Abstract
Articular cartilage injury is a common source of knee pain and dysfunction. Patients in whom conservative treatment fails may benefit from surgical intervention to restore function and alleviate pain. Autologous cartilage procedures are a viable treatment modality for cartilage repair, providing comparable outcomes to osteochondral allografts while leaving the subchondral bone intact. This article discusses the senior author's method of cartilage restoration using BioCartilage (Arthrex, Naples, FL), platelet-rich plasma, and autologous cartilage collected using a designated collection device sealed with activated autologous serum.
Chondral defects are a common cause of knee pain and, if left untreated or undertreated, may progress in size and thickness.1,2 Articular cartilage displays poor healing after injury because of a scarcity of cells in lacunae, poor vascular supply, unique biochemistry, and inevitable mechanical wear.3 Conservative management of symptomatic cartilage defects may include activity modification, weight loss, physical therapy, and/or injections.4 Surgical management of chondral defects is often indicated when nonoperative therapy fails to improve pain and function. Surgical treatment of symptomatic cartilage lesions may be broadly classified into traditional marrow stimulation techniques that induce the formation of fibrocartilage and more recent augmented techniques that aim to restore hyaline cartilage. Traditional techniques include debridement and/or microfracture, which is indicated if the lesion cannot be treated by debridement alone and has minimal subchondral bone involvement.5 However, the disadvantage of this technique is that the resultant fibrocartilage is biomechanically inferior to hyaline cartilage and postoperative outcomes accordingly deteriorate over time.6,7
There are several cartilage repair procedures aimed to restore structural hyaline cartilage to the articular defect, including autologous chondrocyte implantation (ACI), matrix-associated autologous chondrocyte implantation (MACI), osteochondral autograft, and osteochondral allograft (OAG). MACI is a 2-step procedure aiming to create a new hyaline cartilage surface by harvesting, culturing, and reimplanting autologous articular cartilage.8 OAG transplantation is an intervention indicated for large, full-thickness cartilage lesions that have penetrated the subchondral bone. Of all procedures described, OAG and MACI provide the best long-term patient outcomes.9,10
When used appropriately, microfracture can lead to successful outcomes.11 Advancements such as the use of drilling rather than an awl have shown decreased fracture response, although long-term outcomes of microfracture are variable.5,12 BioCartilage (Arthrex, Naples, FL) presents a viable augmentation to microfracture as a single-stage cartilage restoration technique. BioCartilage is a micronized, dehydrated cartilage allograft used as a scaffold for autologous cartilage restoration.13 Studies in animal models have shown chondrogenesis and upregulation of type II collagen when BioCartilage is used in conjunction with microfracture.14,15 In addition, platelet-rich plasma (PRP) can be used to harness anti-catabolic and pro-anabolic growth factors, creating a pro–hyaline tissue environment.16,17 When combined, these augmentations may enhance the structural integrity of the implanted cartilage with the intention of creating more reliable long-term clinical outcomes for patients after microfracture (Table 1).18
Table 1.
Advantages and Disadvantages
| Advantages | Disadvantages |
|---|---|
| Autologous cartilage implants do not cause any structural damage to the bone, and if they fail, the site will still be viable for OAG implantation.26 | |
| The described method restores hyaline cartilage in a single step while being less invasive and resource intensive in comparison to OAG, as well as less expensive than a 2-stage ACI or MACI procedure. | Outcomes after this method of cartilage restoration are less studied than those after OAG, ACI, and MACI procedures because of its recent implementation. |
| Micronized BioCartilage used with PRP and autologous cartilage acts as a scaffold and perhaps may allow for the growth of stronger, structurally sound hyaline cartilage superior to the fibrocartilage resulting from microfracture, thus creating a structurally and biochemically beneficial augmentation to the traditional microfracture procedure. | Preparation of the BioCartilage–PRP–autologous mixture and Thrombinator activated serum is more labor and resource intensive than microfracture alone. |
| The described method poses a viable option for treatment of tibial cartilage defects, which are not reliably treated by microfracture alone.24 | Outcomes after cartilage restoration of the tibia are not well defined. |
ACI, autologous chondrocyte implantation; MACI, matrix-associated autologous chondrocyte implantation; OAG, osteochondral allograft; PRP, platelet-rich plasma.
BioCartilage has traditionally been combined with PRP and sealed with fibrin glue.13,18 Adding autologous cartilage sealed with autologous activated serum may further enhance the regeneration of hyaline cartilage. Devices such as the GraftNet Autologous Tissue Collector (Arthrex) and the Thrombinator system (Arthrex) allow these innovations for the standard BioCartilage technique. This article discusses a method of cartilage restoration using microfracture augmented with a BioCartilage, PRP, and autologous cartilage mixture sealed with activated serum. This technique is indicated for tibial plateau and some femoral condyle defects (Table 1).17 We prefer to use this procedure for contained, small to medium cartilage defects in patients with a body mass index of less than 30.19, 20, 21
Positioning and Preparation
The patient is positioned supine; a tourniquet is placed on the operative knee prior to standard sterile preparation and draping and is subsequently inflated. Standard arthroscopic portals are established in the knee, and a diagnostic arthroscopy is performed (Fig 1, Video 1).
Fig 1.
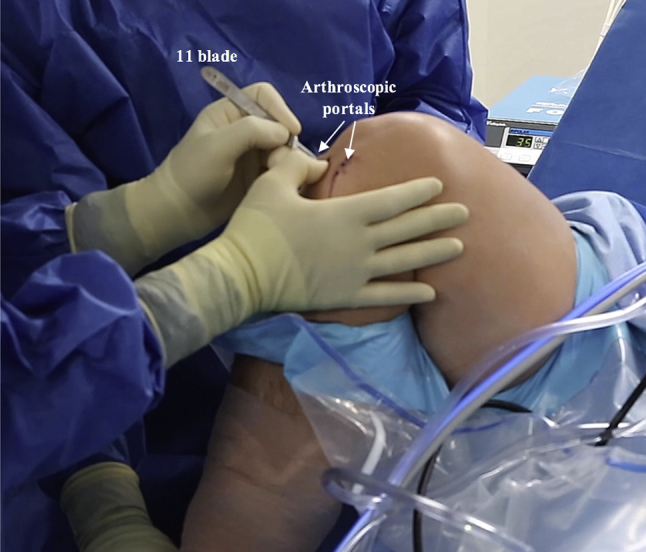
Intraoperative photograph of the right knee with the patient in the supine position. The right knee is prepared and draped in the typical fashion. The knee is flexed, and after the tourniquet has been inflated, standard inferomedial and inferolateral arthroscopic portals are established.
