial portion of the flavanone aglycones are further metabolized to similarly bioavailable catabolites by the colonic microbiota [24], the hypothetical total urinary excretion of Citrus flavanones and their catabolites suggests a total bioavailability of almost 100% of the ingested dose [25]. These catabolites include highly specific breakdown products of hesperetin such as 3-(3′-hydroxy-4′-methoxyphenyl)propionic acid (HMPPA, Fig. 1) [24] as well as less specific catabolites such as hippuric acid (HipA), 4-hydroxyhippuric acid (4-OH-HipA), and 3-(3′-hydroxyphenyl)hydracrylic acid (HPHPA), which may also result from other phenolic sources [24–26].
Figure 1.
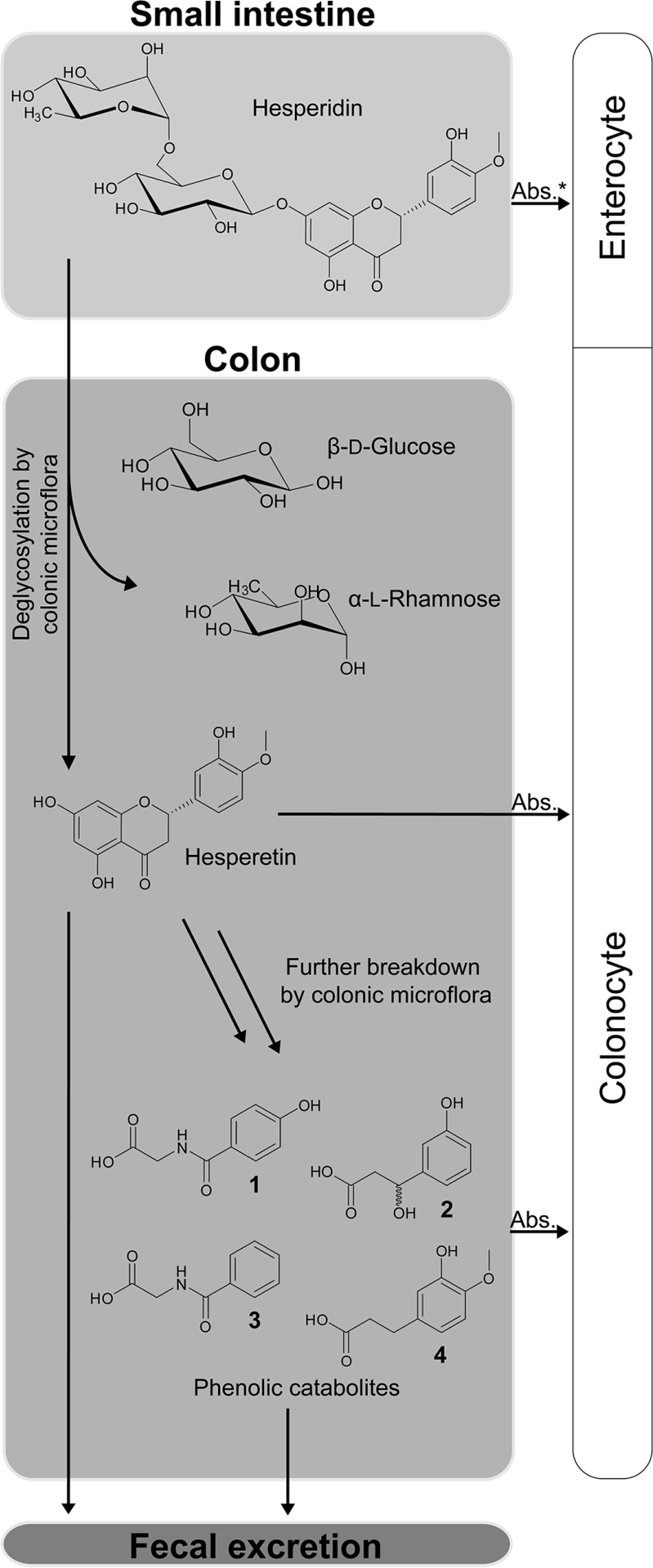
Intestinal absorption pathway of hesperidin and some of its major phenolic catabolites. 1: 4-hydroxyhippuric acid (4-OHHipA), 2: 3-(3′-hydroxyphenyl)hydracrylic acid (HPHPA), 3: hippuric acid (HipA), 4: 3-(3′-hydroxy-4′-methoxyphenyl)propionic acid (HMPPA). Absorption (Abs.) to the enterocyte (marked with an asterisk) has been tentatively reported by Actis-Goretta et al. [18] and Borges et al. [20].
Since flavanones are mainly deposited in segmental membranes and albedo of orange fruit [27], their levels are strongly reduced during orange juice production [4]. However, in our previous study, absolute flavanone bioaccessibilities were similar after in vitro digestion of orange fruit and orange juice, despite up to eightfold higher flavanone contents in orange fruit [4]. Thus, the aim of the present study was to determine total urinary excretion of flavanones and their catabolites after ingestion of a high flavanone dose from orange fruit and a lower dose from orange juice in humans. Total hesperetin and narirutin excretion in 24-h urine was determined after the consumption of either fresh orange fruit or pasteurized orange juice. In addition, levels of four colon-derived flavanone catabolites (HMPPA, HPHPA, 4-OH-HipA, and HipA) were monitored.
2. Materials and methods
2.1. Chemicals and materials
Acetonitrile (ACN), Optima LC/MS grade), diethyl ether (laboratory grade), methanol (Optima LC/MS grade), and water (Optima LC/MS grade) were from Fisher Scientific (Pittsburgh, PA, USA). Sulfatase from Helix pomatia exerting secondary glucuronidase activity (S9626), HipA (98%), hesperetin (>98%), and naringenin (>95%) were obtained from Sigma Aldrich (St. Louis, MO, USA). Narirutin (96%) was from ChromaDex (Irvine, CA, USA) and hesperidin (>90%) from Fluka Chemie (Buchs, Switzerland). HPHPA (>99.9%) and HMPPA (98%) were from Toronto Research Chemicals (Toronto, Canada), whereas 4-OH-HipA (>95%) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Acetic acid (glacial) was from Ricca Chemical (Arlington, TX, USA).
