Abstract
Black mustard (Brassica nigra) was grown in pots amended with 41 nm ZnO (200–600 mg/kg soil) and 47 nm CuO (12.5–50 mg/kg soil) nanoparticles (NPs) to analyze growth response and yield characteristics. B. nigra seed germination was not affected by CuO NPs, but significant toxicity was observed by ZnO NP treatment. Both NPs significantly increased the growth profile of B. nigra, i.e., the stem height, number of leaves, average leaf area, number of branches, and number of nodes per plant. Application of ZnO and CuO NPs brought a significant dose-dependent decrease in primary root length; however, the number of secondary roots increased in the presence of CuO NPs. The average number of flowers and pods per plant significantly increased in the presence of CuO NPs. The seed yield, average seed weight per plant, and seed diameter parameters were observed to be better in the presence of CuO NPs as compared with ZnO NPs. Total protein contents and glucosinolates increased in the seeds grown in the NP-amended soil, while total oil contents decreased. Oil analysis depicted that oleic acid and linolenic acid percentage decreased while erucic acid percentage increased in seeds in the presence of both NPs in the soil. An atomic absorption spectrophotometer showed accumulation of Cu and Zn in B. nigra in the following order: root > stem > leaves > seeds. The study concludes that CuO and ZnO NPs have detrimental effect on the B. nigra plant and yield. The release of NPs and type of metal in NPs might also have a positive effect on the plant; however, their concentration in the soil also matters.
1. Introduction
Nanoparticles (NPs) have diverse properties due to the nanoscale composition of atomic structures. This property makes them attractive for pharmaceutical, energy, electronics, cosmetic, textile, and agriculture industries.1 However, their rapid use accounts for their adverse effects on the ecosystem. Nanoparticles can enter into the environment via various routes, i.e., skin care products, medicines, paints, direct release from industries, combustion, etc.1 Conversely, exposure of NPs to living organisms should not be ignored. Being primary producers, plants can aid in NP/metal ion transportation and bioaccumulation through food chain.2 Various reports widely demonstrate both affirmative and toxic effects of engineered NPs on growth dynamics, photosynthesis, morphology, physiology, and yield of some crops such as soybean, wheat, rice, and maize.3−6
ZnO and CuO NPs have grabbed attention among different metal oxide NPs due to their diverse applications and physiochemical properties. ZnO NPs are used in several industries such as cosmetics, batteries, glass, ceramics, lubricants, food, plastics, paints, adhesives, etc.1 CuO NPs are used in electronics and other technologies due to their unique and excellent thermophysical properties.7 Effects of ZnO and CuO NPs on plants have been demonstrated in various studies, which reveal that potential phytotoxicity depends upon their concentration, shape, size, etc.8 Phytotoxicity of NPs such as reduction in biomass, low seed germination, reduced root length, varying chlorophyll synthesis, and stunted shoot length has been reported in ryegrass, rice, wheat, and mustard plants.6,9−11
Brassica species are considered as model dicotyledonary plants due to the high rate of seed germination, rapid growth of plant, and short life span. Besides these, Brassica species are wild and also grown as food and fodder crops. B. nigra is commonly grown for food, oil extraction, animal cake production, and green manure. B. nigra has been reported as a metal accumulator and potential phytoextractant, and it tolerates the presence of heavy metal concentrations in the soil.12,13 Based on use, growth characteristics, resistivity, and tolerance toward metals, B. nigra is considered as a model plant to study plant growth response in a contaminated soil.14
Most of the studies are carried out in lab-scale experiments to investigate the effect of nanoparticles on plants, but few have been reported under soil conditions.15,16 It has been reported that medium conditions greatly influence dissolution of ions and bioavailability, aggregation, and mobility of nanoparticles. The organic matter in soil reduces the aggregation and enhances the solubility of NPs, which result in diverse phytotoxic effects.17 The NPs released in the environment affect plants, and these effects can be transferred to consumers via food chain due to metal accumulation in outgrowth and alter nutritional contents of seeds and fruits. Therefore, the objective of this study was to analyze the effect of ZnO and CuO NPs on the growth dynamics, yield, and nutritional composition of black mustard. Plants were grown in soil amended with chemically synthesized CuO and ZnO NPs. After harvest, seed characterization and elemental analysis were performed to determine nutritional composition and metal accumulation.
2. Materials and Methods
2.1. Synthesis and Characterization of Nanoparticles
The ZnO nanoparticles (NPs) were synthesized using the coprecipitation method as described by Ali et al.18 Briefly, 100 mL of 1 mM Zn(CH3COO)2·2H2O was dropwise mixed with 50 mL of 2 M NaOH, with constant stirring for 2 h. The white precipitate was collected by centrifugation (Model 5810r, Eppendorf Corporation, Hamburg, Germany) at 950g for 5 min at room temperature (25 ± 2 °C) and washed thrice with distilled water to remove impurities. The ZnO NPs were dried overnight in a drying incubator (Thomas Scientific, Swedesboro, NJ) at 60 °C.
The coprecipitation method was employed for the synthesis of CuO nanoparticles.19 Copper acetate monohydrate (600 mL, 0.2 M) was mixed with 2 mL of glacial acetic acid at 100 °C during continuous stirring. After 5 min, 30 mL of 6 M NaOH solution was dropwise added into the mixture. The reaction was continued for 2 h with stirring to complete the reaction. The black CuO NPs were separated by centrifugation (Model 5810r, Eppendorf Corporation, Hamburg, Germany) at 950g for 5 min at room temperature (25 ± 2 °C), washed thrice with distilled water to remove impurities, and dried overnight in a drying incubator (Thomas Scientific, Swedesboro, NJ) at 60 °C. The CuO NPs were calcinated at 500 °C for 4 h.
The powder patterns were recorded using an Empyrean PANalytical X-ray diffractometer with Bragg–Brentano geometry using Cu Kα radiation (λ = 1.54 Å). The step scan covered the angular range 20–80° with a step of 0.02°. The crystallite size was determined using the Scherrer equation.
where D is the crystallite size, k is a constant (∼0.94), λ is the wavelength of the X-ray radiation, β is the line width at half-maximum intensity of the peak, and θB is the angle of diffraction.
The morphology of the NPs was determined by scanning electron microscopy (SEM) using a Nova NanoSEM 450 (Thermo Fisher Scientific, Waltham, MA), operating at 10 kV. Energy-dispersive X-ray spectroscopy (EDX) was performed for determining the elemental composition of the synthesized material. Fourier transform infrared spectroscopy (FTIR) spectra were recorded using an OPUS Tensor 27 (Bruker, Ettlingen, Germany) with a wavenumber resolution of 1 cm–1.
2.2. Soil Amendment and Plant Growth Conditions
Medium loam natural soil collected from an agriculture field in Islamabad, Pakistan, was used in this study. The physiochemical properties of soil before the addition of nanoparticles analyzed through AOAC protocols20 are given in Table 1. In brief, the soil texture was analyzed by the hydrometer method. For this, 50 g of oven-dried soil was suspended in 50 mL of distilled water and 10 mL of 5% sodium hexametaphosphate solution was added. The mixture was stirred for 10 min and then transferred to a settling cylinder. The hydrometer was placed in a cylinder, and then the cylinder was filled with distilled water. The hydrometer was removed, and the suspension was shaken vigorously in a back-and-forth manner. After 20 s, the hydrometer was inserted again and the reading was noted. The reading was also noted after 40 s and 60 s. Thereafter, the shaking and settling procedure was repeated again and the reading was noted after 2 h. The procedure was performed at 24 °C; therefore, 1.44 was subtracted from the values. The standard textural triangle diagram was used to determine the texture class of the soil.
Table 1. Properties of the Soil Used for Filling of Pots before Addition of Nanoparticlesa.
| pH | 6.8 |
| E.C. (mS cm–1) | 0.9 |
| organic matter (%) | 26.4 |
| total nitrogen (g/kg) | 5.9 |
| available phosphorus (mg/kg) | 27.1 |
| sand (%) | 39 |
| silt (%) | 27 |
| clay | 34 |
Sand, clay, and manure (30:30:40) were mixed to make the active soil.
To determine soil pH, 10 g of soil was suspended in 20 mL of 0.01 M CaCl2 solution. The suspension was thoroughly stirred for 30 min, and pH was recorded by a pH meter. To find electrical conductivity (EC) of soil, 40 g of soil was mixed with 80 mL of distilled water in a flask and shaken for 1 h on a reciprocating shaker. The mixture was filtered thereafter, and EC was determined by an EC meter taking KCl as a standard.
The colorimetric method was followed to determine the organic matter in the soil. One gram of soil was mixed with 10 mL of 0.1667 M K2Cr2O7 and 20 mL of concentrated H2SO4 containing 1.25% of Ag2SO4. The mixture was stirred and allowed to stand for 30 min. The green color of chromium sulfate was read on a spectrophotometer at 660 nm. Sucrose (0.42%) was used as a standard, and percent carbon was calculated as
Percent organic matter was calculated as
To determine available phosphorus, 5 g of soil was extracted with 50 mL of Bray’s extractant (0.03 M NH4F in 0.025 M HCL), shaken well for 5 min, and filtered thereafter. Then, 5 mL of the extract was reacted with 5 mL of the molybdate reagent and 1 mL of SnCl2 solution (40% diluted as 1:66 with distilled water). After 10 min, absorbance was read on a spectrophotometer at 660 nm. The reading was calculated with standard KH2PO4 (0.02195%) that has 50 μg P/mL.
Clay pots (35 cm height × 25 cm diameter) were purchased from a local store. Powdered ZnO and CuO NPs were weighed and mixed with soil to achieve concentrations of 12.5, 25, and 50 mg of CuO NPs and 200, 400, and 600 mg of ZnO NP per kg of the soil. The selection of CuO and ZnO NP concentrations was based on our previous results where these ranges of NPs were found to be optimal as tolerable and toxic for B. nigra.21,22 Seeds of B. nigra (germination efficiency ≥ 98%) were provided by the Oil Seed Crop Division, National Agriculture Research Centre (NARC), Islamabad. Three replicates of each concentration (six pots in each replicate) and control were prepared (approx 5–6 kg soil per pot). After filling the pots with the amended soil, seeds of B. nigra (disinfected with 0.1% mercuric chloride (w/v) and hydrated in distilled water (DW) for 24 h to assist germination) were planted at 1 cm depth in a quincunx pattern and watered with DW. Afterward, pots were transferred to a shady chamber for 7 days to provide a dark environment for breaking seed dormancy, and seed germination parameters were recorded. Initially, in each pot, five seeds were sown, and then the experiment was continued with three plants per pot. After seed germination, pots were relocated to a growth chamber with 14 h photoperiod, 25/20 °C day/night temperature, and 50–55% humidity. Pots were watered with DW when required (approximately 400 ± 10 mL/pot) assuring that water did not leach the ions/NPs to the bottom of the pot. All of the pots were irrigated at the same time.