Surgical Technique
The defect is probed and debrided (Fig 2, Video 1). A ring curette is used to remove the calcified layer at the base of the lesion and create vertical walls on the defect periphery to promote glue adherence (Table 2, Video 1). A PowerPick (Arthrex) is used to microfracture the defect, creating holes 1.5 mm in diameter and 4 mm deep in the subcortical bone (Table 2, Fig 3, Video 1). These holes are drilled 3 to 4 mm apart and are not allowed to become confluent. A GraftNet device, connected in-line with the suction, is attached to the shaver and is used to harvest and capture autologous hyaline cartilage from the intercondylar notch and proximal aspect of the femoral condyle (Fig 4, Fig 5, Fig 6, Video 1). Roughly 72 mL of whole blood is collected from the patient; 60 mL is centrifuged in the Arthrex Angel PRP system, and the remainder is saved for use in the Thrombinator system (Fig 7, Video 1).
Fig 2.
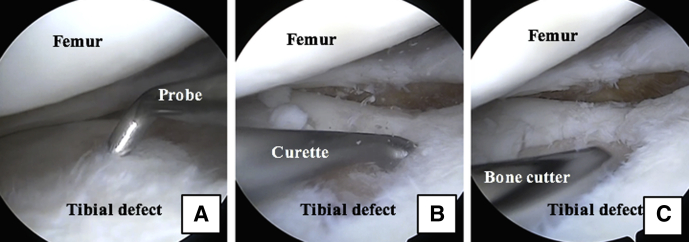
Arthroscopic photographs of the right knee with the patient in the supine position and the leg out straight. The tibial plateau defect is viewed through the inferomedial portal, and instruments are introduced through the inferolateral portal. (A) A probe is used to assess the defect. A curette (B) and bone cutter (C) are used to prepare the defect and establish vertical walls.
Table 2.
Pearls and Pitfalls
| Step | Pearl | Pitfall |
|---|---|---|
| Initial defect preparation | A ring curette should be used to establish vertical walls. | Failure to establish vertical walls may make it difficult to contain the BioCartilage mixture in the defect. |
| Microfracture | Use of a PowerPick ensures drill hole depth precision and minimizes damage to the subchondral plate. | Although use of an awl is not entirely contraindicated, it may pose an increased risk to the subchondral plate and does not control for depth.5,11 |
| Defect preparation for BioCartilage insertion | The microfractured defect should be dried with a pledget to create a dry, adherent surface for the BioCartilage mixture. | Failure to dry the defect may make it difficult to retain the BioCartilage mixture in the defect. |
| BioCartilage insertion | The defect should be filled to just under the surrounding cartilage margin. | Proud application results in a greater chance of subsequent delamination and bone exposure.23 |
| It may be necessary to switch portals or place the bed in the Trendelenburg position to mitigate the effects of gravity. | Failure to mitigate the effects of gravity may make it difficult to contain the BioCartilage mixture in the defect. | |
| Sealant insertion | The sealant should be tested before insertion to ensure proper clotting. | Failure to ensure proper clotting may result in uncontained sealant. |
| The defect should be covered without overfilling so that the construct is flush with the surrounding articular surface. | Proud application results in a greater chance of subsequent delamination and bone exposure.23 |
Fig 3.
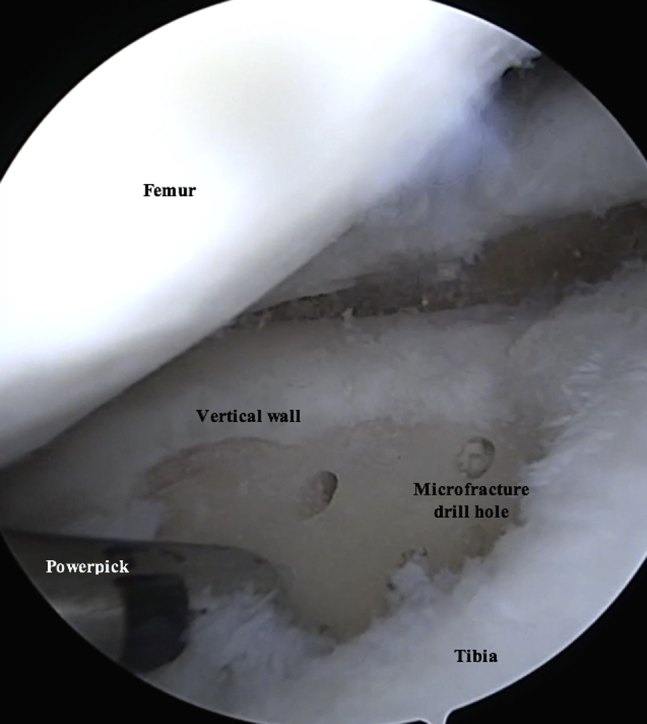
Arthroscopic photograph of the right knee with the patient in the supine position and the leg out straight. The tibial plateau defect is viewed through the inferomedial portal, and instruments are introduced through the inferolateral portal. A PowerPick is used to microfracture the prepared defect, creating holes 1.5 mm in diameter and 4 mm deep in the subcortical bone. These holes are drilled roughly 3 to 4 mm apart and are not allowed to become confluent.
Fig 4.
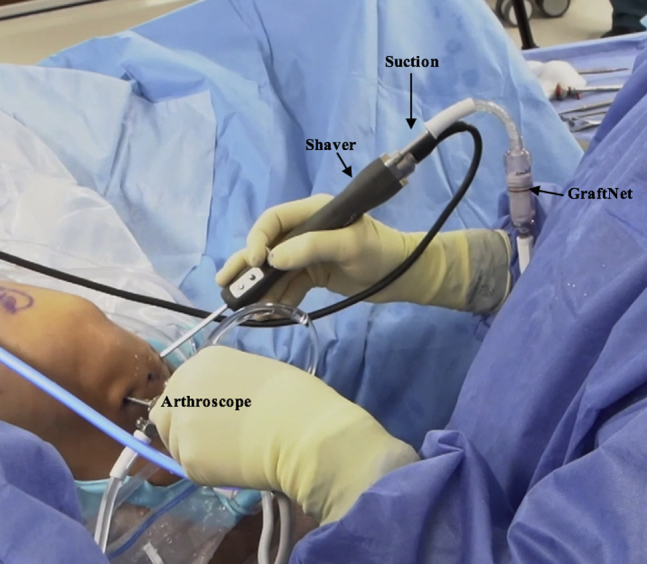
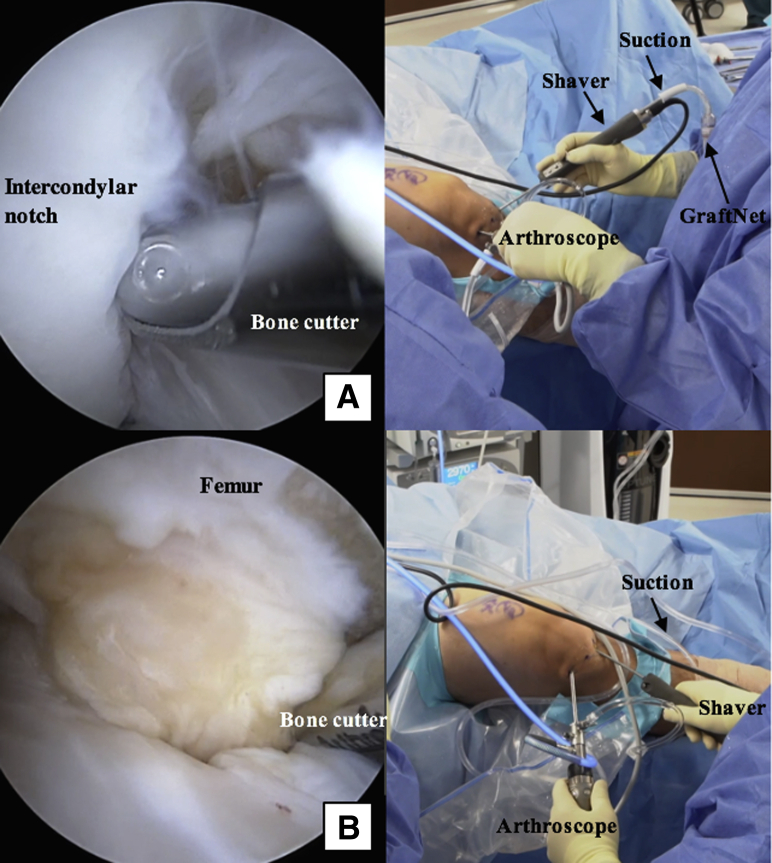
Intraoperative photograph of the right knee with the patient in the supine position. The right knee is hanging off the lateral side of the operating table in flexion. A GraftNet device is attached to the shaver in-line with the suction and introduced through the inferomedial portal, with the arthroscope viewing through the inferolateral portal.