2.2. Study design
Following a randomized two-way cross-over design, 12 healthy subjects participated in this study between February and April 2014 at the University of Hohenheim. Due to the acute intake of antibiotics during the orange juice intervention, urinary excretion data of one subject was excluded. The remaining 11 subjects had a mean BMI of 23.3 ± 1.8 kg/m2 (range: 20.5–26.2) and a median age of 28 years. Details regarding subject characteristics, inclusion and exclusion criteria, and the overall study design were previously published in an article on carotenoid bioavailability from the below-described test meals [6]. Based on equal carotenoid uptake, subjects consumed either 400 g of fresh navel oranges (C. sinensis) or 719 g of pasteurized orange juice (total heat exposure at 90°C: 54 s) produced from the same batch of oranges as used for fresh fruit consumption. The pasteurization value of the orange juice was 0.383 (reference germ: Bacillus coagulans, TRef = 93.3°C, z = 8.9 K). Urine was collected throughout each clinical day in urine collection flasks and subjects returned their 24-h urine samples the next morning. The final urine volume was measured, and aliquots were stored at −80°C until further analyses. The study protocol (F-2013–097) was approved by the ethics committee of the State Chamber of Physicians of Baden-Württemberg (Stuttgart, Germany) and registered at clinicaltrials.gov (NCT02380144). All subjects provided written informed consent.
2.3. Flavanone concentrations in test foods
Frozen orange segments and frozen orange juice were milled under liquid nitrogen using a model 38BL41 blender (Waring, Torrington, CT, USA) prior to flavanone analyses. Hesperidin and narirutin in test foods were quantitated by HPLC-DAD using previously published and validated methods in duplicate analyses [4].
2.4. Flavanone excretion in 24-h urine
For hesperetin and naringenin analyses, 0.5 mL aliquots of the collected 24-h urine were combined with 0.5 mL of aqueous sodium acetate buffer (1 M, pH 5.5). After the addition of 10 μL buffer containing glucuronidase (162.0 U) and sulfatase (5.4 U) mixture, the samples were incubated for 2 h at 37°C. Subsequently, 3 mL of diethyl ether was added, and the mixture was probe sonicated for 8 s (<2°C). After separation of the ether phase, the aqueous phase was reextracted twice as described above. The combined ether extracts were dried under a stream of nitrogen (<20°C), re-dissolved in 1 mL of methanol with bath sonication, and membrane-filtered through a 0.2 μm nylon filter (Fisher Scientific, Hampton, NH, USA). Flavanone quantitation was performed on a series 1200 HPLC (Agilent Technologies, Santa Clara, CA, USA) coupled to a QTRAP 5500 mass spectrometer (AB Sciex, Concord, ON, Canada). Flavanones were separated using an ACE 3 C18-AR bonded phase column (75 × 4.6 mm id, 3 μm particle size; Mac-Mod, Chadds Ford, PA, USA) operated at 40°C. The following gradient, consisting of 0.1% (v/v) formic acid in water (eluent A) and 0.1% (v/v) formic acid in ACN (eluent B), was used at a flow rate of 1.5 mL/min (min/percentage of A): 0/95, 7.5/65, 9/5, 9.01/0, 11.0/0, 11.01/95, 14/95. Eluent flow was split sending approximately half to the mass spectrometer via a t-splitter. The injection volume was 1 μL. Quantitation was carried out by multiple reaction monitoring with electrospray ionization in negative ion mode, using the transitions of 301.1 > 163.6 (collision energy (CE): 35 eV) and 271.1 > 119.1 (CE: 30 eV) for quantification of hesperetin and naringenin, respectively, with optimized dwell times of 120 ms. Qualifier transitions for hesperetin were 301 > 286.0, 135.7, 241.0 and for naringenin 271 > 107.0, 151.0. Flavanones were identified and quantitated with authentic standards. Source parameters included −70 V declustering potential, −10 V entrance potential, −10 V collision cell exit potential, 40 psi curtain gas, −4500 V turbo ion spray, 600[H11034]C source temperature, 60 psi nebulizer gas, and 55 psi turbo jet gas. All flavanone concentrations in the subjects’ urine samples were found to be above the LOQ at >10:1 root mean square S/N.
2.5. Quantitation of flavanone catabolites in 24-h urine
Aliquots of 0.3 mL of urine were mixed with 300 μL of sodium acetate buffer (1 M, pH 5.5) containing glucuronidase (97.2 U) and sulfatase (3.2 U), and incubated for 2 h at 37°C. Subsequently, 0.6 mL of ACN was added, and the sample was vortexed for 30 s. The samples were centrifuged at 21 130 × g for 2 min (model 5424 centrifuge; Eppendorf, Hamburg, Germany), and then directly injected into a series 1290 UHPLC coupled to a 6495 triple quadrupole mass spectrometer (Agilent Technologies). Flavanone catabolites were separated on a Zorbax Eclipse+ C18 HD column (50 × 2.1 mm id, 1.8 μm particle size; Agilent Technologies) using the following gradient (min/percentage of A): 0/95, 0.5/95, 3/43, 3.01/5, 3.5/5, 3.51/95, 6/95, with 0.1% (v/v) acetic acid in water (eluent A) and 0.1% (v/v) acetic acid in methanol (eluent B). Source parameters included: 150°C drying gas, 18 L/min drying gas flow, 30 psi nebulizer, 400°C sheath gas, 12 L/min sheath gas, 3000 V capillary, 1500 V nozzle, and ion funnel RF of 90 high pressure funnel and 60 low pressure funnel. UHPLC retention times and MS/MS transitions of flavanone catabolites are displayed in Table 1 with qualifier and quantifier transitions indicated. Dwell times ranged from 43 to 90 ms depending on peak width for a given compound in order to maintain appropriate duty cycle (15 pts/peak). Flavonoid catabolites were quantified using authentic standards. LOD and LOQ were determined based on the S/N using Masshunter Qualitative Analysis B.07.00 Software (Agilent Technologies). All catabolite concentrations in the subjects’ urine samples were found to be above the LOQ at > 10:1 root mean square S/N.
Table 1.
UHPLC retention times (RT) and fragmentation patterns of flavanone catabolites in 24-h urine after consumption of the orange fruit and orange juice test meal
| No. | Compound | RT (min) | [M – H]− (m/z) | MS2 (mlz)a) |
|---|---|---|---|---|
| 1 | 4-Hydroxyhippuric acid (4-OH-HipA) | 1.45 | 194.1 | 150.0, 100.0, 93.1, 74.2 |
| 2 | 3-(3′-Hydroxyphenyl)hydracrylic acid (HPHPA) | 1.90 | 181.1 | 136.9, 121.0, 119.1 |
| 3 | Hippuric acid (HipA) | 2.13 | 178.1 | 134.0, 132.0, 77.1 |
| 4 | 3-(3′-Hydroxy-4′-methoxypheny)propionic acid (HMPPA) | 2.89 | 195.1 | 136.1, 134.9, 119.1, 107.9 |
Hesperetin and naringenin were quantitated by a separate method (see Materials and methods section).
Transitions printed in bold were used as quantifiers for the respective catabolite, whereas the remaining ones were qualifiers to ascertain catabolite identity and peak purity.