The experiment was continued for approximately 15 weeks until seed harvesting was done. After maturation, pods holding seeds were cut from the pedicel. Seed shattering by opening of mature pods was prevented through continued harvesting for 2 weeks. Afterward, seeds were dried under sunlight for 1 day and then stored in a glass container.
2.3. Morphological Growth Parameters of B. nigra Plants
To investigate the effect of NPs on growth dynamics, various growth morphological parameters, i.e., stem length, number of leaves and branches, etc., were studied during the plant life cycle with 10 day interval, while the area of apical, middle, and basal leaves was measured once using a portable Cl-202 leaf area meter (CID, Inc.). The number of flowers per plant was also recorded at 60th day. After harvesting of plants, the number of nodes, lateral roots, and average primary root length were recorded.
2.4. Yield Analysis and SmartGRAIN Imaging of Seeds
To investigate the effect on yield, various parameters, i.e., the seed diameter, number of seeds, weight of 1000 seeds, and total seed weight per plant, were studied along with analysis of digital images of seed using SmartGrain software developed by Tanabata et al.23 Digital images of the black mustard seeds were quantified with specific dimensions by a digital image analysis technique. Seven photographs of each seed batch were captured using a digital camera (Apple iPhone with 12 megapixel camera) with horizontal angle. The distance between the camera lens and seeds was kept constant, i.e., 20 cm. A black background grid with uniform squares was used to keep the distance of seeds constant. The photos were converted into a JPEG format and further processed with SmartGrain software (version 1.1) to study seed morphological parameters.
2.5. Mineral Composition of Plants and Seeds
Accumulation of Zn and Cu in roots, stems, and leaves was quantified after acid digestion. The plant samples, roots, stem, and leaves, were separated and washed thoroughly under running tap water. Thereafter, each part was rinsed with distilled water, and finally the plant parts were dried at 60 °C for 48 h. The leaves, stem, and roots of the B. nigra plant (both treated and untreated) were ground in an electric grinder. The powdered material (500 mg) was acid-digested with 10 mL of a HNO3/HClO4 solution (4:1) in a conical flask at 110 °C for 90 min until brown fumes turned white and diluted to 50 mL with deionized water. Metal contents in the samples were determined using an atomic absorption spectrophotometer (AAS). Zn and Cu metal contents in the control and amended soil samples collected after plant harvesting were also determined. These results were compared with the Zn and Cu contents of initial control soil samples. The soil samples were treated in the same way as mentioned above. Accumulation of trace and major elements, i.e., Ni, Co, Cd, Zn, Cu, Ca, Mg, and K, in seeds grown in the amended soil was determined through acid digestion and AAS using the above-mentioned protocol.
2.6. Protein, Glucosinolate, and Fatty Acid Analysis of Seeds
For analyses, seed samples from each treatment and control were taken randomly, ground, and subjected to chemical analysis. The total seed protein was determined following the method described by AOAC.24 For that, B. napa seeds were ground in a mortar and pestle and 2 g of the powdered sample was digested in a Kjeldahl digestion flask by boiling with 20 mL of concentrated H2SO4 until the mixture was clear. The digest was filtered, the volume was raised up to 250 mL with distilled water, and the flask was connected for distillation. Ammonia containing 50 mL of 45% sodium hydroxide solution was steam distilled from the digest. The distillate (150 mL) was mixed with 100 mL of 0.1 N HCl and methyl red indicator. The titration was performed against 2 M NaOH till the color changed from red to yellow. %Nitrogen was calculated as follows
Total protein was calculated as follows
The glucosinolate content of the oil was determined following the method of Smith et al.25 In short, 200 mg of powdered seeds was thoroughly mixed with 2 mL of glycine NaOH buffer (50 mM pH 9.0). After 10 min incubation at room temperature, 1 mL of chloroform, 1.0 mL of 100 mM citrate buffer (pH 5.0), 50 mL of 10% chlorhexidine diacetate, and 200 mg of activated charcoal were added. A diastix strip was immersed in the supernatant for 5 s, and after 2 min, the reflectance of the developed color was measured in a precalibrated TRUBLUGLU meter. For seed oil analysis, the oil was extracted in a Soxhlet extractor. The seed oil content was estimated by nuclear magnetic resonance (NMR) spectrometry.26 The oil acid value was determined according to the AOAC method.24 Briefly, 10 g of dried and fine powdered seeds were extracted for 8 h in the Soxhlet apparatus using petroleum ether as the extraction solvent. The extract was filtered through Whatman filter paper No. 1, and the solvent was evaporated under reduced pressure using a rotary evaporator (Büchi, Switzerland). The lipid fraction residues were weighed and dried for 1 h at 105 °C. For quantification of fatty acids, the oil sample (50–100 mg) was converted into its fatty acid methyl esters. The methyl esters of the fatty acids (0.5 mL) were analyzed by gas chromatograph (Shimadzu QP 5050) equipped with a flame ionizing detector (FID) and a fused silica capillary column, MN FFAP (50 m; 0.32 mm i.d.; film thickness, 0.25 mm). Helium was used as a carrier gas. The column temperature was kept at 110 °C for 0.5 min, increased up to 200 °C at 10 °C/min, and then maintained for 10 min. The temperatures of the injector and detector were set at 220 and 250 °C, respectively.
2.7. Statistical Analysis
Two-way analysis of variance (ANOVA) was performed to determine the effects NPs and concentration on plant growth; seed production; nutrient composition of seeds; and metal analyses in soil, seeds, and plants. The analysis of means was done by Tukey’s test. The statistical significance was accepted at a p-value of 0.05. The results are presented as mean ± standard deviation.
3. Results and Discussion
3.1. Synthesis and Characterization of NPs
SEM analysis revealed that ZnO NPs had sheetlike structures along with some sticks (Figure 1A). Zinc was the major element present in the nanoparticles along with oxygen as depicted by EDX analysis (Figure 1B). ZnO NPs, in FTIR analysis, showed an absorption band at around 3400 cm–1 that is assigned to the C–H stretching vibration and bending of the hydroxyl group. The other minor groups were also observed in FTIR analysis (Figure 1C). Most probably, these groups are part of acetate ion or hydroxyl released from the precursors of reactant salts. X-ray diffraction (XRD) analysis shows peaks located at 31.84, 34.52, 36.33, 47.63, 56.71, 62.96, 68.13, and 69.18° (Figure 1D). The size of synthesized ZnO NPs calculated by Scherrer’s formula was 41 nm. The XRD peak analysis also predicted that the NPs were hexagonal wurtzite in nature. ZnO NPs have been produced by different techniques and different precursors. Zinc acetate on reacting with alkali results in the formation of <100 nm nanoparticles with varying shape depending upon other physiological conditions.27−28
Figure 1.
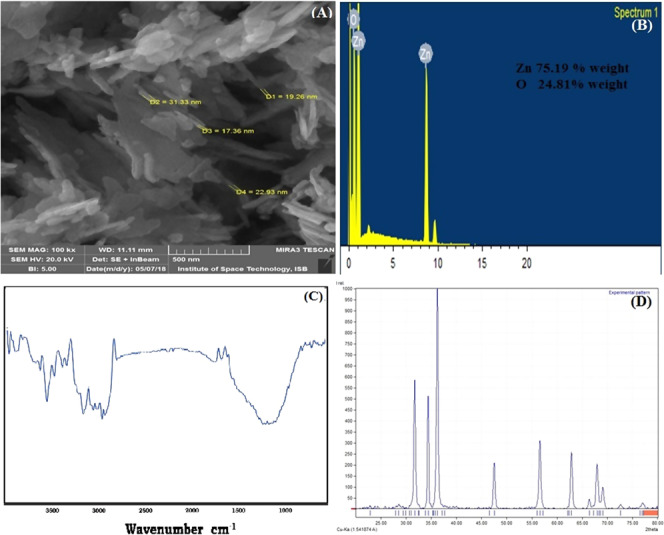
Characterization of synthesized ZnO nanoparticles: (A) scanning electron microscopy image, (B) energy-dispersive X-ray spectroscopy analysis, (C) Fourier transform infrared spectroscopy spectra, and (D) X-ray powder diffraction diffractogram.
In the case of CuO nanoparticles, the SEM micrograph clearly showed irregular-shaped morphologies. The NPs were circular with irregular surface lattice and clumped with each other (Figure 2A). The EDX spectra of CuO nanoparticles showed that there was no other elemental impurity present in the prepared nanoparticles (Figure 2B).
Figure 2.
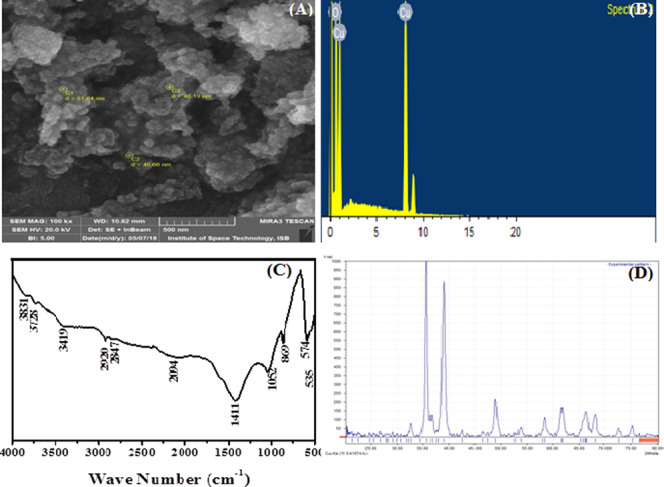
Characterization of synthesized CuO nanoparticles: (A) scanning electron microscopy image, (B) energy-dispersive X-ray spectroscopy analysis, (C) Fourier transform infrared spectroscopy spectra, and (D) X-ray powder diffraction diffractogram.