Fig 5.
Intraoperative photographs of the right knee with the patient in the supine position. The right knee is hanging off the lateral side of the operating table in flexion. A GraftNet device is attached to the shaver in-line with the suction and introduced through the inferomedial portal, with the arthroscope viewing through the inferolateral portal. The GraftNet device is used to harvest and capture autologous hyaline cartilage from the intercondylar notch (A) and proximal aspect of the femoral condyle (B) as they are debrided with a bone cutter.
Fig 6.
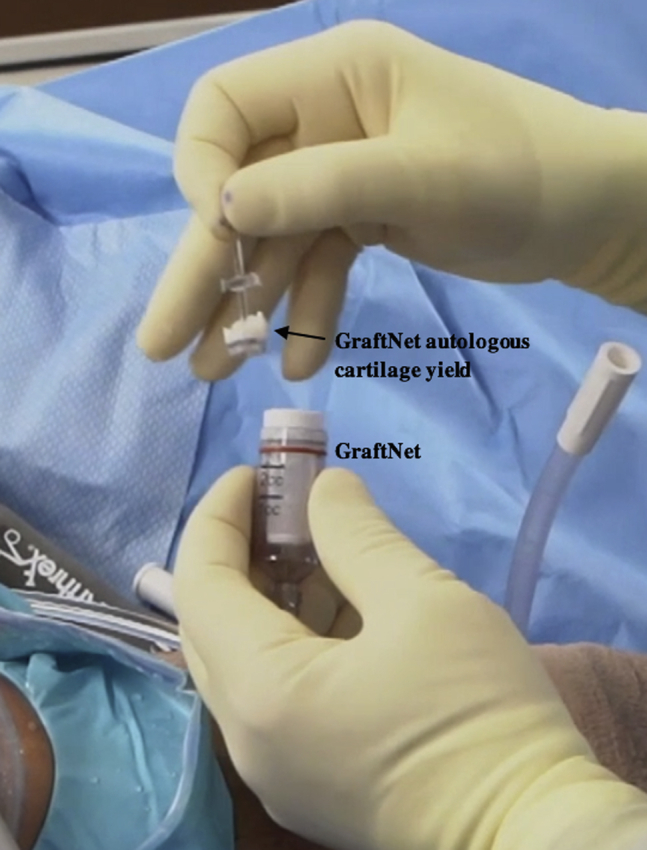
Intraoperative photograph of the right knee with the patient in the supine position and the leg out straight. The GraftNet device is detached from the shaver, and the autologous cartilage yield is saved for BioCartilage preparation.
Fig 7.
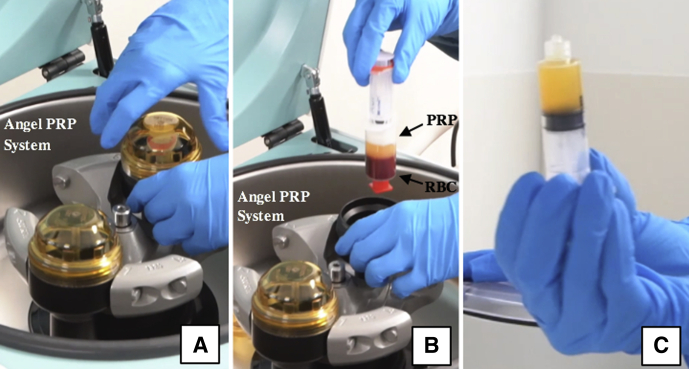
Intraoperative photograph of platelet-rich plasma preparation. Whole blood is centrifuged in the Angel platelet-rich plasma (PRP) system (A, B), yielding PRP (C). (RBC, red blood cells.)
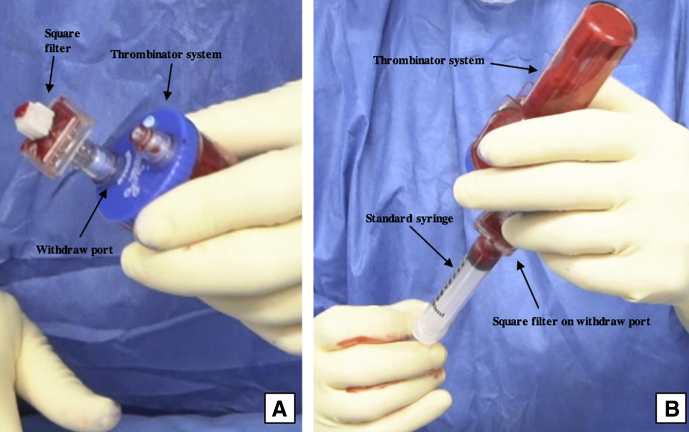
Preparation of the BioCartilage mixture and activated serum is performed on the back table. Four milliliters of whole blood is injected into the “inject” port on the Thrombinator system (Fig 8, Video 1). The system is shaken for 5 seconds to agitate the blood and initiate the clotting cascade and then set to rest horizontally for 10 minutes. During this time, equal parts (roughly 1 mL) BioCartilage, PRP, and autologous cartilage collected with the GraftNet device are combined in an Arthrex Mixing and Delivery Syringe (Fig 9, Video 1). Once the contents have been mixed, the syringe is attached to a Tuohy needle and advanced to fill the needle (Fig 10, Video 1). The syringe is removed, an obturator is attached to the back of the needle, and the BioCartilage mixture is ready to inject (Video 1). After 10 minutes have passed, the Thrombinator is shaken vigorously for 5 seconds to break up the clot, and a subsequent 8 mL of whole blood is injected through the inject portal (Fig 11). This mixture is agitated for 5 seconds and rested for 1 minute. A square, 0.8-μm filter is placed on the Thrombinator withdrawal port, and the system is agitated again for 5 seconds, yielding autologous thrombin serum (Fig 12A). The Thrombinator is inverted to withdraw the sealant through the withdrawal port and filter (Fig 12B). The thrombin serum is attached to a Y syringe containing an identical syringe of PRP (1:1), and the mixtures are advanced to equalize (Fig 13, Video 1).