2.6. Statistics
Statistically significant differences of means (p < 0.05) for flavonoid concentrations in the test foods were identified by Tukey’s post hoc test. Shapiro–Wilk’s test showed nonnormal distribution of the data obtained for urinary flavanone and flavanone catabolite excretion. Consequently, identification of significant differences between means (p < 0.05) was achieved using the nonparametric Cochran–Mantel– Haenszel (CMH) test with the different food sequences as strata, controlling for carry-over, period, and time effects. Logarithmic transformation of the data resulted in closer approximation to a normal distribution, and thus an analysis of variance (ANOVA) was additionally carried out using a linear mixed model with the covariates food sequence (two combinations), period (two clinical visits), participants (n = 11), and test meal (orange fruit and orange juice). p-Values quoted in the text are from the CMH test unless indicated otherwise. Multivariate data analysis was performed for urinary hesperetin and naringenin excretions following data preprocessing by logarithmic transformation, mean centering, and weighting of all variables by their standard deviation. Statistical data evaluation was carried out with SAS 9.3 (SAS Institute, Cary, NC, USA), except for multivariate analyses, where Solo 7.5 (Eigenvector Research, Manson, WA, USA) was used.
3. Results
3.1. Flavanone content in test foods and their urinary excretion
In agreement with previous studies [4, 23], hesperidin and narirutin concentrations in the fresh oranges (269 mg/100 g fresh weight [FW]) were approximately 4.5-fold higher compared to those in the pasteurized orange juice (63 mg/100 g FW). Thus, subjects consumed 1477 ± 88 and 636 ± 17 μmol hesperidin with the orange fruit and the orange juice test meal, respectively, corresponding to a 2.3-fold higher dose provided by the fruit. The delivered narirutin dose was 296 ± 10 μmol from orange fruit and 115 ± 1 μmol from orange juice.
Subjects excreted 1.5 and 2.9% of total consumed hesperidin as hesperetin conjugates after consuming the orange fruit and the orange juice, respectively. However, differences between the absolute urinary hesperetin excretion after consumption of the orange fruit (21.6 ± 8.0 μmol, range: 0.9–93.6 μmol) and that of the orange juice test meal (18.3 ± 7.2 μmol, range: 1.6–78.5 μmol) were statistically not significant (Fig. 2). In contrast to hesperetin recovery (1.5–2.9%), a substantially greater proportion of naringenin was determined in the urine after orange fruit (12.9%) and orange juice (20.3%) ingestion (Fig. 2), although both test foods delivered approximately five times more hesperidin than narirutin. While the nonparametric CMH test yielded no statistical difference between the means of the absolute naringenin excretion after both treatments (p = 0.1190), the ANOVA indicated a significantly higher (p = 0.0022) urinary naringenin excretion after consumption of the orange fruit test meal (38.3 ± 10.2 μmol, range: 6.5–114.0 μmol) compared to that of the orange juice test meal (23.3 7.5 μmol, range: 3.9–74.6 μmol, Fig. 2). Both BMI and gender had no significant effects on flavanone excretion. Total 24-h urine volumes did not differ significantly between the two interventions.
Figure 2.
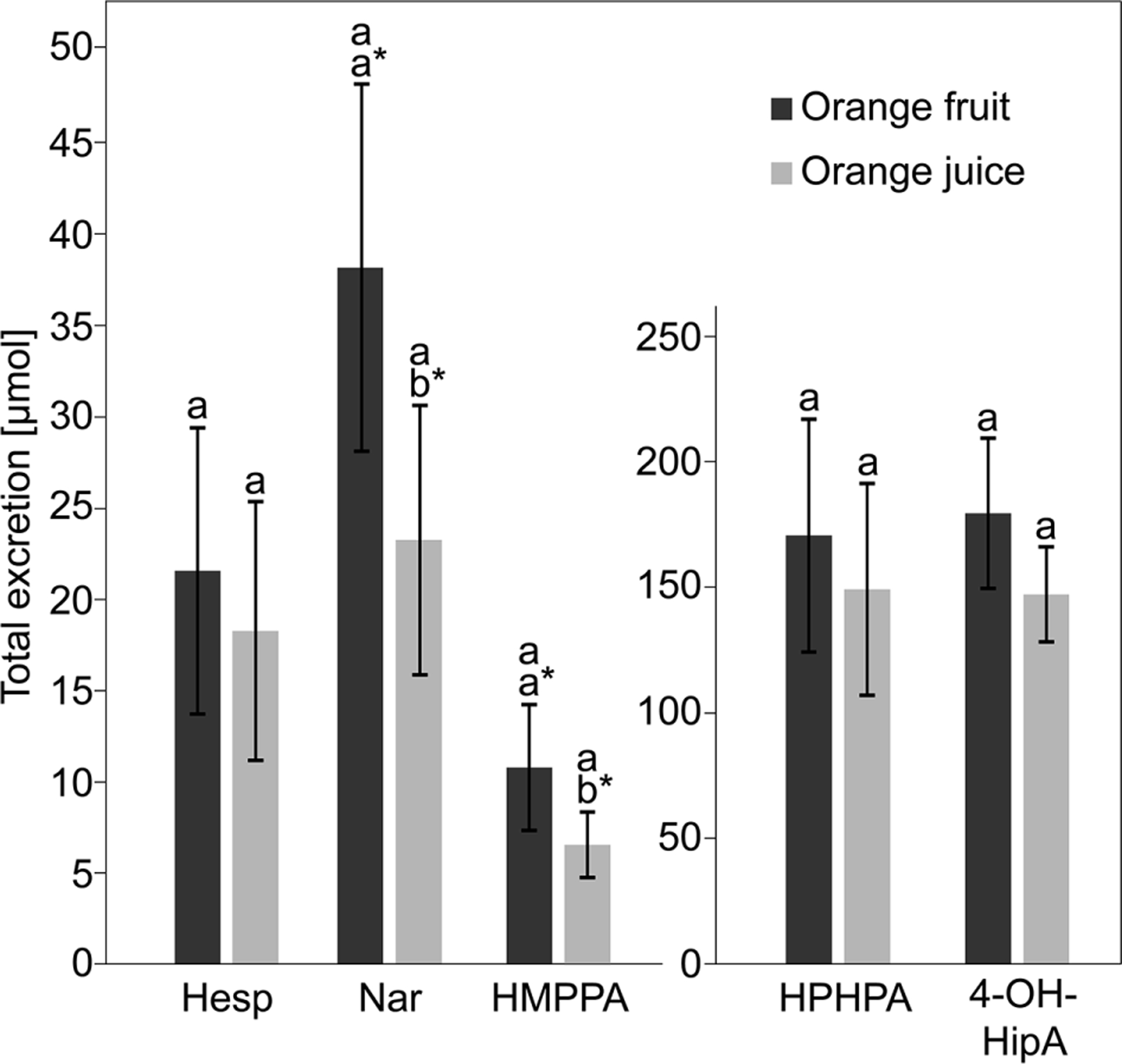
Excretion of hesperetin (Hesp), naringenin (Nar), and the phenolic catabolites 3-(3′-hydroxy-4′-methoxyphenyl)propionic acid (HMPPA), 3-(3′-hydroxyphenyl)hydracrylic acid (HPHPA), and 4-hydroxyhippuric acid (4-OH-HipA) in 24-h urine after consumption of either fresh orange fruit (dark gray bars) or pasteurized orange juice (light gray bars). Columns represent means (n = 11), vertical error bars indicate standard errors of mean. Different letters above the bars indicate significant difference between the two treatments (fruit/juice consumption) as assessed by the nonparametric Cochran–Mantel–Haenszel (CMH) test (p < 0.05). Differences between all treatments were nonsignificant according to the CMH test. When applying an ANOVA to the logarithmically transformed data, excretion of Nar (p = 0.0022) and HMPPA (p = 0.0297) were significantly higher after consumption of orange fruit as compared to orange juice (a*, b*).