In FTIR spectroscopy, well-defined peaks were observed at 3419, 2920, 2847, 1411, 1052, 869, 594, and 535 cm–1. The absorption peaks at around 3419 and 1052 cm–1 are assigned to the O–H and C–O stretching vibrations, respectively (Figure 2C). The stretching vibrations of C–H, C–O, and O–H might be from the acetate of the metal salt used for the metal precursor and the alkali used for reduction of metal ions, respectively, that bound on the surface of the nanoparticles.18 The CuO nanoparticles synthesized by the coprecipitation method produced 47 nm (calculated by Scherrer’s formula) crystalline monoclinic nanoparticles (Figure 2D). The positions of all peaks are in good agreement with the PCPDFWIN data card 895899. These results are also in good concurrence with the literature.19,28
3.2. Effect of ZnO and CuO NPs on Seed Germination and Plant Morphology
Seed germination efficiency is extensively used for the phytotoxicity test. The physiochemical process begins with seed imbibition of water and results in sprouting of seedlings.9 In this study, B. nigra seed germination was not affected by CuO NPs but significant phytotoxicity was observed by ZnO NP treatment. At 600 mg/kg ZnO NPs in soil, 53.3% seeds germinated, while at both lower concentrations (200 and 400 mg/kg soil), the seed germination efficiency was 80%. These findings indicate the phytotoxic effect of ZnO NPs at these concentrations and the neutral effect of CuO NPs on seed germination. Similar results have been reported by Adhikari et al. and Lee et al. who reported no effect of CuO NPs and an inhibitory effect of ZnO NPs on seed germination on different plant species.28,30 It might be the phytotoxic effect of ZnO NPs that decreased the permeability of seed coat that impedes intake of water and oxygen into the cells and results in deceleration of germination and metabolism.31 The application of NPs in the soil following watering may increase the release of ions from respective NPs that may result in a decrease of pH.32 Low pH does not support germination of seeds and greatly influences uptake of nutrients.33 The decrease in soil pH also increases the net negative surface charge on organic matter and soil oxides.34 An optimal soil pH for Indian mustard growth has been documented to be 6.5.35
ZnO and CuO NPs significantly increased the growth profile of B. nigra plants (Figure 4A–F). Application of ZnO NPs at 200 and 600 mg/kg soil resulted in 5.8 and 8.6% average increase in plant height, respectively, as compared to control. However, at 400 mg/kg, 3.4% decrease in plant height was observed at day 60. A steady increase in plant height was observed between days 30 and 40 due to the presence of ZnO NPs. A gradual significant increase in plant height was observed as CuO NP concentration increased up to 50 mg/kg (Figure 3B). It is also evident from the figure that the B. nigra height gradually increased at all of the concentrations of CuO NPs especially after day 30 of seed sowing.
Figure 4.
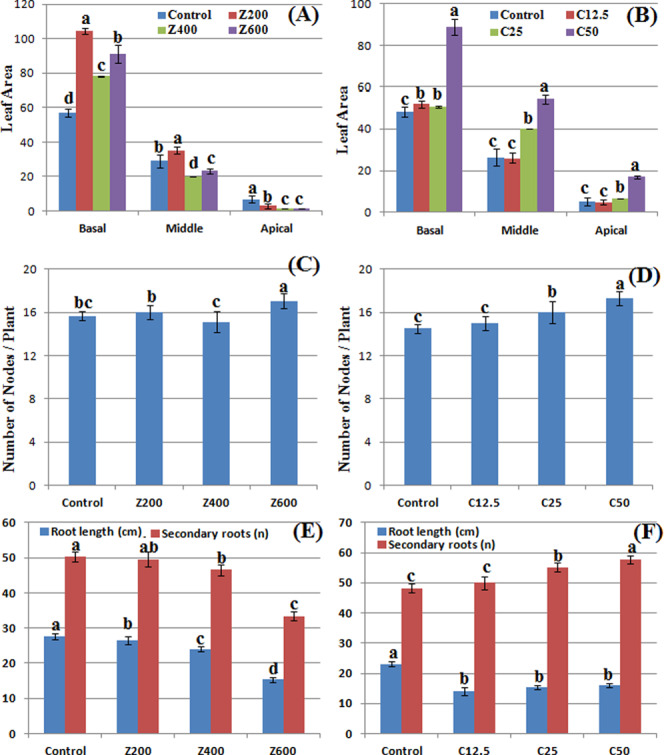
Morphological responses of B. nigra upon exposure to ZnO and CuO nanoparticles. Error bars represent the SE (n = 6), and similar letters mean no statistical difference between treatments in Tukey’s test (p ≤ 0.05). (A, B) Leaf area; (C, D) number of nodes/plant; and (E, F) primary root length/plant and secondary roots/plant in the presence of ZnO and CuO NPs, respectively.
Figure 3.
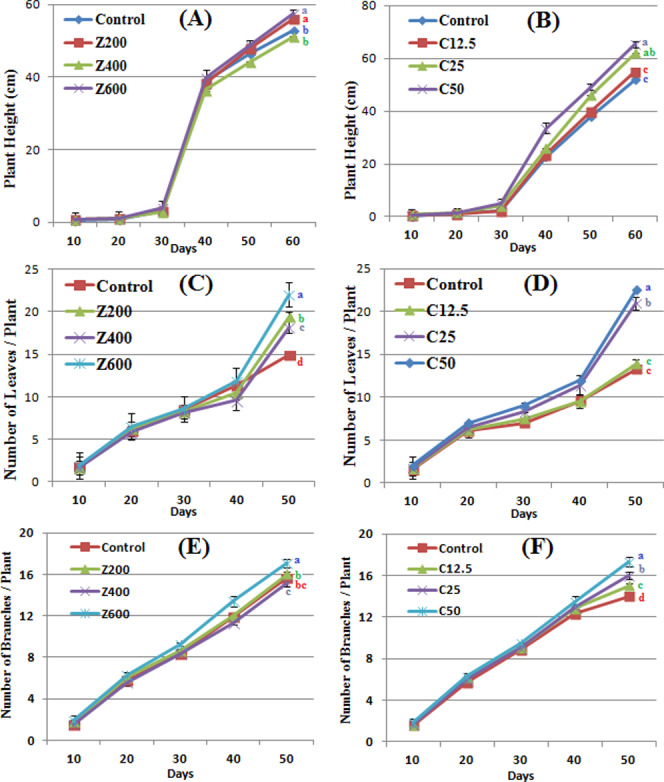
Morphological responses of B. nigra upon exposure to ZnO and CuO nanoparticles. Error bars represent the SE (n = 6), and similar letters mean no statistical difference between treatments in Tukey’s test (p ≤ 0.05). (A, B) Plant height; (C, D) number of leaves/plant; and (E, F) number of branches/plant in the presence of ZnO and CuO NPs, respectively.
ZnO and CuO NPs in the soil also affected the number of nodes per plant. At 600 mg/kg ZnO NPs, 17.07 average number of nodes per plant were counted that was approximately 7% increase from control and 13% increase from 400 mg/kg ZnO (Figure 4C). In the presence of CuO NPs, significant difference was observed at 25 and 50 mg/kg where 10 and 19% increase in the number of nodes, respectively, was observed as compared with control. These results show that the presence of ZnO and CuO NPs in the soil produced a dose-dependent growth-promoting effect on stem height and number of leaves and branches. However, at 400 mg/kg ZnO NPs, the parameters were less variable as compared with other concentrations. An increase in the above-mentioned growth parameters might be due to the nutritional behavior of dissociated ions from nanoparticles, which upregulated growth hormones. It has also been reported that Brassica is a model plant that uptakes and stores high concentration of metals including Zn and Cu. However, this accumulation has a negative effect on plant biomass and growth.36,37
In the present study, the positive effect might be due to application of Zn and Cu as NPs. Plants uptake NPs or the metals that are released from NPs. Findings by Prasad et al.37,38 and Hafeez et al.15 also showed the growth-promoting effect of CuO and ZnO NPs under soil conditions, while Zhao et al. determined that the length of shoots in the cucumber plant was not affected by ZnO NPs at concentrations of 400 and 800 mg/kg in the soil.39 Zinc is among the essential metals required for proper metabolism. Zn also functions as a cofactor for many enzymes as compared with copper and therefore is required at a higher concentration.1 Another possible explanation might be the use of pot experiments, where small volumes of soil influence plant development to a higher extent than field conditions. Furthermore, from the elements immobilized on the root surface, few are effectively absorbed by the roots. Even from the absorbed metals, little trasnlocate from roots to shoots due to the defense barrier.40,41 This has also been proven in later experiments where higher concentrations of metals were detected in roots as compared to shoots.
A significant variation in the number of leaves per plant was observed in the presence of both CuO and ZnO NPs (Figure 3C,D). The number of leaves increased from 22 to 47.6% in the presence of ZnO NPs (200–600 mg/kg) as compared with the control. In control plants and in plants grown at 12.5 mg/kg CuO NPs, there was no significant difference. Augmentation of CuO NPs in soil (25 and 50 mg/kg) also resulted in an increase of the number of leaves. An almost same trend was observed in the case of the number of branches per plant where a gradual increase was observed by addition of NPs. However, in the presence of CuO NPs, more numbers of branches were observed (Figure 3F). A total 17.3 branches per plant were observed at 50 mg/kg CuO as compared with control (14 branches per plant). A significant variation in leaf area was also observed due to ZnO NP application. At 200 mg/kg ZnO NPs, the basal leaf area increased 83%, while it was less when ZnO concentration was increased, however significantly different from the control (Figure 4A). Among apical leaves, control plants had greater leaf area. Application of CuO NPs increased the leaf area at higher concentrations (25 and 50 mg/kg) (Figure 4B). At 50 mg/kg CuO NPs 232, 105, and 84% increase was observed in apical, middle, and basal leaf areas, respectively, as compared with the control. However, at 12.5 mg/kg, nonsignificant difference was observed, whereas at 25 mg/kg, middle leaf area increased significantly. The number of leaves and leaf area are vital indicators of photosynthesis due to the existence of chlorophyll. Increases in leaf area and leaf count indicate a high rate of photosynthesis, and hence, increase in plant growth rate becomes evident. In this study, the concentration-dependent increase shows that CuO NPs have a positive impact on the leaf area, which resulted in an increase of the rate of photosynthesis (result not shown). Similar findings by Hafeez et al. showed a positive effect of soil application of CuO NPs on leaf area.15 The random trend in the leaf area of ZnO-NP-treated plants might be due to variation in accumulation of this NP in the plants. A study by Kisan et al. reported a concentration-dependent increase in the leaf area due to ZnO NP application.41 Therefore, the antagonistic action of ZnO and CuO NPs resulted in an increase in the leaf count of B. nigra. According to another study on Pistia stratiotes, Cu and Zn NP application resulted in an increase in the number of leaves per plant.42 In this study, the concentration-dependent increase shows that CuO NPs have a positive impact on growth and leaf count, while ZnO NPs have a phytotoxic effect on leaf area at higher concentrations.
