Abstract
Background
Cytochrome P450 (CYP) mediated metabolism of chemotherapeutic agents contributes to chemotherapy resistance in multiple malignancies. Adrenocortical carcinoma (ACC) is known to have a poor response to adjuvant therapies; however, the mechanism remains unknown. Recent comprehensive genetic analyses of ACCs demonstrated recurrent copy number gains in multiple CYP genes prompting investigation into whether CYP overexpression potentiates ACC chemoresistance.
Methods
We determined the expression patterns of six CYP genes (CYP2A6, CYP2A7, CYP2A13, CYP2B6, CYP2S1, & CYP4F2) predicted to be amplified in ACC (n=29) relative to normal adrenal cortex (n=10). Gene copy numbers were determined with TaqMan copy number assay. Gene silencing was performed via small interfering RNA (siRNA) in the ACC cell line NCI-H295R and treated with mitotane and cisplatin.
Results
Of the six CYP genes tested, CYP2A6 was significantly overexpressed with a 55-fold mean increase compared to normal adrenal samples (P < .05). Immunohistochemical analysis confirmed overexpression. Copy gains of CYP2A6 were found in 26% (7/27) of ACC specimens. Silencing of CYP2A6 in NCI-H295R cells resulted in decreased cell viability and increased chemosensitivity (P < .05).
Conclusions
Frequent upregulation in ACCs and the reversal of chemoresistance in ACC cells via enforced silencing, suggest a role for CYP2A6 in adrenocortical malignancy.
BACKGROUND
Adrenocortical carcinoma (ACC) is a genetically heterogeneous tumor characterized by its lethality and commonly advanced stage at diagnosis.1 Despite the growing understanding of the genetic drivers of familial ACC, the basic metabolic pathways that promote the aggressive and resistant behavior of sporadic ACC have yet to be fully clarified. Currently, there are few recurrent ACC biomarkers that are amenable to therapeutic targeting and intervention.
Advancements in the quality and availability of genetic sequencing have allowed for a more complete comprehension of the underlying genetic complexity of ACC, but have also highlighted intrinsic chromosomal aberrations.2,3 A recent analysis of copy number variations (CNV) in ACC using depth of coverage comparison demonstrated pervasive CNVs with large-scale recurrent copy number gains and losses.4 Gene-level analyses of loci with abnormal copy numbers revealed multiple cytochrome P450 (CYP) genes to be overrepresented in the cohort.
The cytochrome P450 superfamily is a group of more than 50 heme-thiolate enzymes that are critical for physiologic homeostasis. These monooxygenases catalyze the biosynthesis of lipids, cholesterol, and steroids with wide-ranging and multisystemic effects. They also constitute the major determinants of drug elimination and half-life in humans.5 In addition to their roles in metabolism, abnormal expression and function of CYP enzymes have been linked to lung, colon, pancreatic, and adrenal cancer.6–9 Purported causes for this association include changes in steroid production and the activation of benign xenobiotic compounds into carcinogenic or cytotoxic metabolites. Steroid hormones have been shown to promote cancer development and have the potential to be especially important in endocrine organs like the adrenal gland.10 Additionally, because the majority of pharmaceuticals are enzymatically processed by one or more of the CYP proteins, the group plays an important role in chemotherapy metabolism and, in some cases, can confer chemoresistance.11
A cornerstone of ACC treatment for decades, mitotane (1,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl) ethane) is a potent adrenocorticolytic compound that can reduce tumor burden and inhibits hormone synthesis. Since it was first used in the late 1950s, mitotane has been a beneficial adjunct to surgery in the appropriate clinical setting.12,13 However, even with modern combination chemotherapy regimens using cisplatin and others agents, the long-term survival rate in advanced ACC remains dismal.14 Despite decades of use, the mechanism of action of mitotane is still not clear. Multiple studies have demonstrated in vivo induction of CYP3A4 in patients treated with mitotane, while others have illustrated the accumulation of toxic lipids as a possible pro-apoptotic pathway.15,16
The copy number gains of CYP genes in ACC predicted by Rubinstein et al. and the superfamily’s known association with carcinogenesis and anti-cancer drug metabolism prompted further investigation into the potential influences of the studied CYP enzymes in ACC progression or chemoresistance.4
METHODS
Patients and tissue collection
Patients included in this study were treated either at Yale-New Haven Hospital (New Haven, United States) or Karolinska University Hospital (Stockholm, Sweden). ACC (n = 29) and normal adrenal samples (n = 10) were independently reviewed by experienced endocrine pathologists for identification. Non-tumor samples were extracted from histopathologically normal adrenocortical tissue adjacent to adrenocortical adenomas. Additional formalin-fixed paraffin-embedded (FFPE) samples from the same tumors were used for immunohistochemical analysis. Informed consent was obtained from all patients involved in this study. The acquisition and use of protected health information and tissue specimens were performed as specified by the Health Insurance Portability and Accountability Act (Yale) or in agreement with Swedish Biobank laws (Karolinska). The consent waiver and all procedural aspects of this study were approved by the Yale University and Karolinska Institutet Institutional Review Boards.