3.2. Flavanone catabolite concentrations in urine
Urinary excretion of the flavanone catabolites HMPPA, HPHPA, and 4-OH-HipA is shown in Fig. 2, showing nonsignificant differences after consumption of either the orange fruit or the orange juice test meal. However, when the data were logarithmically transformed and subsequently analyzed via ANOVA, urinary excretion of HMPPA was found to be significantly higher (p = 0.0297) after orange fruit (10.8 ± 3.6 μmol, range: 0.0−38.2 μmol) compared to or ange juice (6.5 ± 1.9 μmol, range: 0.0–19.5 [H9262]mol) consumption. In addition, HMPPA was only found in urine samples of 10 and nine subjects out of 11 after consumption of orange fruit and orange juice, respectively. In contrast, 4-OHHipA, HipA, and HPHPA were identified in urine of all 11 subjects, irrespective of the test meal. HipA excretions were 2406 ± 267 and 2844 ± 468 μmol after consumption of orange fruit and orange juice, respectively. Since HipA may result from the metabolism of phenolic compounds other than Citrus flavanones, the total excretion of monitored catabolites excluding HipA was 361.1 and 303.1 μmol after consumption of the orange fruit and orange juice test meal, respectively. When combined with the data of hesperetin and naringenin excretion, total flavanone recoveries ranged between 23.7 and 45.9% for orange fruit and juice, respectively. The effect of BMI on flavanone catabolite excretions was negligible to moderate, and statistically significant only regarding a slight trend toward HMPPA excretion (orange fruit: Spearman’s r 0.60, p = 0.05; orange juice: r = 0.54, p = 0.09). Thus, irrespective of the test food consumed, subjects with a higher BMI tended to excrete more HMPPA compared to those with lower BMI.
3.3. Interindividual differences in flavanone excretion
To determine the interindividual differences in flavanone absorption, urinary hesperetin, naringenin, and HMPPA excretions were logarithmically transformed and subsequently grouped into tertiles representing high, intermediate, and low excretors. The results clearly indicate subjects 1, 2, and 3 to be high hesperetin excretors (range: 23.4−93.6 μmol), consistently being in the high tertile, irrespective of the test meal consumed. In contrast, subjects 9, 10, and 11 were classified as low to intermediate hesperetin excretors (range: 0.9−5.5 μmol). Similar results were obtained for naringenin, where subjects 1, 2, and 3 showed high excretions (range: 40.3−114.0 μmol), while subjects 9, 10, and 11 were classified as low excretors (range: 3.9−13.0 μmol). These findings were consistent with principal components analysis, clustering groups of subjects based on absorption behaviors (Fig. 3). Excretion of HMPPA, a highly specific hesperetin catabolite [24, 25], was inversely related to hesperetin excretion after consumption of either test food (Fig. 4). HMPPA excretion from subjects 1, 2, and 3 was low (range: 0.0−2.1 μmol), while that of subjects 9, 10, and 11 was intermediate to high (range: 5.8−24.2 μmol). In contrast to HMPPA, however, the excretion of 4-OH-HipA and HPHPA showed no such clear relationship.
Figure 3.
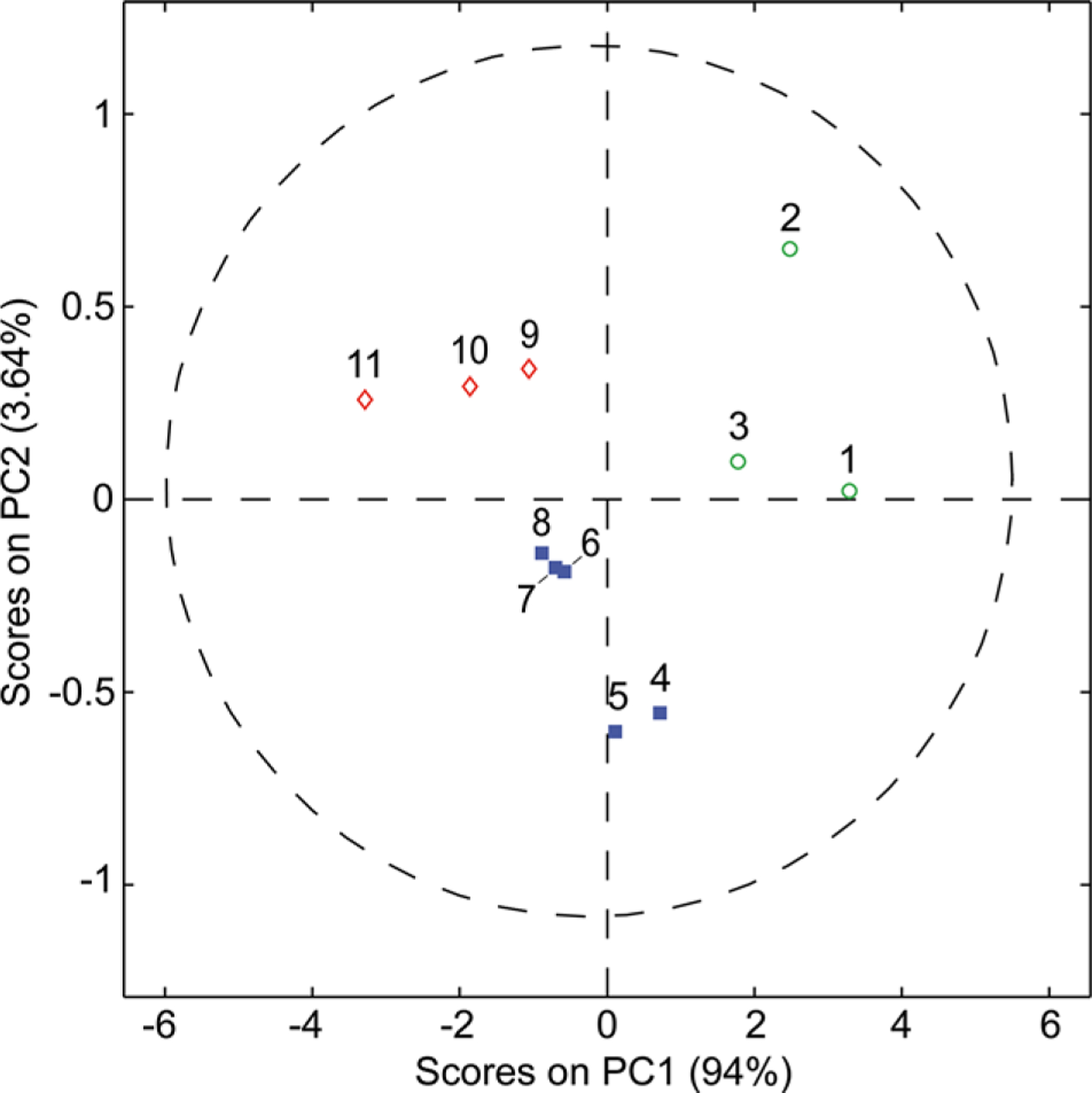
Principal component analysis (PCA) scores plot of hesperetin and naringenin excretion in 24-h urine of 11 subjects after the consumption of either the orange fruit or orange juice test meal. Groups showing different absorption behaviors are distinctively clustered in different sectors of the PCA plot. A confidence level of 95% is displayed by the dashed circle. ○ = high; ■ = intermediate; ◊ = low flavanone responders.