Roots are the first target to confront higher concentration of NPs; hence, noxious symptoms are more likely to appear in roots as compared to stems and leaves.43 Although the phytotoxicity mechanism is not clear, but it is postulated that NPs can enter in seeds, which results in alteration of seed chemistry.44 The NPs might also adhere on emerging root plumule, inhibiting the growth. Application of ZnO and CuO NPs brought about a significant dose-dependent decrease in primary root length (Figure 4E,F). A steady decrease in the root length was observed at increasing concentration of ZnO NPs. At 600 mg/kg ZnO NPs, 15.32 cm root length was observed (44% decrease than control). CuO NPs at applied concentrations had a less toxic effect than ZnO. An average decrease of 30–39% in the root length was observed at 12.5–50 mg/kg CuO NPs. The results show that ZnO NPs have a toxic effect on secondary roots of B. nigra plants where a decrease was observed by increasing the NP concentration in the soil. ZnO NPs also decreased the number of secondary roots of B. nigra plants with maximum 44.3% decrease at 600 mg/kg soil as compared with the control. On the contrary, application of CuO NPs increased the number of secondary roots, and this increase was more when CuO NP concentration was increased. Similar results have been reported by Hafeez et al.,15 Boonyanitipong et al.,43 and Zafar et al.9 showing a growth-promoting impact of CuO NPs and phytotoxic effect of ZnO NPs on root elongation and lateral roots.
NPs affect plant growth starting from seed germination. They reduce the length and biomass of the plants and alter different biochemical processes. Many other studies also described the effect of CuO on different plants.45−48 It has been proven that toxicological and growth-stimulatory effects depend upon the characteristics of NPs used, the growth medium, and the plant type. However, NPs are found to be more toxic than ions and microparticles.3,49
3.3. Effect of CuO and ZnO NP Treatment on the Yield of B. nigra
Exposure of CuO and ZnO NPs did not impose any effect on the average life cycle of B. nigra. In this study, the number of days from plantation to seed harvesting were ∼95 without any variation in treated and untreated plants. Similarly, no effect of NPs on the average life cycle was found in a study on tomato.50
CuO NPs brought a significant linear promotive effect on the average number of flowers and pods, seed weight, and seed diameter as compared with control (Figure 5A–F). The control plants bore 162 flowers at day 60, while 168, 178, and 198 flowers were counted on plants grown in the presence of 12.5, 25, and 50 mg/kg CuO NPs in the soil. All of the flowers converted into pods and bore seeds. However, a significant decrease in the above parameters was recorded when plants were grown in the presence of ZnO NPs. At 400 mg/kg ZnO NPs, maximum decreases of 41 and 40% in the number of flowers and pods, respectively, were recorded (Figure 5A) as compared with control.
Figure 5.
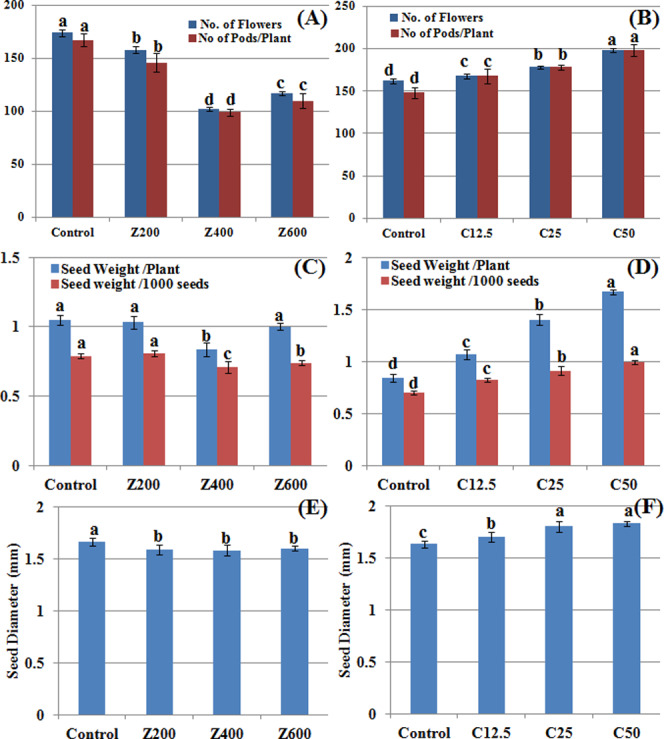
Reproductive and yield responses of B. nigra upon exposure to ZnO and CuO nanoparticles. Error bars represent the SE (n = 6), and similar letters mean no statistical difference between treatments in Tukey’s test (p ≤ 0.05). 5 (A) and (B) Number of flowers and pods per plant; (C) and (D) seed weight per plant and seed weight/1000 seeds; and (E) and (F) seed diameter in the presence of ZnO and CuO NPs, respectively.
It was observed that a higher zinc concentration in the soil also caused falling or withering of flowers before pod formation. Application of CuO and ZnO NPs in the soil also changed seed weight per plant, weight per 1000 seeds (Figure 5C,D), and seed diameter (Figure 5E,F). A decrease in all of these parameters was observed while applying ZnO NPs. The seed yield decreased up to 0.83 g per plant as compared to control (1.04 g per plant). However, CuO NPs increased all of these parameters with a gradual increase in concentration. The seed yield doubled (1.67 g/plant) at 50 mg/kg CuO NPs (Figure 5D). The weight of 1000 seeds also increased up to 41% at 50 mg/kg CuO NPs as compared with control. The increase in seed diameter was also observed to develop dose dependently in plants grown in the presence of CuO NPs (Figure 5F). Pods play developmental role in encapsulating seeds and protect them from biotic and abiotic stress. Their count is an important parameter for quantification of yield.51−53 Seed diameter and seed weight are vital physical indicators of seed quality, which influence the yield. Sowing affects nutritive quality of subsequent plants, which is an important agronomic aspect.54 The promising effect of CuO NPs on the yield of wheat is reported by Hafeez et al.,15 while a positive impact of ZnO NPs at low concentration and an inhibitory effect at higher concentrations are reported in a study on spinach.50 This might be due to the detrimental effect of ZnO NP stress during flowering at a higher concentration, which results in abnormal gynoecia and causes flower abortion; reduction in seed count, seed weight, and pod number; and ultimately low yield of black mustard.
According to smartGRAIN software image analysis, >70% increase in seed area was found at 50 mg/kg CuO NPs and 600 mg/kg ZnO NPs as compared to control (Table 2). This increase was significant dose dependently for CuO NPs. However, 200 mg/kg ZnO NP treatment was not significantly different from control. A similar trend was observed in seed perimeter length. However, the length-to-width ratio was nonsignificantly different in the case of the ZnO NPs, while a significant difference in the length-to-width ratio was observed in the case of CuO NP treatment. The circularity index indicates sphericity of seeds. Results show that the seed circularity index was mostly nonsignificant except for the control set in CuO NPs (Table 2).
Table 2. Analysis of the Effect of CuO and ZnO NPs on Seed Physical Parameters Using SmartGRAIN Softwarea.
| NPs | conc. (mg/kg) | area size (m2) | perimeter length (mm) | length (mm) | width (mm) | length-to-width ratio | circularity index |
|---|---|---|---|---|---|---|---|
| ZnO | control | 98.3 ± 2.7b | 39.3 ± 1.3b | 15.0 ± 0.4b | 9.1 ± 0.3b | 1.7 ± 0.2a | 0.8a |
| Z200 | 98.3 ± 2.4b | 39.3 ± 1.2b | 15.0 ± 0.3b | 9.1 ± 0.4b | 1.7 ± 0.1a | 0.8a | |
| Z400 | 94.3 ± 1.4c | 38.3 ± 1.7b | 14.5 ± 0.2c | 8.9 ± 0.2b | 1.7 ± 0.3a | 0.81a | |
| Z600 | 171.2 ± 3.1a | 51.9 ± 1.8a | 19.3 ± 1.0a | 12.3 ± 0.8a | 1.6 ± 0.4a | 0.79a | |
| CuO | Control | 100.3 ± 1.3d | 40.1 ± 0.9c | 15.2 ± 0.3c | 9.1 ± 0.6c | 1.6 ± 0.1a | 0.79b |
| C12.5 | 116.5 ± 1.7c | 42.4 ± 1.0b | 15.7 ± 0.3b | 10.0 ± 0.3bc | 1.6 ± 0.2a | 0.82ab | |
| C25 | 120.5 ± 1.4b | 42.3 ± 1.1b | 15.3 ± 0.4c | 10.6 ± 0.5b | 1.4 ± 0.1b | 0.84a | |
| C50 | 174.3 ± 2.0a | 51.1 ± 1.5a | 18.1 ± 0.7a | 13.1 ± 0.4a | 1.4 ± 0.1b | 0.84a |
The results are presented as mean (n = 3). The similar letters within the column mean no statistical difference between treatments in Turkey’s test (p ≤ 0.05).
