Abstract
Stricturing Crohn’s disease (CD) is a severe phenotype that presents unique challenges to therapeutic management. Emerging literature suggests that anti-TNF monoclonal antibody (mAb) therapies are inadequate for preventing progression to stricture. We hereby present a case of a patient with refractory CD who required multiple surgical resections despite several anti-TNF treatment regimens. Subsequent surgical complications were avoided after changing to combination vedolizumab and ustekinumab therapies every 4 weeks. This case argues for a tailored approach to CD therapy based on disease phenotype and demonstrates that combination therapy with ustekinumab and vedolizumab is a viable option for patients with stricturing disease.
Keywords: double biologic therapy (DBT), vedolizumab, ustekinumab, stricture, Crohn’s disease
To the Editors,
Stricturing Crohn’s disease (CD) constitutes a severe phenotype associated with bowel obstruction and high morbidity.1 Surgical resection is a first-line therapy for symptomatic strictures, but disease recurs without subsequent medical therapy.2, 3 Though antitumor necrosis factor (TNF) biologics induce and maintain remission in CD, many patients are refractory.4 New therapies such as ustekinumab (UST) and vedolizumab (VDZ), biologic agents that block IL-12p40/IL-23 cytokines and mucosal recruitment of α4β7-expressing lymphocytes, respectively, may be more appropriate for stricturing phenotypes. Given the relative safety of these drugs and distinct therapeutic profiles, we believe these agents (UST/VDZ) may provide synergistic benefits. Reports of UST/VDZ dual biologic therapy (DBT) for refractory CD are sparse, yet encouraging.5 In this report, we present the case of an anti-TNF mAb refractory patient with stricturing CD who benefited from DBT.
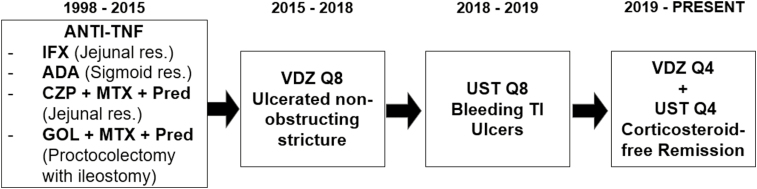
A 35-year-old white male with a history of multiple strictures requiring surgical resection was referred to our clinic in 2014 (Fig. 1). Previously failed treatments included azathioprine combined with infliximab, adalimumab, and certolizumab. Each failure resulted in intestinal stricture requiring resection. Subsequently, he failed both combination of methotrexate (MTX) and certolizumab, and MTX with golimumab, leading to a proctocolectomy with end ileostomy in 2015. He initiated vedolizumab every 8 weeks (Q8) postoperatively and felt well for 3 years despite exhibiting elevated fecal calprotectin. Vedolizumab was switched to ustekinumab in 2018 after ileoscopy revealed inflammation and a nonobstructing ileal stricture. Unfortunately, soon after therapeutic switch, he developed hematochezia from 2 ileal ulcers, requiring hospitalization and blood transfusion. Ustekinumab dosing was increased to every 4 weeks (Q4), and azathioprine (175 mg) and budesonide (9 mg/d) were added. After 3 months, ileoscopy revealed 2 ulcers in the neoterminal ileum (TI). Vedolizumab Q8 was reinitiated in addition to ustekinumab Q4 with azathioprine. After 4 months on this regimen, the patient improved symptomatically, but the neo-TI ulcers persisted. Vedolizumab was increased to Q4, and a decadron taper was initiated. Ileoscopy performed 4 months later demonstrated a normal neo-TI, and histologic evaluation confirmed deep remission. Remission persisted through completion of the decadron taper and budesonide discontinuation. He remains on azathioprine with DBT. No adverse side effects have occurred with maximum dosage of combination ustekinumab and vedolizumab therapy. We believe this case speaks to the importance of tailoring therapeutic regimens based on CD phenotype. We believe avoidance of anti-TNF mAb agents may be preferable in stricturing CD and demonstrate that UST/VDZ combination therapy is a safe and efficacious alternative.
FIGURE 1.
Timeline of treatment modalities employed in CD patient from 1998 to 2020. Anti-TNF therapies utilized from 1998 to 2015 resulted in recurrent strictures and surgical resections. Vedolizumab (VDZ) every 8 weeks (Q8) and ustekinumab (UST) Q8 failed to induce remission but did not result in surgical or stricture formation. Dose escalation of VDZ and UST every 4 weeks (Q4) allowed the patient to achieve steroid-free deep remission.
Supported by: CP is supported by the National Institutes of Health, Grant TL1TR001997 of CCTS funding. The authors’ work is also supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R21DK118954. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was supported in part by Merit Review Award #I01CX001353 to TAB from the United States Department of Veterans Affairs Laboratory Research and Development Program.
Conflicts of Interest: TAB has consulted and received honoraria for speaker’s bureau activities for Takeda and AbbVie pharmaceutical companies.
REFERENCES
- 1. Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013;62:1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Regueiro M, Schraut W, Baidoo L, et al. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology. 2009;136:441–50. [DOI] [PubMed] [Google Scholar]
- 3. Wolters FL, Russel MG, Stockbrügger RW. Systematic review: has disease outcome in Crohn’s disease changed during the last four decades? Aliment Pharmacol Ther. 2004;20:483–496. [DOI] [PubMed] [Google Scholar]
- 4. Yoon SM, Haritunians T, Chhina S, et al. Colonic phenotypes are associated with poorer response to anti-TNF therapies in patients with IBD. Inflamm Bowel Dis. 2017;23:1382–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kwapisz L, Raffals LE, Bruining DH, et al. Combination biologic therapy in inflammatory bowel disease: experience from a tertiary care center. Clin Gastroenterol Hepatol. 2020. doi: 10.1016/j.cgh.2020.02.017 [DOI] [PubMed] [Google Scholar]