Fig 8.
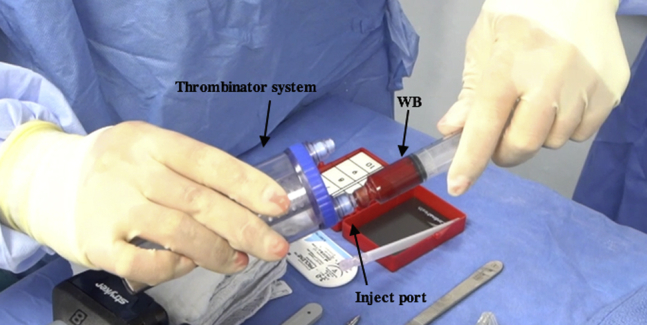
Intraoperative photograph of Thrombinator serum being prepared on the back table. Four milliliters of whole blood (WB) is injected into the “inject” port on the Thrombinator system.
Fig 9.
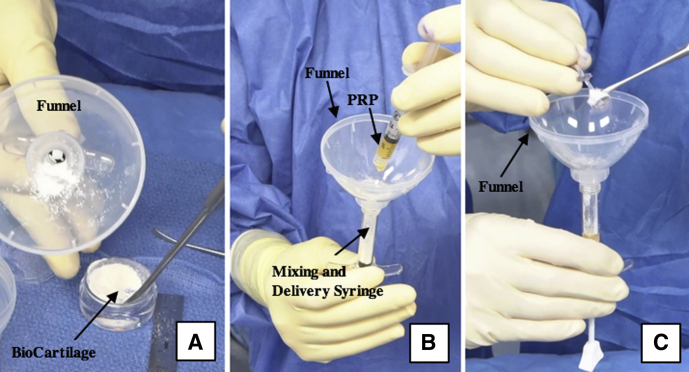
Intraoperative photograph of the BioCartilage mixture being prepared on the back table. Equal parts (roughly 1 mL each) BioCartilage (A), platelet-rich plasma (PRP) (B), and GraftNet-harvested autologous cartilage (C) are combined in an Arthrex Mixing and Delivery Syringe using a funnel.
Fig 10.
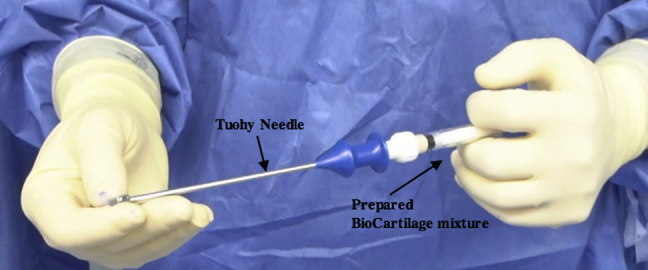
Intraoperative photograph of the BioCartilage mixture being prepared on the back table. The prepared mixing syringe is attached to a Tuohy needle and advanced to fill the needle.
Fig 11.
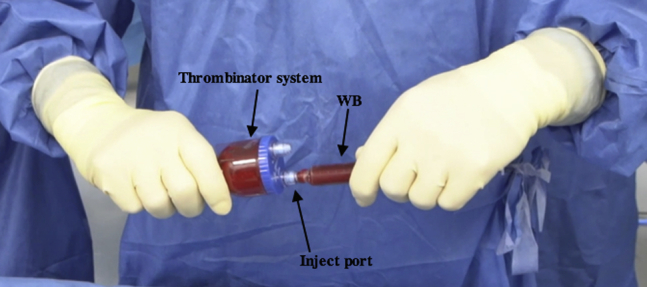
Intraoperative photograph of the Thrombinator serum being prepared on the back table. After the Thrombinator system has rested horizontally for 10 minutes, it is shaken vigorously for 5 seconds and 8 mL of whole blood (WB) is injected through the inject portal.
Fig 12.
Intraoperative photograph of the Thrombinator activated serum being prepared on the back table. A square filter is placed on the withdrawal port (A), and the Thrombinator system is inverted to withdraw the thrombin serum through the withdrawal port using a syringe (B).
Fig 13.
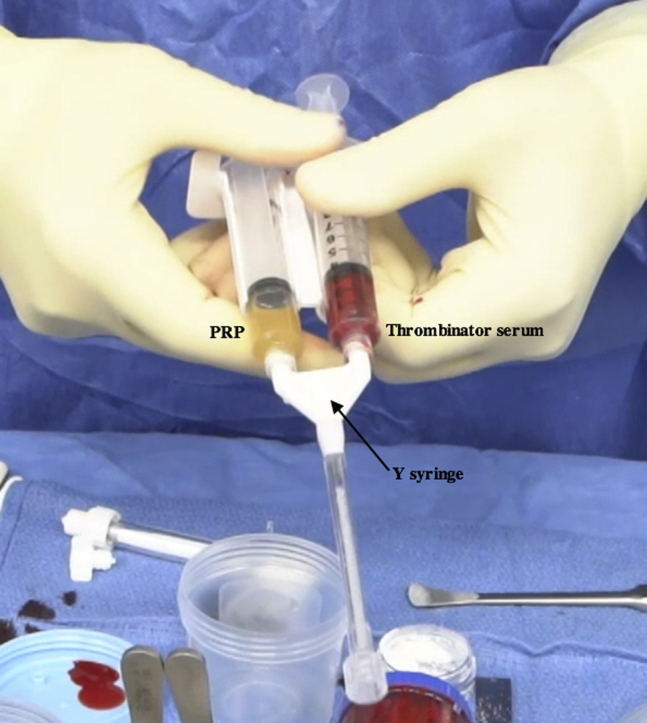
Intraoperative photograph of the Thrombinator serum and platelet-rich plasma (PRP) mixture being prepared on the back table. The syringes containing PRP and thrombin serum are attached to a Y syringe, and the mixtures are advanced to equalize.