3.4. Seed Macronutrient Profiling
Protein and total oil content are the most valuable nutrient contents of B. nigra seeds. Results of relative protein and total oil content in seeds are shown in Table 3. It was observed that protein contents increased in the seeds grown in the NP-contaminated soil, while total oil contents decreased as compared with control. Zinc is closely involved in nitrogen metabolism and protein synthesis, so availability of Zn increases the protein content in plants, fruits and seeds.55,56 It has also been reported that application of Zn improves the quality of fruits by influencing sugar and acid contents.57,58 A significant decrease in oleic acid was evident in CuO and ZnO NP-treated seeds. Less significant difference was observed in linoleic acid in NP-treated seeds as compared with control. Erucic acid significantly increased by ∼12–18% as compared with control. A significant, ∼17–29%, increase in glucosinolates was also observed in NP-treated seeds (Table 3). An almost same trend was observed in the presence of CuO NPs, where the protein content and erucic acid significantly increased as compared with control while the total oil content, oleic acid and linolenic acid, decreased. A significant increase in glucosinolates was also observed due to amendment of soil by CuO NPs (Table 3). The low availability of Zn in soil results in poor oil contents in mustard.59 Contrary to these results, a study on mustard exposed to NaCl stress showed reduction in protein content.60 Another study on the protein content of spinach leaves treated with ZnO NPs showed a significant increase at higher concentrations in comparison with control.39
Table 3. Effect of ZnO and CuO NPs on B. nigra Seed Analysisa.
| NPs | conc. (mg/kg) | oil content (%) | protein content (%) | glucosinolates (μmol/g) | moisture (%) | oleic acid 18:01 (%) | linolenic acid 18:03 (%) | erucic acid 22:01 (%) |
|---|---|---|---|---|---|---|---|---|
| ZnO | control | 34.8 ± 2.9a | 26.5 ± 0.6c | 105.7 ± 2.1c | 7.0 ± 0.02a | 22.4 ± 2.3a | 14.5 ± 0.4a | 22.9 ± 2.1c |
| Z200 | 32.2 ± 2.1ab | 30.6 ± 0.9ab | 137.6 ± 2.4a | 6.2 ± 0.01c | 13.8 ± 1.4bc | 14.1 ± 0.3ab | 38.0 ± 3.1a | |
| Z400 | 32.9 ± 1.8ab | 29.3 ± 0.7b | 126.9 ± 1.9b | 7.0 ± 0.01a | 14.5 ± 1.9b | 14.4 ± 0.3a | 36.8 ± 2.4b | |
| Z600 | 30.5 ± 1.4b | 31.7 ± 1.3a | 100.7 ± 2.0d | 6.7 ± 0.03b | 12.6 ± 2.0c | 13.8 ± 0.2b | 39.2 ± 2.8a | |
| CuO | control | 34.7 ± 2.1a | 28.0 ± 0.4c | 106.8 ± 1.5d | 6.9 ± 0.04a | 22.8 ± 2.1a | 14.3 ± 0.4a | 23.1 ± 1.8c |
| C12.5 | 30.7 ± 1.7b | 31.2 ± 0.8b | 124.9 ± 2.3b | 6.7 ± 0.03b | 14.3 ± 2.0b | 13.9 ± 0.4ab | 38.1 ± 2.3ab | |
| C25 | 27.2 ± 1.6c | 33.8 ± 1.4a | 135.3 ± 1.8a | 6.8 ± 0.02ab | 12.8 ± 1.4c | 13.3 ± 0.3b | 40.4 ± 1.7a | |
| C50 | 31.4 ± 2.0ab | 30.5 ± 1.2b | 121.9 ± 2.1c | 6.7 ± 0.03b | 15.1 ± 1.8b | 13.9 ± 0.3ab | 35.9 ± 2.2b |
The results are presented as mean (n = 3). The similar letters within the column mean no statistical difference between treatments in Turkey’s test (p ≤ 0.05).
It is important to note that in this study the average protein content found in treated seeds is slightly higher (∼3–6%) than that reported by Danlami et al.53 Nutritional properties of seed oil depend on the fatty acid composition, mainly the quantity of oleic, linoleic, and erucic acids, which in turn has great importance in human nutrition.61,62 Among them, erucic acid is the most vital fatty acid present in the genus Brassica.55 It has been reported that there is positive correlation of the oil content with oleic and linoleic acid and negative correlation with erucic acid.63 Findings of this study agree with the above-mentioned reports and show that a decrease in the total oil content is positively correlated with oleic and linoleic acids and a significant increase in erucic acid confirms its negative correlation with the total oil content. It is also reported that these principle fatty acids, especially erucic acid, are valuable for industrial applications, but at a higher level (54%), they are noxious to human health.61 However, the quantity of erucic acid used in this study is not likely to impose toxic effects and can be further exploited in several industrial applications. Glucosinolates are natural mustard oil glycosides that act as secondary metabolites and are involved in stress tolerance.64 They are also called sinigrin, which release volatile aggressive allyl isothiocyanate, responsible for pungent taste of B. nigra.65 These findings agree with results of a study supporting the significant promoting effect of CuO NPs on glucosinolate accumulation in Brassica rapa. The metals, i.e., Cu, that have a metal chelating effect due to variation in the valance number have different affinity with organic molecules. Therefore, they form tetrahedral complexes with ligands; form stable metalloenzymes, and interact with enzyme systems.66 Jahangir et al. also reported accumulation of different metabolites (amino acids, phenolics, and glucosinolates) in B. rapa plants under metal toxicity.66 Zinc is also a key regulator for auxin and lipid biosynthesis. The essential fatty acids i.e., 18:7, 22:1 are the first target for these regulations.66−70
3.5. Elemental Analysis of Cu and Zn Contents in Soil and Plants
Results obtained from an atomic absorption spectrophotometer have shown accumulation of Cu and Zn in B. nigra. The soil analysis before sowing of seeds (after addition of NPs) and after harvesting of plants shows that the concentration of Cu and Zn decreased in the soil (Table 4). In the control experiment, 30 and 33% decrease in Zn and Cu concentration, respectively, was observed. The Zn content decreased from 219.6 mg/kg (before plantation) to 153.5 mg/kg (after harvesting) in control pots. While Cu decreased up to 14.8 from 21.5 mg/kg. The addition of NPs resulted in an increase of available ions, which resulted in accumulation of metals in plant parts. The results also show that a decrease of Cu and Zn in soil was approximately consistent at all concentrations applied. A total of 42% decrease in Zn concentration was observed at 200 mg/kg ZnO NP application while 49% at 600 mg/kg ZnO NPs. An almost same trend, decrease in Cu in soil before and after, was observed in the case of CuO NP application.
Table 4. Analysis of Zn and Cu in Soil before (after Addition of NPs) and after the Experimentsa.
| NPs | conc. (mg/kg) | before | after |
|---|---|---|---|
| Zinc Analysis | |||
| ZnO | control | 219.6 ± 9.8d | 153.5 ± 8.3d |
| Z200 | 364.1 ± 10.9c | 208.9 ± 11.5c | |
| Z400 | 438.3 ± 14.4b | 261.0 ± 10.8b | |
| Z600 | 972.8 ± 15.0a | 495.3 ± 13.2a | |
| Copper Analysis | |||
| CuO | control | 21.5 ± 3.7d | 14.8 ± 2.9d |
| C12.5 | 89.2 ± 6.1c | 42.5 ± 7.2c | |
| C25 | 108.4 ± 7.5b | 74.2 ± 7.9b | |
| C50 | 176.2 ± 7.1a | 91.3 ± 4.6a | |
The results are presented as mean (n = 3). The similar letters within the column mean no statistical difference between treatments in Turkey’s test (p ≤ 0.05).
Results show that incorporation of Cu and Zn nanoparticles in the soil increased the accumulation of Cu and Zn in all parts of plants (Table 5). Roots were the major part of plant to accumulate metals. It was observed that Cu accumulated in roots at a higher concentration as compared with controls. The analysis of leaves showed that basal leaves accumulated more metal as compared with middle and apical leaves. However, in the case of 200 mg/kg ZnO NPs, zinc accumulation was higher in middle leaves while copper accumulation was less in middle leaves in plants grown in the presence of 12.5 mg/kg CuO NPs. The presence of excessive amounts of metals in the soil affects their uptake and even that of other elements, but no clear trend can be established. The uptake of metals by roots from the soil and translocation to shoots are based on bioavailability, interaction with the roots through root surface organic molecules, and translocation efficiency. Therefore, most of the metal concentration is quantified in roots followed by stem and leaves. Although depending upon the plant characteristics based on the storage of metals in the tissues, metal concentrations may vary in roots, stem, and leaves.71
Table 5. Effect of ZnO and CuO NPs on Accumulation of Zn and Cu in B. nigraa.
| |
leaves |
|||||
|---|---|---|---|---|---|---|
| treatment (mg/kg) | roots | stem | apical | middle | basal | |
| Accumulation of Zn | ||||||
| ZnO NPs | control | 106.2 ± 8.7Ad | 86.1 ± 6.5Bd | 81.5 ± 6.9BCd | 52.9 ± 3.6Cd | 46.3 ± 2.7Dd |
| Z200 | 189.1 ± 9.8Ac | 178.6 ± 10.1Bc | 108.3 ± 5.7Cc | 95.2 ± 4.1Dc | 69.5 ± 3.8Ec | |
| Z400 | 354.5 ± 10.9Ab | 208.3 ± 10.8Bb | 162.1 ± 9.6Cb | 134.6 ± 7.7Db | 127.2 ± 5.9DEb | |
| Z600 | 573.9 ± 14.5Aa | 361.5 ± 12.4Ba | 308.5 ± 7.2Ca | 184.0 ± 6.8Ea | 214.3 ± 6.1Da | |
| Accumulation of Cu | ||||||
| CuO NPs | control | 7.4 ± 2.3Dd | 11.7 ± 0.8Cd | 21.4 ± 2.5Ac | 17.3 ± 1.7Bb | 18.2 ± 2.5ABc |
| C12.5 | 19.1 ± 5.4Cc | 24.6 ± 3.2Bc | 28.0 ± 4.9ABb | 18.5 ± 2.1Cb | 31.4 ± 3.5Ab | |
| C25 | 35.8 ± 8.4Bb | 42.8 ± 3.9Ab | 30.6 ± 3.4Cab | 26.1 ± 3.8Da | 39.4 ± 4.1ABab | |
| C50 | 41.6 ± 7.3ABa | 49.1 ± 4.3Aa | 34.8 ± 2.7Ba | 25.4 ± 5.1Ca | 43.2 ± 3.8ABa | |
The results are presented as mean (n = 3). The similar capital letters within the row and small letters within the column mean no statistical difference between treatments in Turkey’s test (p ≤ 0.05).