Copy number analysis
Histologically confirmed ACC (n = 27) and normal adrenal reference samples (n = 3) were analyzed for CYP2A6, CYP2A7, CYP2A13, CYP2B6, CYP2S1, and CYP4F2 copy number alterations. The endogenous control ribonuclease P (RNase P) was used for normalization purposes. Twenty ng aliquots of genomic DNA isolated from fresh frozen tissue samples were subjected to quadruplicate multiplex reactions containing individual CYP TaqMan copy number assays and RNase P TaqMan copy number reference assay (all from Life Technologies, Carlsbad, CA) as previously described.17 Experiments were conducted with a StepOnePlus System (Life Technologies) and outlier replicates were manually omitted. CYP gene copy number was analyzed using CopyCaller Software v2.0 (Applied Biosystems, Foster City, CA).
CYP gene expression
RNA was isolated from 29 fresh frozen ACC samples, 10 normal adrenal samples, and two human ACC cell lines (NCI-H295R and SW-13) using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany). This cohort included all 27 samples accrued for the CNV analysis and 2 additional ACC tumors acquired at a later date. Quantity and quality of isolated RNA was assessed by spectrophotometry (NanoDrop Technologies, Wilmington, DE). The synthesis of cDNA was performed with the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) using 200 ng of RNA per sample. Quantitative RT-PCR was performed in a CFX96 Real-Time System thermocycler (Bio-Rad) using TaqMan PCR master mix with primers and probes specific to the CYP genes CYP2A6, CYP2A7, CYP2A13, CYP2B6, CYP2S1, & CYP4F2 (all from Applied Biosystems). Relative expression between samples was normalized to that of endogenous ribosomal protein, large, P0 (RPLP0) expression. The presented data are representative of two independent experiments performed with duplicate samples for all assays. Relative mRNA expression levels were calculated by first normalizing the mean expression in the non-tumor adrenal samples to 1.0. This arbitrary value was then compared to the expression level in tumor samples and fold-change was calculated using the Livak method.18
Immunohistochemical analysis
Five μm thick FFPE sections of histologically confirmed ACC and normal adrenal tissue were used in this study. Representative ACC (n = 2) and normal adrenocortical samples (n = 2) were selected for immunostaining. Using a standard immunohistochemistry protocol, target epitopes were detected using mouse monoclonal antibody to cytochrome P450 2A6 (Abcam Inc., Cambridge, MA) followed by bovine anti-mouse IgG-HRP conjugated secondary antibody (Santa Cruz, Dallas, TX). DAB (3,3’-diaminobenzidine tetrahydrochloride) was utilized for antigen detection (Life Technologies). Sections were counterstained with hematoxylin (VWR International, Radnor, PA) and mounted using immunohistomount (Santa Cruz). Anonymous samples of histologically normal liver were used as a positive control for CYP2A6 expression.