All inflow of fluids into the knee is turned off, the joint capsule is drained, and the defect is dried with a pledget (Table 2, Fig 14, Video 1). The prepared Tuohy needle is inserted into the joint, and the BioCartilage–PRP–autologous cartilage mixture is injected into the microfractured defect and gently pressed into place, filling the defect to just under the surrounding cartilage margin (Table 2, Figs 15 and 16, Video 1). It may be necessary to switch portals to access different portions of the lesion, and placing the bed in the Trendelenburg position can help mitigate the effects of gravity (Table 2). A freer elevator can be used to smooth the mixture into place (Fig 17). A spinal needle is attached to the Y syringe containing the activated serum to facilitate injection and placement over the defect (Fig 18). The sealant should be tested before insertion to ensure proper clotting (Table 2). The spinal needle on the Y syringe is inserted into the joint capsule, and the activated serum is dripped over the defect (Fig 18, Video 1). It is critical to cover the defect without overfilling so that the construct is flush with the surrounding articular surface (Table 2, Fig 19).22,23 The sealant is allowed to dry for 20 to 30 seconds, and the knee is closed in the typical fashion.
Fig 14.
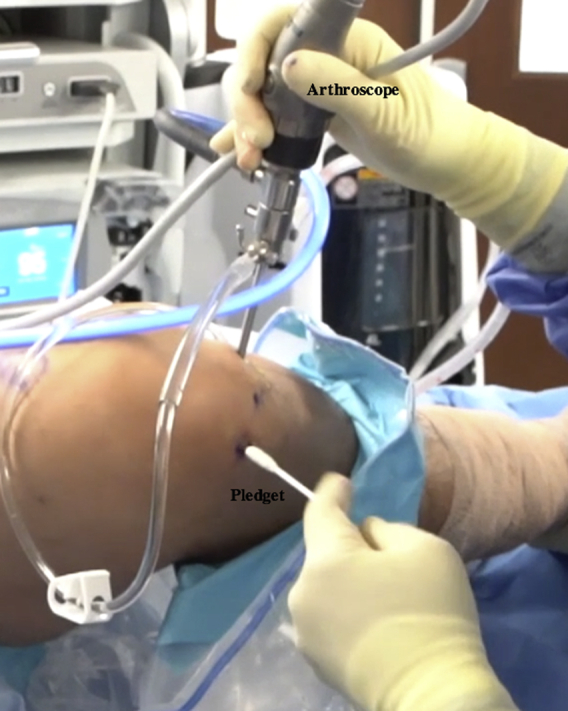
Intraoperative photograph of the right knee with the patient in the supine position. An assistant is elevating the foot roughly 6 inches while maintaining roughly 70° of flexion. Inflow into the knee is turned off, and the defect is dried using a pledget inserted through the inferolateral portal.
Fig 15.
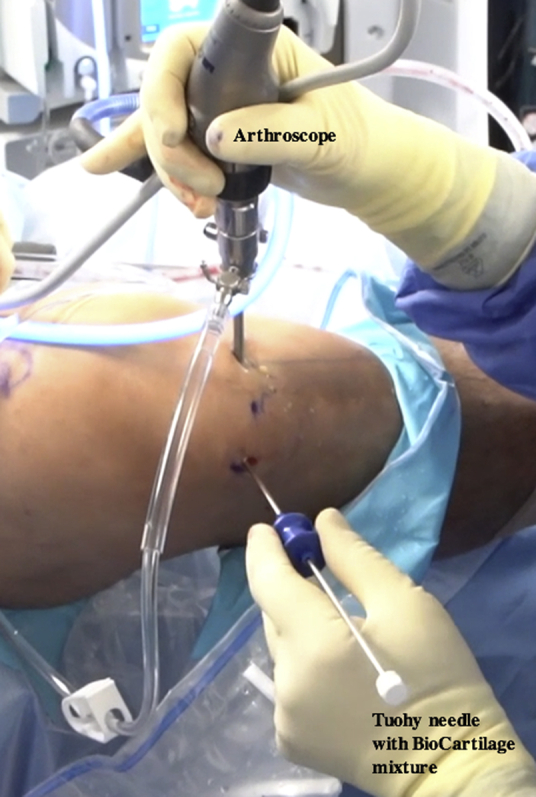
Intraoperative photograph of the right knee with the patient in the supine position. An assistant is elevating the foot roughly 6 inches while maintaining roughly 70° of flexion. The prepared Tuohy needle is inserted into the joint through the inferolateral portal while viewing through the inferomedial portal.
Fig 16.
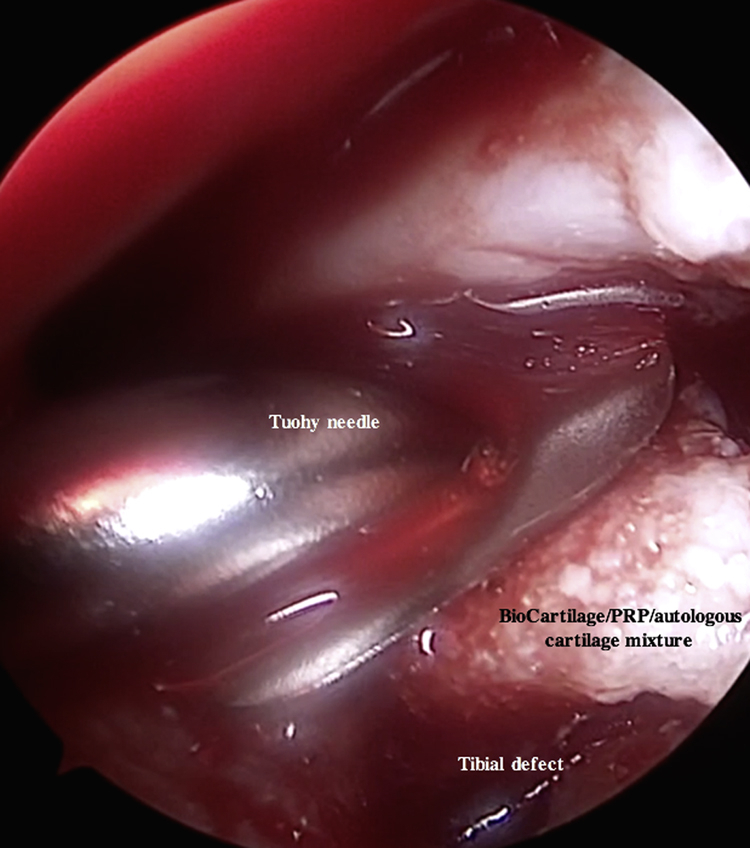
Arthroscopic photograph of the right knee with the patient in the supine position and the bed placed in the Trendelenburg position. An assistant is elevating the foot roughly 6 inches while maintaining roughly 70° of flexion. The tibial plateau defect is viewed through the inferomedial portal, and instruments are introduced through the inferolateral portal. The BioCartilage–platelet-rich plasma (PRP)–autologous cartilage mixture is injected into the microfractured defect using a Tuohy needle inserted through the inferolateral portal.