However, excessive amount of metal in the soil may facilitate other metals to be taken up by plants and also may hinder absorbance; hence, no specified reason can be drawn.72,73 Zn is a structural component of several enzymes and thus affects the metabolism, pollen structure, and yield.3,49 Therefore, the influence on pod development, seed number, and weight was observed.74,75 Similarly, Cu at less than threshold level stimulates plant growth and also influences the uptake of other nutrients and metal ions from the soil.4,76,77
3.6. Elemental Analysis of Trace and Major Elements Accumulation in Yield
Accumulation of trace and major elements, i.e., Zn, Cu, Ni, Co, Ca, Mg, Cd, etc., was evaluated in seeds. Data shows that the treatments of metallic nanoparticles significantly influenced the accumulation of respective metals in seeds (Table 6). Approximately 10 times higher accumulation of zinc was observed in ZnO NPs (600 mg/kg)-treated plants, while in the case of 50 mg/kg CuO NP treatment, 6.8 μg/mg copper was observed as compared with 0.26 μg/mg in the seeds of control. It was observed that nanoparticle stress also significantly influenced accumulation of Ca in seeds while it was nonsignificant for Mg. Although Ni was not detected in treated and untreated plant seeds, traces of cobalt were observed in treated plant seeds that were not detected in control plants. Copper and Zn have similar ionic size; the presence of Cu in the soil can therefore have some negative effect on Zn as both elements compete for the same adsorption sites showing antagonistic behavior.75,78 Metal accumulation in plants depends on the concentration of metals in soil, soil physicochemical characteristics, and uptake mechanism. Furthermore, seeds are storage organs that have low transpiration rate. Loaded with phloem, they accumulate less metals as compared with other plant organs.79 This might be the reason for the less accumulation of metals in the seeds of the plants, though leafy vegetables have greater potential of accumulating heavy metals in their edible parts.69 Accumulation of metals also affects lipid synthesis and composition by metal inactivation of physiologically essential thiol-containing enzymes and cofactors.80−82 This is in accordance with our results, where, in the presence of CuO and ZnO NPs, low protein and oil contents and high metal accumulation are observed in seeds of B. nigra.
Table 6. Effect of ZnO and CuO NPs on Accumulation of Metals in B. nigra Seedsa.
| treatment (mg/kg) | Zn | Cu | Ni | Mg | Co | Cd | Ca | |
|---|---|---|---|---|---|---|---|---|
| ZnO NPs | control | 2.3 ± 0.8d | 0.29 ± 0.04a | ND | 111.1 ± 2.9a | ND | ND | 221.6 ± 6.2d |
| Z200 | 10.7 ± 1.5c | 0.28 ± 0.02a | ND | 110.1 ± 3.4a | 0.17 ± 0.02b | ND | 262.3 ± 3.8b | |
| Z400 | 18.3 ± 1.1b | 0.17 ± 0.02b | ND | 107.1 ± 2.8b | 0.2 ± 0.02a | ND | 234.5 ± 5.1c | |
| Z600 | 21.4 ± 1.3a | 0.21 ± 0.01c | ND | 111.1 ± 3.1a | 0.17 ± 0.01b | ND | 273.9 ± 5.7a | |
| CuO NPs | control | 2.2 ± 0.1bc | 0.3 ± 0.03d | ND | 110.5 ± 2.5a | ND | ND | 119.8 ± 4.2d |
| C12.5 | 1.9 ± 0.2c | 3.2 ± 0.7c | ND | 110.1 ± 2.8a | 0.02 ± 0.01b | ND | 231.6 ± 5.6c | |
| C25 | 2.6 ± 0.2a | 5.5 ± 0.6b | ND | 112.1 ± 2.1a | 0.02 ± 0.01b | 0.06 | 278.6 ± 4.9a | |
| C50 | 2.2 ± 0.1b | 6.8 ± 0.4a | ND | 111.9 ± 3.0a | 0.06 ± 0.02a | ND | 256.6 ± 5.3b | |
The results are presented as mean (n = 3). The similar letters within the column mean no statistical difference between treatments in Turkey’s test (p ≤ 0.05). (ND = not detected).
The decrease in Cu accumulation might be attributed to immobilization of Cu by humic substances in the soil.65 Some metals are important cofactors for drought resistance enzymes and promote nitrogen fixation in legumes, and their increased accumulation results in a significant increase in protein production.83 The role of Cu in nitrogen fixation can be correlated with the increase in protein content (Table 4).
In this study, seed calcium content was positively affected by NP exposure and resulted in a significant increase in its accumulation in all treatments, which is indicative of healthy seeds. In the case of CuO NP treatment, the Ca level increased up to ∼4–20%, while in ZnO NPs, accumulation was ∼5–19% higher than in control. These results are supported by studies on beans and rice. It has been reported that Ca and Mg enter into the root system through cation-permeable channels.17,65
4. Conclusions
This study was intended to determine the effect of chemically synthesized CuO and ZnO NPs on the growth morphology, yield, and nutrient composition of B. nigra. Results of the study indicate that application of CuO NPs up to 50 mg/kg soil has a positive impact on the growth of B. nigra. However, application of ZnO NPs (200–600 mg/kg soil) has noxious effect on the growth of the plant. Plant height and the number of branches, leaves, and secondary roots were significantly higher in the presence of CuO NPs, while primary root length decreased due to both CUO and ZnO NPs. Flower, pod, and seed characteristics were also better in the presence of CuO NPs. Plant elemental analysis showed high accumulation of Cu and Zn in plant parts as compared with control. Application of NPs also resulted in a higher accumulation of Ca, Co, Cu, and Zn in seeds. It is worth mentioning that the protein content increased along with glucosinolates and erucic acid in all treated seeds, which makes them significant for industrial applications. Hence, diverse behaviors of CuO and ZnO NPs under soil conditions open a new door for further investigation to clarify the mechanisms of action at hormonal and molecular levels. Furthermore, in agriculture fields, these NPs will also increase seed yield and nutritional content. However, this needs further investigation.
Author Contributions
All authors contributed equally to the experiment design, performance, and writing of the manuscript.
The authors are thankful to the Higher Education Commission Pakistan for partial funding to H.Z. under Indigenous Ph.D. Fellowship program.
The authors declare no competing financial interest.
References
- Ali A.; Phull A. R.; Zia M. Elemental zinc to zinc nanoparticles: Is ZnO NPs crucial for life? Synthesis, toxicological and environmental concerns. Nanotechnol. Rev. 2018, 7, 413–442. 10.1515/ntrev-2018-0067. [DOI] [Google Scholar]
- Yang J.; Cao W.; Rui Y. Interactions between nanoparticles and plants: phytotoxicity and defense mechanisms. J. Plant Interact. 2017, 12, 158–169. 10.1080/17429145.2017.1310944. [DOI] [Google Scholar]
- Shaw A. K.; Hossain Z. Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere 2013, 93, 906–915. 10.1016/j.chemosphere.2013.05.044. [DOI] [PubMed] [Google Scholar]
- Nair P. M. G.; Chung I. M. A mechanistic study on the toxic effect of copper oxide nanoparticles in soybean (Glycine max L.) root development and lignification of root cells. Biol. Trace Elem. Res. 2014, 162, 342–352. 10.1007/s12011-014-0106-5. [DOI] [PubMed] [Google Scholar]
- Mahmoodzadeh H.; Aghili R.; Nabavi M. Physiological effects of TiO2 nanoparticles on wheat (Triticum aestivum). J. Appl. Sci. Eng. Technol. 2013, 3, 1365–1370. [Google Scholar]
- Dimkpa C. O.; McLean J. E.; Latta D. E.; Manangón E.; Britt D. W.; Johnson W. P.; Boyanov M. I.; Anderson A. J. CuO and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanopart. Res. 2012, 14, 1125–1130. 10.1007/s11051-012-1125-9. [DOI] [Google Scholar]
- Hänsch R.; Mendel R. R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. 10.1016/j.pbi.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Naz S.; Gul A.; Zia M. On the Toxicity Copper Oxide Nanoparticles: A Review Study. IET Nanobiotechnol. 2020, 14, 1–13. 10.1049/iet-nbt.2019.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar H.; Ali A.; Zia M. CuO nanoparticles inhibited root growth from Brassica nigra seedlings but induced root from stem and leaf explants. Appl. Biochem. Biotechnol. 2017, 181, 365–378. 10.1007/s12010-016-2217-2. [DOI] [PubMed] [Google Scholar]
- Zafar H.; Ali A.; Ali J. S.; Haq I. U.; Zia M. Effect of ZnO Nanoparticles on Brassica nigra Seedlings and Stem Explants: Growth Dynamics and Antioxidative Response. Front. Plant Sci. 2016, 7, 535. 10.3389/fpls.2016.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Liu H.; Zhang Y.; Xin H. The effect of CuO NPs on reactive oxygen species and cell cycle gene expression in roots of rice. Environ. Toxicol. Chem. 2015, 34, 554–561. 10.1002/etc.2826. [DOI] [PubMed] [Google Scholar]
- Van Ginneken L.; Meers E.; Guisson R.; Ruttens A.; Elst K.; Tack F. M. G.; Vangronsveld J.; Diels L.; Dejonghe W. Phytoremediation for heavy metal-contaminated soils combined with bioenergy production. J. Environ. Eng. Landscape Manage. 2007, 15, 227–236. 10.3846/16486897.2007.9636935. [DOI] [Google Scholar]
- Angelova V.; Ivanov K. Bioaccumulation and distribution of heavy metals in black mustard (Brassica nigra Koch). Environ. Monit. Assess. 2009, 153, 449–459. 10.1007/s10661-008-0370-y. [DOI] [PubMed] [Google Scholar]
- Ali A.; Phull A. R.; Zia M.; Shah A. M. A.; Malik R. N.; ul-Haq I. Phytotoxicity of River Chenab sediments: in vitro morphological and biochemical response of Brassica napus L. Environ. Nanotechnol. Monit. Manage. 2015, 4, 74–84. 10.1016/j.enmm.2015.09.003. [DOI] [Google Scholar]
- Hafeez A.; Razzaq A.; Mahmood T.; Jhanzab H. M. Potential of copper nanoparticles to increase growth and yield of wheat. J. Nanosci. Adv. Technol. 2015, 1, 6–11. 10.24218/jnat.2015.02. [DOI] [Google Scholar]
- Rawat S.; Pullagurala V. L. R.; Hernandez-Molina M.; Sun Y.; Niu G.; Hernandez-Viezcas J. A.; Peralta-Videa J. R.; Gardea-Torresdey J. L. Impacts of copper oxide nanoparticles on bell pepper (Capsicum annum L.) plants: a full life cycle study. Environ. Sci.: Nano 2018, 5, 83–95. 10.1039/C7EN00697G. [DOI] [Google Scholar]
- Medina-Velo I. A.; Dominguez O. E.; Ochoa L.; Barrios A. C.; Hernández-Viezcas J. A.; White J. C.; Peralta-Videa J. R.; Gardea-Torresdey J. L. Nutritional quality of bean seeds harvested from plants grown in different soils amended with coated and uncoated zinc oxide nanomaterials. Environ. Sci.: Nano 2017, 4, 2336–2347. 10.1039/C7EN00495H. [DOI] [Google Scholar]
- Ali A.; Ambreen S.; Javed R.; Tabassum S.; ul Haq I.; Zia M. ZnO nanostructure fabrication in different solvents transforms physio-chemical, biological and photodegradable properties. Mater. Sci. Eng., C 2017, 74, 137–145. 10.1016/j.msec.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Javed R.; Ahmed M.; ul-Haq I.; Nisa S.; Zia M. PVP and PEG doped CuO nanoparticles are more biologically active: antibacterial, antioxidant, antidiabetic and cytotoxic perspective. Mater. Sci. Eng., C 2017, 79, 108–115. 10.1016/j.msec.2017.05.006. [DOI] [PubMed] [Google Scholar]
- AOAC . Methods of Soil Analysis; 12th ed.; Association of Official Analytical Chemists: Washington DC, 2005. [Google Scholar]
- Zafar H.; Abbasi B. H.; Zia M. Physiological and antioxidative response of Brassica nigra (L.) to ZnO nanoparticles grown in culture media and soil. Toxicol. Environ. Chem. 2019, 101, 281–299. 10.1080/02772248.2019.1691555. [DOI] [Google Scholar]
- Zafar H.; Haq I. U.; Nasreen S.; Zia M. Toxicological effect of cuo nanoparticles to brassica nigra l. Seedlings: a comparative in vivo and in vitro response. Pak. J. Bot. 2019, 51, 427–434. 10.30848/PJB2019-2(15). [DOI] [Google Scholar]
- Tanabata T.; Shibaya T.; Hori K.; Ebana K.; Yano M. SmartGrain: high-throughput phenotyping software for measuring seed shape through image analysis. Plant Physiol. 2012, 160, 1871–1880. 10.1104/pp.112.205120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association of Official Analytical Chemists (AOAC) Official methods of analysis of the Association of Official Analytical Chemists (Vol. 1). The Association, 1990.