Figure 4.
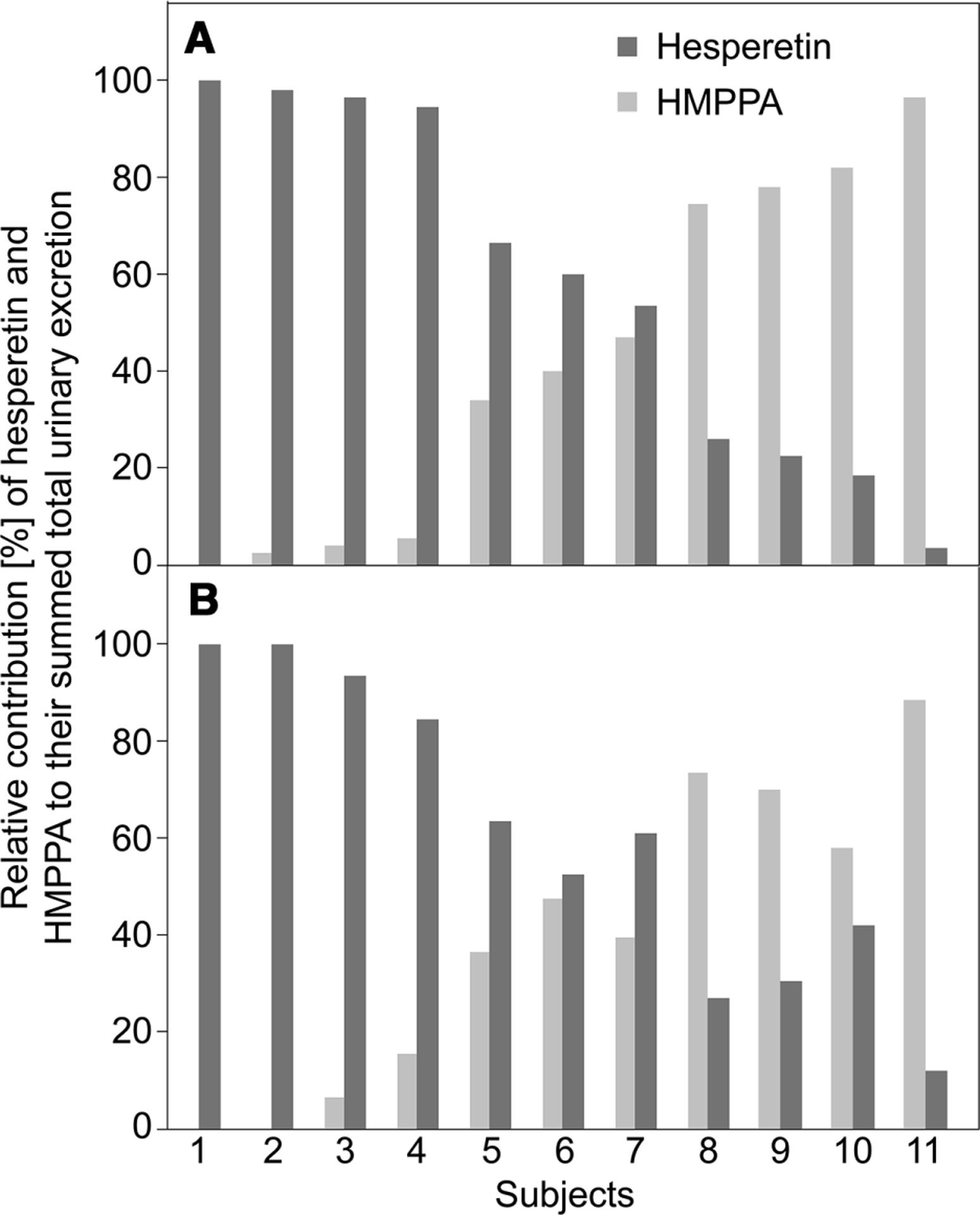
Relative contribution of hesperetin and its colonic microbiota derived catabolite 3-(3′-hydroxy-4′-methoxyphenyl) propionic acid (HMPPA) to their summed total excretions in 24-h urine after consumption of orange fruit (A) and orange juice (B).
4. Discussion
4.1. Urinary hesperetin and naringenin excretion
Epidemiological and prospective studies have related Citrus flavanone intake to a variety of health beneficial effects, promoting further research regarding their intestinal absorption and metabolism [8, 28]. Although partial uptake from the small intestine has previously been shown in ileostomists [20], most dietary hesperidin and narirutin is believed to reach the colon, where they are metabolized by the colonic microbiota. After eventual deglycosylation and subsequent absorption to the colonocyte (Fig. 1) [29, 30], the aglycones are rapidly conjugated with glucuronic acid or sulfate catalyzed by enzymes of the phase II metabolism and released into the portal vein [21,22]. Flavanone conjugates have been reported to reach maximal postprandial concentrations in plasma between 3.6 and 6.8 h [16, 17, 31, 32] and urinary excretion to be complete within 24 h after consumption of the test meal [16,32].