Fig 17.
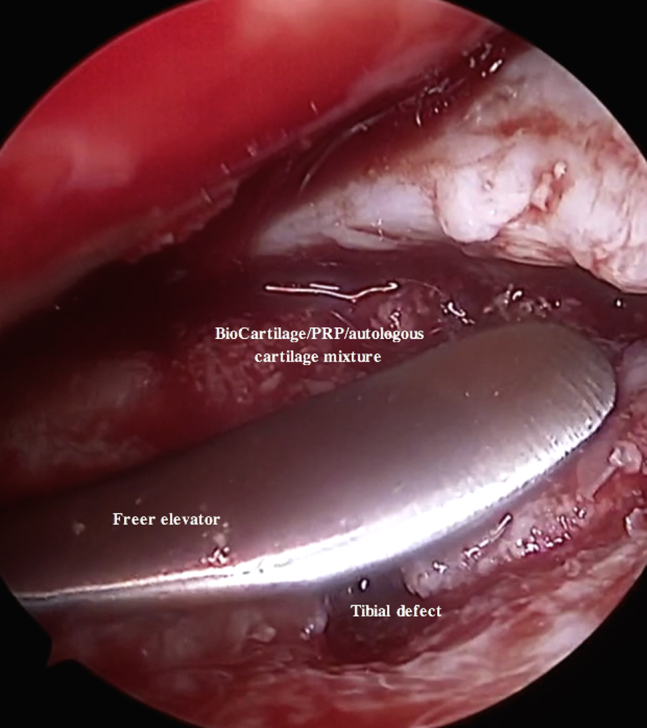
Arthroscopic photograph of the right knee with the patient in the supine position and the bed placed in the Trendelenburg position. An assistant is elevating the foot roughly 6 inches while maintaining roughly 70° of flexion. The tibial plateau defect is viewed through the inferomedial portal, and instruments are introduced through the inferolateral portal. The BioCartilage–platelet-rich plasma (PRP)–autologous cartilage mixture is gently pressed into place using a freer elevator inserted through the inferolateral portal, filling the defect to just under the surrounding cartilage margin.
Fig 18.
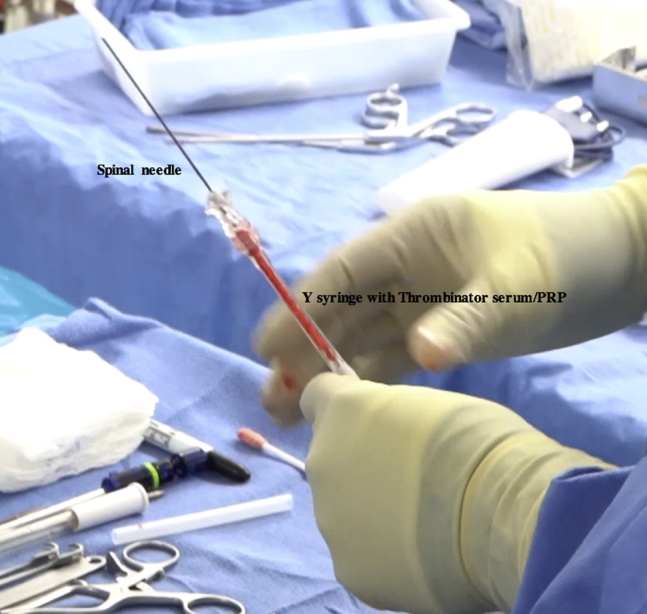
Intraoperative photograph of the prepared Thrombinator serum–platelet-rich plasma (PRP) insertion device on the back table. A spinal needle is attached to the Y syringe containing the Thrombinator serum and PRP to facilitate injection and placement over the defect.
Fig 19.
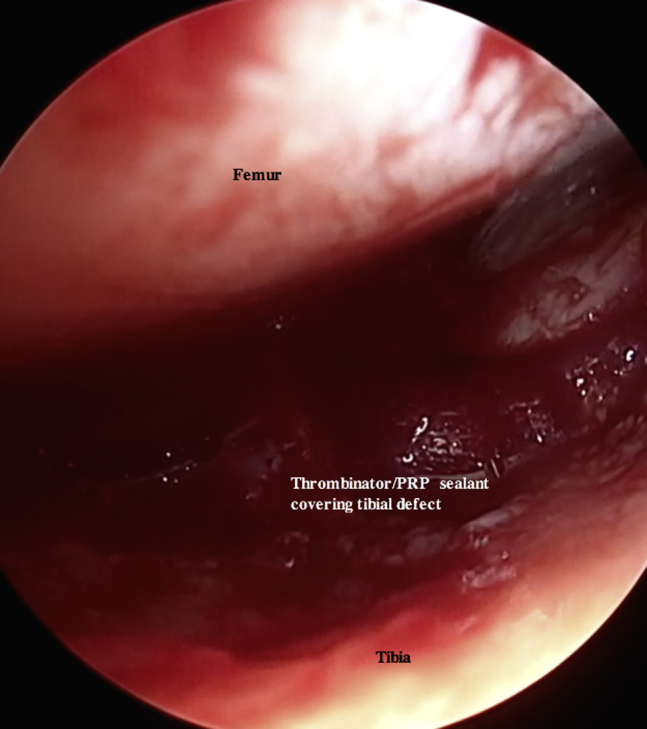
Arthroscopic photograph of the right knee with the patient in the supine position and the bed placed in the Trendelenburg position. An assistant is elevating the foot roughly 6 inches while maintaining roughly 70° of flexion. The tibial plateau defect is viewed through the inferomedial portal, and instruments are introduced through the inferolateral portal. The thrombin serum is dripped over the previously placed BioCartilage–platelet-rich plasma (PRP)–autologous cartilage defect using the prepared spinal needle on the Y syringe inserted through the inferolateral portal.
A post-microfracture rehabilitation protocol is used. Patients are restricted to toe-touch weight bearing for 6 weeks while using crutches. For patellofemoral lesions, we allow full weight bearing in extension in a brace that limits flexion to avoid overloading the healing defect. Continuous passive motion is used for 6 weeks for up to 6 hours a day.
Discussion
OAGs are often used as the last restorative resort for articular cartilage defects, with most data showing they lead to better clinical outcomes.24,25 Autologous cartilage implants are sometimes used before OAG because they do not cause any structural damage to the bone and, if they fail, the site will still be viable for OAG implantation.26 This article provides an efficient and effective technique for treating articular cartilage defects without subchondral bone degradation in the knee. This method may be advantageous in its ability to restore hyaline cartilage in a single step while being less invasive and perhaps less resource intensive than OAG transplantation, as well as less expensive than a 2-stage ACI or MACI procedure (Table 1).