- Smith D. B.; Parson D. G.; Starr C. A simple and rapid method of qualitatively measuring the glucosinolate concentration of rapeseed. J. Agric. Sci. 1985, 597–603. 10.1017/S0021859600059505. [DOI] [Google Scholar]
- Madsen E. Nuclear magnetic resonance spectrometry as a quick method of determination of oil content in rapeseed. J. Am. Oil Chem. Soc. 1976, 53, 467–469. 10.1007/BF02636813. [DOI] [Google Scholar]
- Javed R.; Mohamed A.; Yücesan B.; Gürel E.; Kausar R.; Zia M. CuO nanoparticles significantly influence in vitro culture, steviol glycosides, and antioxidant activities of Stevia rebaudiana Bertoni. Plant Cell, Tissue Organ Cult. 2017, 131, 611–620. 10.1007/s11240-017-1312-6. [DOI] [Google Scholar]
- Shamhari N. M.; Wee B. S.; Chin S. F.; Kok K. Y. Synthesis and characterization of zinc oxide nanoparticles with small particle size distribution. Acta Chim. Slov. 2018, 65, 578–585. 10.17344/acsi.2018.4213. [DOI] [PubMed] [Google Scholar]
- Adhikari T.; Kundu S.; Biswas A. K.; Tarafdar J. C.; Rao A. S. Effect of copper oxide nano particle on seed germination of selected crops. J. Agric. Sci. Technol. 2012, 2, 815–820. [Google Scholar]
- Lee S.; Chung H.; Kim S.; Lee I. The genotoxic effect of ZnO and CuO nanoparticles on early growth of buckwheat, Fagopyrum esculentum. Water, Air, Soil Pollut. 2013, 224, 1668 10.1007/s11270-013-1668-0. [DOI] [Google Scholar]
- Veselova T. V.; Veselovskii V. A.; Usmanov P. D.; Usmanova O. V. Hypoxia and imbibition injuries to aging seeds. Russ. J. Plant Physiol. 2003, 50, 835–842. 10.1023/B:RUPP.0000003283.24523.82. [DOI] [Google Scholar]
- Bandyopadhyay S.; Plascencia-Villa G.; Mukherjee A.; Rico C. M.; José-Yacamán M.; Peralta-Videa J. R.; Gardea-Torresdey J. L. Comparative phytotoxicity of ZnO NPs, bulk ZnO, and ionic zinc onto the alfalfa plants symbiotically associated with Sinorhizobium meliloti in soil. Sci. Total Environ. 2015, 515–516, 60–69. 10.1016/j.scitotenv.2015.02.014. [DOI] [PubMed] [Google Scholar]
- Motior M. R.; Abdou A. S.; Fareed H. A. I. D.; Sofian A. M. Responses of sulfur, nitrogen and irrigation water to the uptake of nutrients by Zea mays grown in sandy calcareous soil. Aust. J. Crop Sci. 2011, 5, 347–357. [Google Scholar]
- McBride M. B.Environmental Chemistry of Soils; Oxford University Press: New York, 1994. [Google Scholar]
- Zaurov D. E.; Perdomo P.; Raskin I. Optimizing soil fertility and pH to maximize cadmium removed by Indian mustard from contaminated soils. J. Plant Nutr. 1999, 22, 977–986. 10.1080/01904169909365687. [DOI] [Google Scholar]
- Ebbs S. D.; Kochian L. V. Toxicity of zinc and copper to Brassica species: implications for phytoremediation. J. Environ. Qual. 1997, 26, 776–781. 10.2134/jeq1997.00472425002600030026x. [DOI] [Google Scholar]
- Prasad T. N. V. K. V.; Sudhakar P.; Sreenivasulu Y.; Latha P.; Munaswamy V.; Reddy K. R.; Sreeprasad T. S.; Sajanlal P. R.; Pradeep T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–992. 10.1080/01904167.2012.663443. [DOI] [Google Scholar]
- Prasad K. V. S. K.; Saradhi P. P.; Sharmila P. Concerted action of antioxidant enzymes and curtailed growth under zinc toxicity in Brassica juncea. Environ. Exp. Bot. 1999, 42, 1–10. 10.1016/S0098-8472(99)00013-1. [DOI] [Google Scholar]
- Zhao L.; Sun Y.; Hernandez-Viezcas J. A.; Servin A. D.; Hong J.; Niu G.; Peralta-Videa J. R.; Duarte-Gardea M.; Gardea-Torresdey J. L. Influence of CeO2 and ZnO nanoparticles -on cucumber physiological markers and bioaccumulation of Ce and Zn: A life cycle study. J. Agric. Food Chem. 2013, 61, 11945–11951. 10.1021/jf404328e. [DOI] [PubMed] [Google Scholar]
- Lasat M. M. Phytoextration of metals from contaminated soil: a review of plant/soil/metal interaction and assessment of pertinent agronomic issues. J. Hazard. Subst. Res. 1999, 2, 5. 10.4148/1090-7025.1015. [DOI] [Google Scholar]
- Kisan B.; Shruthi H.; Sharanagouda H.; Revanappa S. B.; Pramod N. K. Effect of nano-zinc oxide on the leaf physical and nutritional quality of spinach. Agrotechnology 2015, 5, 135. [Google Scholar]
- Olkhovych O.; Volkogon M.; Taran N.; Batsmanova L.; Kravchenko I. The effect of copper and zinc nanoparticles on the growth parameters, contents of ascorbic acid, and qualitative composition of amino acids and acylcarnitines in Pistia stratiotes L. (Araceae). Nanoscale Res. Lett. 2016, 11, 218 10.1186/s11671-016-1422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyanitipong P.; Kositsup B.; Kumar P.; Baruah S.; Dutta J. Toxicity of ZnO and TiO2 nanoparticles on germinating rice seed Oryza sativa L. Int. J. Biosci., Biochem. Bioinf. 2011, 1, 282. 10.7763/IJBBB.2011.V1.53. [DOI] [Google Scholar]
- Ochoa L.; Medina-Velo I. A.; Barrios A. C.; Bonilla-Bird N. J.; Hernandez-Viezcas J. A.; Peralta-Videa J. R.; Gardea- Torresdey J. L. Modulation of CuO nanoparticles toxicity to green pea (Pisum sativum Fabaceae) by the phytohormone indole-3-acetic acid. Sci. Total Environ. 2017, 598, 513–524. 10.1016/j.scitotenv.2017.04.063. [DOI] [PubMed] [Google Scholar]
- Apodaca S. A.; Tan W.; Dominguez O. E.; Hernandez-Viezcas J. A.; Peralta-Videa J. R.; Gardea-Torresdey J. L. Physiological and biochemical effects of nanoparticulate copper, bulk copper, copper chloride, and kinetin in kidney bean (Phaseolus vulgaris) plants. Sci. Total Environ. 2017, 599–600, 2085–2094. 10.1016/j.scitotenv.2017.05.095. [DOI] [PubMed] [Google Scholar]
- Tamez C.; Hernandez-Molina M.; Hernandez-Viezcas J. A.; Gardea-Torresdey J. L. Uptake, transport, and effects of nano-copper exposure in zucchini (Cucurbita pepo). Sci. Total Environ. 2019, 665, 100–106. 10.1016/j.scitotenv.2019.02.029. [DOI] [PubMed] [Google Scholar]
- Bonilla-Bird N. J.; Paez A.; Reyes A.; Hernandez-Viezcas J. A.; Li C.; Peralta-Videa J. R.; Gardea-Torresdey J. L. Two-photon microscopy and spectroscopy studies to determine the mechanism of copper oxide nanoparticle uptake by sweetpotato roots during postharvest treatment. Environ. Sci. Technol. 2018, 52, 9954–9963. 10.1021/acs.est.8b02794. [DOI] [PubMed] [Google Scholar]
- Rajput V. D.; Minkina T.; Suskova S.; Mandzhieva S.; Tsitsuashvili V.; Chapligin V.; Fedorenko A. Effects of Copper Nanoparticles (CuO NPs) on Crop Plants: a Mini Review. BioNanoSci. 2018, 8, 36–42. 10.1007/s12668-017-0466-3. [DOI] [Google Scholar]
- Zuverza-Mena N.; Medina-Velo I. A.; Barrios A. C.; Tan W.; Peralta-Videa J. R.; Gardea-Torresdey J. L. Copper nanoparticles/compounds impact agronomic and physiological parameters in cilantro (Coriandrum sativum). Environ. Sci.: Processes Impacts 2015, 17, 1783–1793. 10.1039/C5EM00329F. [DOI] [PubMed] [Google Scholar]
- Raliya R.; Nair R.; Chavalmane S.; Wang W. N.; Biswas P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 2015, 7, 1584–1594. 10.1039/C5MT00168D. [DOI] [PubMed] [Google Scholar]
- Muhammad N.; Cheema M. A.; Wahid M. A.; Ahmad N.; Zaman M. Effect of source and method of nitrogen fertilizer application on seed yield and quality of canola (Brassica napus L.). Pak. J. Agric. Sci. 2007, 44, 74–78. [Google Scholar]
- Bennett E. J.; Roberts J. A.; Wagstaff C. The role of the pod in seed development: strategies for manipulating yield. New Phytol. 2011, 190, 838–853. 10.1111/j.1469-8137.2011.03714.x. [DOI] [PubMed] [Google Scholar]
- Danlami U.; Orishadipe Abayomi T.; Lawal D. R. Phytochemical, nutritional and antimicrobial evaluations of the aqueous extract of Brassica nigra (Brassicaceae) Seeds. Am. J. Appl. Chem. 2016, 4, 161. 10.11648/j.ajac.20160404.17. [DOI] [Google Scholar]