The main question of this study was to evaluate whether subjects were able to absorb and metabolize the high flavanone dose delivered by the orange fruit test meal. Previously, after in vitro digestion of orange fruit, their levels of bioaccessible hesperidin (26–32 mg/100 g FW) were observed to be similar to those found after digestion of orange juices (27–28 mg/100 g FW), despite the eightfold higher flavanone content of the fruit [4]. These findings prompted us to hypothesize that the limited solubility of the flavanones in the digestive fluids (~52–64 mg/L) might have caused the apparent saturation effect, which had presumably been reached even with the comparably low flavanone contents of orange juice. In line with this interpretation, low aqueous hesperidin solubilities ranging from 5 to 20 mg/L have been reported earlier [33, 34], and thus standard analytical methods for the quantitation of hesperidin in foods recommended the use of polar aprotic solvents such as DMF and DMSO to ensure complete dissolution of the analyte [35].
In the present study, the relative urinary hesperetin recoveries observed after consumption of orange fruit (1.5%) and orange juice (2.9%) were slightly lower than those reported in previous studies (from fruit: 4.5%; from juice: 4.1–5.4%, [16, 21, 23]). Most importantly, despite the 2.3-fold higher hesperidin dose delivered with the orange fruit, urinary hesperetin excretion did not differ from that observed after the consumption of orange juice. These results support one or both hypotheses that release, absorption, and metabolism of dietary flavanones is saturated when intake exceeds a certain limit, perhaps due to poor solubility and saturation of transport into the enterocyte, and/or is hindered because of entrapment of the flavonoids within the complex fibrous orange fruit matrix. As previously reported, dietary fiber contents within the orange fruit (2.16 g/100 g FW) were approximately 16-fold higher compared to those in the orange juice (0.13 g/100 g FW) [6]. In accordance, Vallejo et al. reported a strong correlation between dissolved Citrus flavanones and urinary flavanone excretion (r2 = 0.8563) when comparing the consumption of three different orange juices and two beverages supplemented with a flavanone extract [16]. Higher hesperidin concentrations in orange fruit are therefore unlikely to boost the potential health benefit when compared to lower hesperidin intake provided by orange juice consumption.
In contrast to hesperidin, urinary naringenin excretion was higher after orange fruit compared to orange juice, both being generally higher than that of hesperetin. These findings are in line with a study by Kanaze et al., reporting an approximately twofold higher urinary naringenin excretion compared to hesperetin after intake of equimolar doses of the flavanones by six healthy subjects [32]. In a cross-over study with eight healthy subjects, Mullen et al. reported a significantly higher recovery of naringenin (17.7% of the fed dose) compared to hesperetin (6.3%) after the consumption of 250 mL of orange juice fortified with hesperidin [31]. Lastly, in a single treatment study with 129 healthy subjects, Brett et al. demonstrated urinary recoveries of naringenin (14.5%) to be almost fourfold higher than those of hesperetin (3.9%) [23]. Although higher urinary hesperetin recoveries (17.5%) compared to those of naringenin (12.7%) have also been found in a study with 12 healthy subjects [25], the majority of reports [23, 31, 32] provided evidence that naringenin is absorbed and excreted to a greater extent than hesperetin. The enhanced excretion of naringenin may be related to the higher polarity of the molecule imparted by its 4′-hydroxyl group, thereby increasing its solubility in aqueous media. Therefore, in our study, the subjects have been able to utilize the higher narirutin dose delivered by the orange fruit meal, whereas the lower aqueous solubility of hesperidin may have limited urinary hesperetin excretions irrespective of the consumed test meal.
To the best of our knowledge, only one previous study has directly compared the bioavailability of Citrus flavanones from fresh oranges and orange juice. In a randomized cross-over trial comprising 20 subjects, Brett et al. reported no significant difference in urinary flavanone excretion after consumption of 150 g of fresh oranges or 300 mL of orange juice [23]. Noteworthy, in their study, the two test foods delivered similar doses of hesperidin (237–264 μmol) and narirutin (35–43 μmol), thus rather evaluating the effect of different food matrices.
4.2. Flavanone catabolite excretion
The monitored catabolites in our study were selected based on their function as key indicators of orange flavanone consumption. HMPPA is a direct catabolite of hesperetin (Fig. 1), resulting from colonic fission of the C-ring and subsequent dehydrogenation [24, 25]. Pereira-Caro et al. have shown excretion of HPHPA and 4-OH-HipA to increase 15- and 95-fold after consumption of 250 mL orange juice, respectively, as compared to the consumption of a polyphenol-free placebo drink [25]. Although HipA levels were higher after orange juice consumption in the study of Pereira-Caro et al., it is not a Citrus flavanone specific catabolite. HipA is formed as a catabolic end product of colonic microbiota [24] by acylation of benzoic acid with glycine [26], and may thus result from phenolic precursors other than flavanones. Since baseline urine samples were unavailable in our study, it cannot be definitively stated that the increased excretion of HipA after orange juice consumption was causally related to the test meal.
The aforementioned catabolites may not only act as bioactives regarding human health, but may also alter the gut microbiome. For instance, in vitro studies by Hidalgo et al. provided evidence that colonic anthocyanin catabolites may positively modulate Bifidobacterium spp. and Lactobacillus-Enterococcus spp. growth and protect against atherosclerosis [36, 37]. Urolithins (i.e., colonic ellagitannin catabolites) inhibited proliferation and induced apoptosis of colon cancer cells in an in vitro study by González-Sarrías et al. [38]. Larrosa et al. linked several phenolic catabolites such as hydrocaffeic, dihydroxyphenyl acetic, and hydro-ferulic acid to the inhibition of prostaglandin E2 synthesis in IL-1β stimulated colon fibroblast cells. Furthermore, anti-inflammatory effects have been shown in rodents [39]. In a cross-over study with 12 healthy volunteers, Pereira-Caro et al. reported 10 distinctive flavanone derived catabolites in 24-h urine following consumption of 250 mL of orange juice [25]. In their study, urinary flavanone recovery drastically rose from 16 to 100% when these catabolites were included to flavanone glucuronide and sulfate metabolite excretion. In accordance, but to a lesser extent, urinary flavanone recovery after orange fruit and juice consumption in our study markedly increased from 3.4 and 5.5% to 23.7 and 45.9%, respectively, when HMPPA, HPHPA, and 4-OH-HipA were included. Flavanone recovery in the study by Pereira Caro increased to 26.6% when the same catabolites were included as considered in our study [25].
Further experiments using stable isotope-labeled flavanones may allow comprehensive insights into the future. Nonetheless, excretion data of the major known flavanone catabolites analyzed in our study were in line with those of hesperetin and naringenin, showing only nonsignificant differences after consumption of either test meal. However, further studies are needed to assess the effect of repeated high doses of dietary Citrus flavanones on the mid- and long-term modulation of gut microbiota, irrespective of their total amounts excreted in urine.