Although the described method is a recently introduced procedure and there are no completed clinical trials looking at this specific method, BioCartilage with PRP and microfracture have both been independently validated (Table 1).17,18 In addition, numerous animal studies showed improved outcomes when BioCartilage and PRP were used in conjunction with microfracture versus microfracture alone.19 These treatment augmentations have been shown to produce hyaline-like growth and chondrogenesis.18,19 Micronized BioCartilage used with PRP and autologous cartilage acts as a scaffold and perhaps may allow for the growth of stronger, structurally sound hyaline cartilage superior to fibrocartilage.
In conclusion, this article presents the senior author's (B.J.C.) method of cartilage restoration using microfracture augmented with a BioCartilage, PRP, and autologous cartilage mixture sealed with activated serum. Further studies will determine the role of this procedure in restoring hyaline cartilage and reducing pain and related symptoms.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: B.J.C. receives research support and intellectual property royalties from Arthrex and is a paid consultant for Arthrex. B.J.C. receives research support from Aesculap, National Institutes of Health, and Regentis; receives other financial or material support from Athletico, JRF Ortho, and Smith & Nephew; intellectual property royalties from Elsevier; receives publishing royalties and financial or material support from Operative Techniques in Sports Medicine; receives stock options from Ossio; and is a paid consultant for Regentis, outside the submitted work. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Disclosures and introduction (0-19 seconds). The right knee is prepared and draped in the typical fashion with the patient in the supine position (20 seconds). Standard arthroscopic portals are established in the knee, and a diagnostic arthroscopy is performed. The defect is probed and is prepared and debrided using a ring curette, bone cutter, and/or shaver (21-25 seconds). A ring curette is used to remove the calcified layer at the base of the lesion and create vertical walls on the defect periphery to promote glue adherence (26-29 seconds). A PowerPick is used to microfracture the defect, creating holes 1.5 mm in diameter and 4 mm deep in the subcortical bone (30-37 seconds). A GraftNet device, connected in-line with the suction, is attached to the shaver and is used to harvest and capture autologous hyaline cartilage from the intercondylar notch and proximal aspect of the femoral condyle (40 seconds to 1 minute 8 seconds). The GraftNet is disconnected, and the harvested autologous cartilage is set aside. Roughly 72 mL of whole blood is collected from the patient (1 minute 9 seconds to 1 minute 10 seconds); 60 mL is centrifuged in the Angel platelet-rich plasma (PRP) system (1 minute 21 seconds to 1 minute 28 seconds), and the remainder is saved for use in the Thrombinator system. Four milliliters of whole blood is injected into the “inject” port on the Thrombinator system (1 minute 11 seconds to 1 minute 20 seconds). Equal parts (roughly 1 mL) BioCartilage, PRP, and autologous cartilage collected with the GraftNet device are combined in an Arthrex Mixing and Delivery Syringe (1 minute 29 seconds to 2 minutes 9 seconds). The syringe is attached to a Tuohy needle and advanced to fill the needle (2 minute 1 second to 2 minutes 11 seconds). The syringe is removed, an obturator is attached to the back of the needle, and the BioCartilage mixture is ready to inject (2 minutes 12 seconds to 2 minutes 18 seconds). The thrombin serum is attached to a Y syringe containing an identical syringe of PRP (1:1), and the mixtures are advanced to equalize (2 minutes 19 seconds to 2 minutes 24 seconds). All inflow of fluids into the knee is turned off, the joint capsule is drained, and the defect is dried with a pledget (2 minutes 25 seconds to 2 minutes 32 seconds). The prepared Tuohy needle is inserted into the joint, and the BioCartilage–PRP–autologous cartilage mixture is injected into the microfractured defect and gently pressed into place, filling the defect to just under the surrounding cartilage margin (2 minutes 33 seconds to 2 minutes 55 seconds). A freer elevator can be used to smooth the mixture into place. A spinal needle is attached to the Y syringe containing the activated serum to facilitate injection and placement over the defect (2 minutes 56 seconds to 3 minutes 12 seconds). The spinal needle on the Y syringe is inserted into the joint capsule, and the activated serum is dripped over the defect.
References
- 1.Hjelle K., Solheim E., Strand T., Muri R., Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18:730–734. doi: 10.1053/jars.2002.32839. [DOI] [PubMed] [Google Scholar]
- 2.Roos E.M., Östenberg A., Roos H., Ekdahl C., Lohmander L.S. Long-term outcome of meniscectomy: Symptoms, function, and performance tests in patients with or without radiographic osteoarthritis compared to matched controls. Osteoarthritis Cartilage. 2001;9:316–324. doi: 10.1053/joca.2000.0391. [DOI] [PubMed] [Google Scholar]
- 3.Cole B.J., Pascual-Garrido C., Grumet R.C. Surgical management of articular cartilage defects in the knee. J Bone Joint Surg Am. 2009;91:1778–1790. [PubMed] [Google Scholar]
- 4.Levy D.M., Petersen K.A., Scalley Vaught M., Christian D.R., Cole B.J. Injections for knee osteoarthritis: Corticosteroids, viscosupplementation, platelet-rich plasma, and autologous stem cells. Arthroscopy. 2018;34:1730–1743. doi: 10.1016/j.arthro.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Fortier L.A., Cole B.J., McIlwraith C.W. Science and animal models of marrow stimulation for cartilage repair. J Knee Surg. 2012;25:3–8. doi: 10.1055/s-0032-1310389. [DOI] [PubMed] [Google Scholar]
- 6.Krych A.J., Harnly H.W., Rodeo S.A., Williams R.J., III Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee: A retrospective comparative study. J Bone Joint Surg Am. 2012;94:971–978. doi: 10.2106/JBJS.K.00815. [DOI] [PubMed] [Google Scholar]
- 7.Mithoefer K., McAdams T., Williams R.J., Kreuz P.C., Mandelbaum B.R. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: An evidence-based systematic analysis. Am J Sports Med. 2009;37:2053–2063. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]
- 8.Basad E., Wissing F.R., Fehrenbach P., Rickert M., Steinmeyer J., Ishaque B. Matrix-induced autologous chondrocyte implantation (MACI) in the knee: Clinical outcomes and challenges. Knee Surg Sports Traumatol Arthrosc. 2015;23:3729–3735. doi: 10.1007/s00167-014-3295-8. [DOI] [PubMed] [Google Scholar]
- 9.Cotter E.J., Frank R.M., Wang K.C., Cole B.J. Rehabilitation and return to play following osteochondral allograft transplantation in the knee. Oper Tech Sports Med. 2017;25:208–213. [Google Scholar]
- 10.Kreuz P.C., Kalkreuth R.H., Niemeyer P., Uhl M., Erggelet C. Long-term clinical and MRI results of matrix-assisted autologous chondrocyte implantation for articular cartilage defects of the knee. Cartilage. 2019;10:305–313. doi: 10.1177/1947603518756463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber A.E., Locker P.H., Mayer E.N. Clinical outcomes after microfracture of the knee: Midterm follow-up. Orthop J Sports Med. 2018;6 doi: 10.1177/2325967117753572. 2325967117753572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Namdari S., Baldwin K., Anakwenze O., Park M.-J., Russell Huffman G., Sennett B.J. Results and performance after microfracture in National Basketball association athletes. Am J Sports Med. 2009;37:943–948. doi: 10.1177/0363546508330150. [DOI] [PubMed] [Google Scholar]
- 13.Mascarenhas R., Saltzman B.M., Fortier L.A., Cole B.J. Cartilage e-book. CIC Edizioni Internazionali; Rome: 2015. BioCartilage: New frontiers in cartilage restoration; pp. 183–193. [Google Scholar]
- 14.Chadha N, Dang A, Sampson E, et al. Porous cartilagederived matrix scaffolds for repair of articular cartilage defects. Presented at Orthopaedic Research Society 2012 Annual Meeting. February 4-7, 2012. San Francisco, CA.