- Zhao L.; Peralta-Videa J. R.; Ren M.; Varela-Ramirez A.; Li C.; Hernandez-Viezcas J. A.; Aguilera R. J.; Gardea-Torresdey J. L. Transport of Zn in a sandy loam soil treated with ZnO NPs and uptake by corn plants: Electron microprobe and confocal microscopy studies. Chem. Eng. J. 2012, 184, 1–8. 10.1016/j.cej.2012.01.041. [DOI] [Google Scholar]
- Nan C. W.; Clarke D. R. Effect of variations in grain size and grain boundary barrier heights on the current-voltage characteristics of ZnO varistors. J. Am. Ceram. Soc. 1996, 79, 3185–3192. 10.1111/j.1151-2916.1996.tb08094.x. [DOI] [Google Scholar]
- Chatterjee C.; Khurana N. Zinc Stress–Induced Changes in Biochemical Parameters and Oil Content of Mustard. Commun. Soil Sci. Plant Anal. 2007, 38, 751–761. 10.1080/00103620701220718. [DOI] [Google Scholar]
- Pullagurala V. L. R.; Adisa I. O.; Rawat S.; Kim B.; Barrios A. C.; Medina-Velo I. A.; Hernandez-Viezcas J. A.; Peralta-Videa J. R.; Gardea-Torresdey J. L. Finding the conditions for the beneficial use of ZnO nanoparticles towards plants-A review. Environ. Pollut. 2018, 241, 1175–1181. 10.1016/j.envpol.2018.06.036. [DOI] [PubMed] [Google Scholar]
- Olama V.; Ronaghi A.; Karimian N.; Yasrebi J.; Hamidi R.; Tavajjoh M.; Kazemi M. R. Seed quality and micronutrient contents and translocations in rapeseed (Brassica napus L.) as affected by nitrogen and zinc fertilizers. Arch. Agron. Soil Sci. 2014, 60, 423–435. 10.1080/03650340.2013.796588. [DOI] [Google Scholar]
- Sharafi Y.; Majidi M. M.; Goli S. A. H.; Rashidi F. Oil content and fatty acids composition in Brassica species. Int. J. Food Prop. 2015, 18, 2145–2154. 10.1080/10942912.2014.968284. [DOI] [Google Scholar]
- Riahi-Madvar A.; Aminizadeh M.; Mohammadi M. Nano-Metal oxides induced sulforaphane production and peroxidase activity in seedlings of Lepidium draba (Brassicaceae). Prog. Biol. Sci. 2016, 6, 75–83. 10.22059/pbs.2016.59010. [DOI] [Google Scholar]
- Nieschlag H. J.; Wolff I. A. Industrial uses of high erucic oils. J. Am. Oil Chem. Soc. 1971, 48, 723–727. 10.1007/BF02638529. [DOI] [Google Scholar]
- Variyar P. S.; Banerjee A.; Akkarakaran J. J.; Suprasanna P.. Role of glucosinolates in plant stress tolerance. In Emerging Technologies and Management of Crop Stress Tolerance; Academic Press, 2014; pp 271–291. [Google Scholar]
- Chung I.; Rekha K.; Venkidasamy B.; Thiruvengadam M. Effect of copper oxide nanoparticles on the physiology, bioactive molecules, and transcriptional changes in Brassica rapa ssp. Rapa seedlings. Water, Air, Soil Pollut. 2019, 230, 48 10.1007/s11270-019-4084-2. [DOI] [Google Scholar]
- Barth C. A. Nutritional value of rapeseed oil and its high oleic/low linolenic variety–A call for differentiation. Eur. J. lipid Sci. Technol. 2009, 111, 953–956. 10.1002/ejlt.200900019. [DOI] [Google Scholar]
- Fox T. C.; Guerinot M. L. Molecular Biology of Cation Transport in Plants. Annu. Rev. Plant Biol. 1998, 49, 669–696. 10.1146/annurev.arplant.49.1.669. [DOI] [PubMed] [Google Scholar]
- Jahangir M.; Abdel-Farid I. B.; Choi Y. H.; Verpoorte R. Metal ion-inducing metabolite accumulation in Brassica rapa. J. Plant Physiol. 2008, 165, 1429–1437. 10.1016/j.jplph.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Vankova R.; Landa P.; Podlipna R.; Dobrev P. I.; Prerostova S.; Langhansova L.; Gaudinova A.; Motkova K.; Knirsch V.; Vanek T. ZnO nanoparticle effects on hormonal pools in Arabidopsis thaliana. Sci. Total Environ. 2017, 593–594, 535–542. 10.1016/j.scitotenv.2017.03.160. [DOI] [PubMed] [Google Scholar]
- Malfatti H.; Vallee J. C.; Perdrizet E.; Carre M.; Martin C. Acides-aminés et amines libres? explants foliaires de Nicotiana tabacum cultivés in vitro sur des milieux induisant la rhizogenèse ou la caulogenèse. Physiol. Plant. 1983, 57, 492–498. 10.1111/j.1399-3054.1983.tb02774.x. [DOI] [Google Scholar]
- Munshi S. K.; Vats S.; Dhillon K. S.; Sukhija P. S. Lipid biosynthesis in seeds of mustard (Brassica juncea) influenced by zinc and sulphur deficiency. Physiol. Plant. 1990, 80, 102–108. 10.1111/j.1399-3054.1990.tb04381.x. [DOI] [Google Scholar]
- Ebbs S. D.; Kochian L. V. Toxicity of zinc and copper to brassica species: Implications for phytoremediation. J. Environ. Qual. 1997, 26, 776–781. 10.2134/jeq1997.00472425002600030026x. [DOI] [Google Scholar]
- Weis J. S.; Weis P. Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Environ. int. 2004, 30, 685–700. 10.1016/j.envint.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kaur R.; Bhardwaj R.; Thukral A. K.; Narang U. Interactive effects of binary combinations of manganese with other heavy metals on metal uptake and antioxidative enzymes in Brassica juncea L. Seedlings. J. Plant Interact. 2011, 6, 25–34. 10.1080/17429145.2010.516407. [DOI] [Google Scholar]
- Wierzbicka M.; Obidzinska J. The uptake of lead on seed imbibition and germination in different plant species. Plant Sci. 1998, 137, 155–171. 10.1016/S0168-9452(98)00138-1. [DOI] [Google Scholar]
- Azeez M. O.; Adesanwo O. O.; Adepetu J. A. Effect of Copper (Cu) application on soil available nutrients and uptake. Afr. J. Agric. Res. 2015, 10, 359–364. 10.5897/AJAR2014.9010. [DOI] [Google Scholar]
- Lidon F. C.; Henriques F. S. Effects of copper toxicity on growth and the uptake and translocation of metals in rice plants. J. Plant Nutr. 1993, 16, 1449–1464. 10.1080/01904169309364626. [DOI] [Google Scholar]
- Karlsson H. L.; Gustafsson J.; Cronholm P.; Möller L. Size-dependent toxicity of metal oxide particles—a comparison between nano- and micrometer size. Toxicol. Lett. 2009, 188, 112–118. 10.1016/j.toxlet.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Podar D.; Ramsey M. H.; Hutchings M. J. Effect of cadmium, zinc and substrate heterogeneity on yield, shoot metal concentration and metal uptake by Brassica juncea: Implications for human health risk assessment and phytoremediation. New Phytol. 2004, 163, 313–324. 10.1111/j.1469-8137.2004.01122.x. [DOI] [PubMed] [Google Scholar]
- Gupta A. K.; Sinha S. Antioxidant response in sesame plants grown on industrially contaminated soil: effect on oil yield and tolerance to lipid peroxidation. Bioresour. Technol. 2009, 100, 179–185. 10.1016/j.biortech.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Sinha S.; Gupta A. K.; Bhatt K.; Pandey K.; Rai U. N.; Singh K. P. Distribution of metals in the edible plants grown at Jajmau, Kanpur (India) receiving treated tannery wastewater: relation with physico-chemical properties of the soil. Environ. Monit. Assess. 2006, 115, 1–22. 10.1007/s10661-006-5036-z. [DOI] [PubMed] [Google Scholar]
- Jones G. L.; Nichols P. D.; Johns R. B.; Smith J. D. The effect of mercury and cadmium on the fatty acid and sterol composition of the marine diatom Asterionella glacialis. Phytochemistry 1987, 26, 1343–1348. 10.1016/S0031-9422(00)81809-9. [DOI] [Google Scholar]
- Van Assche F.; Clijsters H. Inhibition of photosynthesis in Phaseolus vulgaris by treatment with toxic concentration zinc: effects on ribulose-1,5-bis phoshphate carboxylase oxygenase. J. Plant Physiol. 1986, 125, 355–360. 10.1016/S0176-1617(86)80157-2. [DOI] [Google Scholar]
- Alia Prasad K. V. S. K.; Saradhi P. Effect of zinc on free radical and proline in Brassica juncea and Cajanus cajan. Phytochemistry 1995, 39, 45–47. 10.1016/0031-9422(94)00919-K. [DOI] [Google Scholar]
- Palit S.; Sharma A.; Talukder G. Effects of cobalt on plants. Bot. Rev. 1994, 60, 149–181. 10.1007/BF02856575. [DOI] [Google Scholar]