4.3. Interindividual differences in flavanone excretion
Since flavanone absorption highly depends on gut microbiota, differences in the composition of the colonic microbiota may explain the observed large interindividual differences. Erlund et al. first reported high interindividual variations in Citrus flavanone bioavailability after consumption of orange and grapefruit juice by eight healthy subjects, suggesting a causal relationship to differences in the colonic microbiota [22]. In agreement, Brett et al. reported drastic interindividual differences in urinary hesperetin recovery after consumption of 300 g of orange juice by 129 subjects, ranging from 0.0 to 25.4%, irrespective of the subject’s age, gender, or BMI [23]. In our study, hesperetin recoveries ranged from 0.01 to 6.3% and 0.3 to 12.3% after consumption of the orange fruit and juice test meal, respectively, thus highlighting considerable differences among the subjects. Recall that the highest hesperetin excretors tended to be the lowest HMPPA excretors and vice versa, which would in part explain the large variance in hesperetin excretion.
Similarly, observing large interindividual variations, Vallejo et al. grouped the subjects into low, medium, and high flavanone excretors, most likely reflecting the diversity of their colonic microbiota [16]. The same authors noted that stratification of subjects into high and low absorbers was of utmost relevance when the effect of technological treatments on flavanone bioavailability was assessed in clinical trials, emphasizing the importance of cross-over study designs [40]. Our results additionally suggest an inverse relationship between urinary recovery of hesperetin conjugates and its catabolite HMPPA (Fig. 4). Since various microbial strains were shown to be responsible for flavanone deglycosylation and ring fission [19], the colonic microbiota of high hesperetin excretors (i.e., low HMPPA excretors) might preferentially contain strains being able to deglycosylate the flavanones but only poorly able to cleave the C-ring, while that of low hesperetin excretors (i.e., high HMPPA excretors) may be characterized by strains being capable of both deglycosylation and efficient flavanone C-ring cleavage.
Taken together, the consumption of flavanones from orange juice resulted in a similar urinary hesperetin excretion compared to that of a 2.3-fold higher hesperidin dose from orange fruit. In accordance, differences in the excretion of flavanone catabolites after consumption of either test food were nonsignificant. Thus, orange juice should be considered an excellent dietary source of flavanones, being widely equivalent to fresh orange fruit regarding flavanone uptake. Considering the large interindividual variability in flavanone degradation, further studies should assess the possible effect of long-term consumption of orange fruit and juice on modulation of the colonic microbiome.
Acknowledgments
The technical assistance of Svenja Baur, Julia Kahlhöfer, Judith Karschin, and Christa Rolke during the clinical trial is gratefully acknowledged. We thank Jochen Ziegler for his helpful support in multivariate data analysis. LC-MS experiments were conducted in the Nutrient and Phytochemical Analytic Shared Resource, part of the Ohio State University Comprehensive Cancer Center (NIH P30 CA016058). This study was funded by the University of Hohenheim and The Ohio State University.
Abbreviations:
- ANOVA
analysis of variance
- CMH
Cochran–Mantel–Haenszel
- FW
fresh weight
- HipA
hippuric acid
- HMPPA
3-(3′-hydroxy-4′-methoxyphenyl)propionic acid
- HPHPA
3-(3′-hydroxyphenyl)hydracrylic acid
- 4-OH-HipA
4-hydroxyhippuric acid
Footnotes
The authors have declared no conflict of interest.
5 References
- [1].USDA, US Department of Agriculture, Foreign Agricultural Service. Citrus: World Markets and Trade. Available from: http://apps.fas.usda.gov/psdonline/circulars/citrus.pdf (accessed: March 2016).
- [2].USDA, US Department of Agriculture. Orange juice: production, supply and distribution in selected countries. Available from: http://apps.fas.usda.gov/psdonline/ (accessed: March 2016).
- [3].AIJN, Association of the Industry of Juices and Nectars from Fruits and Vegetables of the European Union. Market Report. Available from: http://www.aijn.org/files/default/aijn2015-report.pdf (accessed: March 2016).
- [4].Aschoff JK, Kaufmann S, Kalkan O, Neidhart S et al. , In vitro bioaccessibility of carotenoids, flavonoids, and vitamin C from differently processed oranges and orange juices [Citrus sinensis (L.) Osbeck]. J. Agric. Food Chem 2015, 63, 578–587. [DOI] [PubMed] [Google Scholar]
- [5].Kawaii S, Tomono Y, Katase E, Ogawa K et al. , Quantitation of flavonoid constituents in Citrus fruits. J. Agric. Food Chem 1999, 47, 3565–3571. [DOI] [PubMed] [Google Scholar]
- [6].Aschoff JK, Rolke CL, Breusing N, Bosy-Westphal A et al. , Bioavailability of β-cryptoxanthin is greater from pasteurized orange juice than from fresh oranges: a randomized cross-over study. Mol. Nutr. Food Res 2015, 59, 1896–1904. [DOI] [PubMed] [Google Scholar]
- [7].Erlund I, Review of the fLavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res 2004, 24, 851–874. [Google Scholar]
- [8].Khan MK, Zill-E-Huma Dangles O, A comprehensive review on flavanones, the major Citrus polyphenols. J. Food Comp. Anal 2014, 33, 85–104. [Google Scholar]
- [9].Kumpulainen JT, Lehtonen M, Mattila P, in: Kumpulainen JT, Salonen JT (Eds.), Natural Antioxidants in Nutrition, Health and Disease, The Royal Society of Chemistry, Cambridge: 1999, pp. 141–150. [Google Scholar]
- [10].Chun OK, Chung SJ, Song WO, Estimated dietary flavonoid intake and major food sources of U.S. adults. J. Nutr 2007, 137, 1244–1252. [DOI] [PubMed] [Google Scholar]
- [11].Cassidy A, Rimm EB, O’Reilly EJ, Logroscino G et al. , Dietary flavonoids and risk of stroke in women. Stroke 2012, 43, 946–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Knekt P, Kumpulainen J, Järvinen R, Rissanen H et al. , Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr 2002, 76, 560–568. [DOI] [PubMed] [Google Scholar]
- [13].Morand C, Dubray C, Milenkovic D, Lioger D et al. , Hesperidin contributes to the vascular protective effects of orange juice: a randomized crossover study in healthy volunteers. Am. J. Clin. Nutr 2011, 93, 73–80. [DOI] [PubMed] [Google Scholar]