- 15.Cheng N.C., Estes B.T., Awad H.A., Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng Part A. 2009;15:231–241. doi: 10.1089/ten.tea.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhollander A.A., De Neve F., Almqvist K.F. Autologous matrix-induced chondrogenesis combined with platelet-rich plasma gel: Technical description and a five pilot patients report. Knee Surg Sports Traumatol Arthrosc. 2011;19:536–542. doi: 10.1007/s00167-010-1337-4. [DOI] [PubMed] [Google Scholar]
- 17.Hapa O., Cakici H., Yuksel H.Y., Firat T., Kukner A., Aygun H. Does platelet-rich plasma enhance microfracture treatment for chronic focal chondral defects? An in-vivo study performed in a rat model. Acta Orthop Traumatol Turc. 2013;47:201–207. doi: 10.3944/aott.2013.2928. [DOI] [PubMed] [Google Scholar]
- 18.Abrams G.D., Mall N.A., Fortier L.A., Roller B.L., Cole B.J. BioCartilage: Background and operative technique. Oper Tech Sports Med. 2013;21:116–124. [Google Scholar]
- 19.Fortier L.A., Chapman H.S., Pownder S.L. BioCartilage improves cartilage repair compared with microfracture alone in an equine model of full-thickness cartilage loss. Am J Sports Med. 2016;44:2366–2374. doi: 10.1177/0363546516648644. [DOI] [PubMed] [Google Scholar]
- 20.Lewis P.B., McCarty L.P., III, Yao J.Q., Williams J.M., Kang R., Cole B.J. Fixation of tissue-engineered human neocartilage constructs with human fibrin in a caprine model. J Knee Surg. 2009;22:196–204. doi: 10.1055/s-0030-1247749. [DOI] [PubMed] [Google Scholar]
- 21.Carter A.H., Guttierez N., Subhawong T.K. MR imaging of BioCartilage augmented microfracture surgery utilizing 2D MOCART and KOOS scores. J Clin Orthop Trauma. 2018;9:146–152. doi: 10.1016/j.jcot.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melugin H.P., Bernard C.D., Camp C.L. tibial plateau cartilage lesions: A systematic review of techniques, outcomes, and complications. Cartilage. 2019 doi: 10.1177/1947603519855767. 1947603519855767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bogunovic L., Wetters N.G., Jain A., Cole B.J., Yanke A.B. In vitro analysis of micronized cartilage stability in the knee: Effect of fibrin level, defect size, and defect location. Arthroscopy. 2019;35:1212–1218. doi: 10.1016/j.arthro.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Bentley G., Biant L.C., Carrington R.W. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223–230. doi: 10.1302/0301-620x.85b2.13543. [DOI] [PubMed] [Google Scholar]
- 25.Dozin B., Malpeli M., Cancedda R. Comparative evaluation of autologous chondrocyte implantation and mosaicplasty: A multicentered randomized clinical trial. Clin J Sport Med. 2005;15:220–226. doi: 10.1097/01.jsm.0000171882.66432.80. [DOI] [PubMed] [Google Scholar]
- 26.Cole B.J., Redondo M.L., Cotter E.J. Articular cartilage injuries of the knee: Patient health literacy, expectations for management, and clinical outcomes. Cartilage. 2018 doi: 10.1177/1947603518816429. 1947603518816429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosures and introduction (0-19 seconds). The right knee is prepared and draped in the typical fashion with the patient in the supine position (20 seconds). Standard arthroscopic portals are established in the knee, and a diagnostic arthroscopy is performed. The defect is probed and is prepared and debrided using a ring curette, bone cutter, and/or shaver (21-25 seconds). A ring curette is used to remove the calcified layer at the base of the lesion and create vertical walls on the defect periphery to promote glue adherence (26-29 seconds). A PowerPick is used to microfracture the defect, creating holes 1.5 mm in diameter and 4 mm deep in the subcortical bone (30-37 seconds). A GraftNet device, connected in-line with the suction, is attached to the shaver and is used to harvest and capture autologous hyaline cartilage from the intercondylar notch and proximal aspect of the femoral condyle (40 seconds to 1 minute 8 seconds). The GraftNet is disconnected, and the harvested autologous cartilage is set aside. Roughly 72 mL of whole blood is collected from the patient (1 minute 9 seconds to 1 minute 10 seconds); 60 mL is centrifuged in the Angel platelet-rich plasma (PRP) system (1 minute 21 seconds to 1 minute 28 seconds), and the remainder is saved for use in the Thrombinator system. Four milliliters of whole blood is injected into the “inject” port on the Thrombinator system (1 minute 11 seconds to 1 minute 20 seconds). Equal parts (roughly 1 mL) BioCartilage, PRP, and autologous cartilage collected with the GraftNet device are combined in an Arthrex Mixing and Delivery Syringe (1 minute 29 seconds to 2 minutes 9 seconds). The syringe is attached to a Tuohy needle and advanced to fill the needle (2 minute 1 second to 2 minutes 11 seconds). The syringe is removed, an obturator is attached to the back of the needle, and the BioCartilage mixture is ready to inject (2 minutes 12 seconds to 2 minutes 18 seconds). The thrombin serum is attached to a Y syringe containing an identical syringe of PRP (1:1), and the mixtures are advanced to equalize (2 minutes 19 seconds to 2 minutes 24 seconds). All inflow of fluids into the knee is turned off, the joint capsule is drained, and the defect is dried with a pledget (2 minutes 25 seconds to 2 minutes 32 seconds). The prepared Tuohy needle is inserted into the joint, and the BioCartilage–PRP–autologous cartilage mixture is injected into the microfractured defect and gently pressed into place, filling the defect to just under the surrounding cartilage margin (2 minutes 33 seconds to 2 minutes 55 seconds). A freer elevator can be used to smooth the mixture into place. A spinal needle is attached to the Y syringe containing the activated serum to facilitate injection and placement over the defect (2 minutes 56 seconds to 3 minutes 12 seconds). The spinal needle on the Y syringe is inserted into the joint capsule, and the activated serum is dripped over the defect.