- [14].Aptekmann NP, Cesar TB, Long-term orange juice consumption is associated with low LDL-cholesterol and apolipoprotein B in normal and moderately hypercholesterolemic subjects. Lipids Health Dis. 2013, 12, 119, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ghanim H, Sia CL, Upadhyay M, Korzeniewski K et al. , Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am. J. Clin. Nutr 2010, 91, 940–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vallejo F, Larrosa M, Escudero E, Zafrilla MP et al. , Concentration and solubility of flavanones in orange beverages affect their bioavailability in humans. J. Agric. Food Chem 2010, 58, 6516–6524. [DOI] [PubMed] [Google Scholar]
- [17].Bredsdorff L, Nielsen Inge Lise F., Rasmussen SE, Cornett C et al. , Absorption, conjugation and excretion of the flavanones, naringenin and hesperetin from alpha-rhamnosidase-treated orange juice in human subjects. Br. J. Nutr 2010, 103, 1602–1609. [DOI] [PubMed] [Google Scholar]
- [18].Actis-Goretta L, Dew TP, Lévèques A, Pereira-Caro G et al. , Gastrointestinal absorption and metabolism of hesperetin-7-O-rutinoside and hesperetin-7-O-glucoside in healthy humans. Mol. Nutr. Food Res 2015, 59, 1651–1662. [DOI] [PubMed] [Google Scholar]
- [19].Selma MV, Espín JC, Tomás-Barberán FA, Interaction between phenolics and gut microbiota: role in human health. J. Agric. Food Chem 2009, 57, 6485–6501. [DOI] [PubMed] [Google Scholar]
- [20].Borges G, Lean Michael E. J., Roberts SA, Crozier A, Bioavailability of dietary (poly)phenols: a study with ileostomists to discriminate between absorption in small and large intestine. Food Funct. 2013, 4, 754–762. [DOI] [PubMed] [Google Scholar]
- [21].Nielsen Inge Lise F., Chee Winnie S. S., Poulsen L, Offord-Cavin E et al. , Bioavailability is improved by enzymatic modification of the Citrus flavonoid hesperidin in humans: a randomized, double-blind, crossover trial. J. Nutr 2006, 136, 404–408. [DOI] [PubMed] [Google Scholar]
- [22].Erlund I, Meririnne E, Alfthan G, Aro A, Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J. Nutr 2001, 131, 235–241. [DOI] [PubMed] [Google Scholar]
- [23].Brett GM, Hollands W, Needs PW, Teucher B et al. , Absorption, metabolism and excretion of flavanones from single portions of orange fruit and juice and effects of anthropometric variables and contraceptive pill use on flavanone excretion. Brit. J. Nutr 2009, 101, 664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pereira-Caro G, Borges G, Ky I, Ribas A et al. , In vitro colonic catabolism of orange juice (poly)phenols. Mol. Nutr. Food Res 2015, 59, 465–475. [DOI] [PubMed] [Google Scholar]
- [25].Pereira-Caro G, Borges G, van der Hooft Justin, Clifford MN et al. , Orange juice (poly)phenols are highly bioavailable in humans. Am. J. Clin. Nutr 2014, 100, 1378–1384. [DOI] [PubMed] [Google Scholar]
- [26].Clifford MN, Copeland EL, Bloxsidge JP, Mitchell LA, Hippuric acid as a major excretion product associated with black tea consumption. Xenobiotica 2000, 30, 317–326. [DOI] [PubMed] [Google Scholar]
- [27].Barthe GA, Jourdan PS, McIntosh CA, Mansell RL, Radioimmunoassay for the quantitative determination of hesperidin and analysis of its distribution in Citrus sinensis. Phytochem. 1988, 27, 249–254. [Google Scholar]
- [28].Tripoli E, La Guardia M, Giammanco S, Di Majo D et al. , Citrus flavonoids: molecular structure, biological activity and nutritional properties: a review. Food Chem. 2007, 104, 466–479. [Google Scholar]
- [29].Kim D-H, Jung E-A, Sohng I-S, Han J-A et al. , Intestinal bacterial metabolism of flavonoids and its relation to some biological activities. Arch. Pharm. Res 1998, 21, 17–23. [DOI] [PubMed] [Google Scholar]
- [30].Roowi S, Mullen W, Edwards CA, Crozier A, Yoghurt impacts on the excretion of phenolic acids derived from colonic breakdown of orange juice flavanones in humans. Mol. Nutr. Food Res 2009, 53(Suppl 1), S68–S75. [DOI] [PubMed] [Google Scholar]
- [31].Mullen W, Archeveque M-A, Edwards CA, Matsumoto H et al. , Bioavailability and metabolism of orange juice flavanones in humans: impact of a full-fat yogurt. J. Agric. Food Chem 2008, 56, 11157–11164. [DOI] [PubMed] [Google Scholar]
- [32].Kanaze FI, Bounartzi MI, Georgarakis M, Niopas I, Pharmacokinetics of the Citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur. J. Clin. Nutr 2007, 61, 472–477. [DOI] [PubMed] [Google Scholar]
- [33].Majumdar S, Srirangam R, Solubility, stability, physico-chemical characteristics and in vitro ocular tissue permeability of hesperidin: a natural bioflavonoid. Pharm. Res 2009, 26, 1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Merck Research Laboratories, The Merck Index, 14th Edition, Merck & Co, Whitehouse Station, NY: 2001. [Google Scholar]
- [35].IFU, International Federation of Fruit Juice Producers. Method of Analysis no. 58 (1991), Paris, France: 1991. [Google Scholar]
- [36].Hidalgo M, Oruna-Concha MJ, Kolida S, Walton GE et al. , Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem 2012, 60, 3882–3890. [DOI] [PubMed] [Google Scholar]
- [37].Hidalgo M, Martin-Santamaria S, Recio I, Sanchez-Moreno C et al. , Potential anti-inflammatory, anti-adhesive, anti/estrogenic, and angiotensin-converting enzyme inhibitory activities of anthocyanins and their gut metabolites. Genes Nutr. 2012, 7, 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].González-Sarrías A, Núñez-Sánchez MÁ, Tomé-Carneiro J, Tomás-Barberán FA et al. , Comprehensive characterization of the effects of ellagic acid and urolithins on colorectal cancer and key associated molecular hallmarks: microRNA-cell specific induction of CDKN1A (p21) as a common mechanism involved. Mol. Nutr. Food Res 2015, 60, 701–716. [DOI] [PubMed] [Google Scholar]
- [39].Larrosa M, Luceri C, Vivoli E, Pagliuca C et al. , Polyphenol metabolites from colonic microbiota exert anti-inflammatory activity on different inflammation models. Mol. Nutr. Food Res 2009, 53, 1044–1054. [DOI] [PubMed] [Google Scholar]
- [40].Tomás-Navarro M, Vallejo F, Sentandreu E, Navarro JL, Tomás-Barberán FA, Volunteer stratification is more relevant than technological treatment in orange juice flavanone bioavailability. J. Agric. Food Chem 2014, 62, 24–27. [DOI] [PubMed] [Google Scholar]