Silencing of CYP2A6 expression in ACC cells
Cell culture experiments were performed applying previously described methodology.19 The human ACC cell line NCI-H295R was purchased from the American Type Culture Collection (Manassas, VA) and grown in DMEM/F-12 containing 5% NuSerum, 0.1% ITS premix, and 10,000 U/mL penicillin/streptomycin (Life Technologies). Gene silencing was accomplished with the use of small interfering RNA (siRNA) according to the manufacturer’s recommendations (OriGene Technologies, Rockville, MD). Two hundred thousand cells were plated 24 hours prior to transfection for 80% confluency. In Opti-MEM medium (Thermo Fisher Scientific, Waltham, MA), cells were transfected with either Lipofectamine 2000 alone (Thermo Fisher Scientific), negative scrambled siRNA, or CYP2A6-specific siRNAs (OriGene Technologies). Two unique 27-mer siRNA duplexes (siA & siB) targeting CYP2A6 were tested at incremental concentrations (20 nM, 40 nM, 80 nM) for gene silencing efficacy. Transfection medium was replaced with complete medium after 6 hours of transfection and cells were lysed after 24 hours for RNA extraction. SiB at a transfection concentration of 40 nM was selected for in vitro gene silencing based on the effective CYP2A6 downregulation as determined by qRT-PCR.
Twenty-four hours after transfection, cells were treated with mitotane alone or mitotane and cisplatin (Spectrum Chemical, New Brunswick, NJ) while control groups were treated with the appropriate amounts of respective diluents. The conditions included (1) no treatment, (2) 5 μM mitotane, (3) 50 μM mitotane, (4) 50 μM mitotane and 700 nM cisplatin, or (5) mock treatment. After 24 hours, cell viability was measured using the trypan blue exclusion assay (Life Technologies) and counted manually with a hemocytometer (Hausser Scientific, Horsham, PA). A minimum of two independent experiments were performed with triplicate samples in each assay.
Statistical analysis
Clinical parameters such as patient age, gender, tumor diameter, pathological stage, metastatic status, and hormone secretion profile were compared with CYP gene expression. Continuous variables were assessed for a normal distribution using the D’Agostino and Pearson omnibus normality test, then analyzed using a 2-tailed t test for normally distributed variables, or the Mann-Whitney U test for non-normally distributed variables. For variables with greater than 2 dependent values a 1-way analysis of variance and Kruskal-Wallis tests were used for normally and non-normally distributed populations, respectively. Spearman correlation was used to compare matched continuous variables. For categorical variables, the Pearson χ2 test or Fisher exact test were used, as appropriate. Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software).
RESULTS
Patient characteristics
Of the 29 patients recruited for this study, 19 were female (65.5%) and the remainder were male (10/29; 34.5%). More than a third of the tumors were nonfunctional (11/29; 37.9%) while a quarter were cortisol-secreting (7/29; 24.1%). The majority of patients were pathologic stage II per the American Joint Committee on Cancer, 7th edition (16/29; 55.2%). Mean age was 57.5 years and the mean tumor diameter was 13.4 cm (Table I).
Table I.
Clinical features of patients with adrenocortical carcinoma (ACC)
| Variable | n (%) |
|---|---|
| Total | 29 (100) |
| Age ± SD, years | 57.5 ± 14.5 |
| Female | 19 (65.5) |
| Male | 10 (34.5) |
| Stage* | |
| I | 0 (0) |
| II | 16 (55.2) |
| III | 5 (17.2) |
| IV | 8 (27.6) |
| Tumor diameter, cm, mean (range) | 13.4 (7.0–21.0) |
| Hormonal status | |
| Aldosterone | 1 (3.5) |
| Cortisol | 7 (24.1) |
| Multiple | 3 (10.3) |
| Non-functional | 11 (37.9) |
| Testosterone | 2 (6.9) |
| Unknown | 5 (17.2) |
Staging criteria per American Joint Committee on Cancer (AJCC), 7th edition
SD = Standard deviation
Copy number analysis
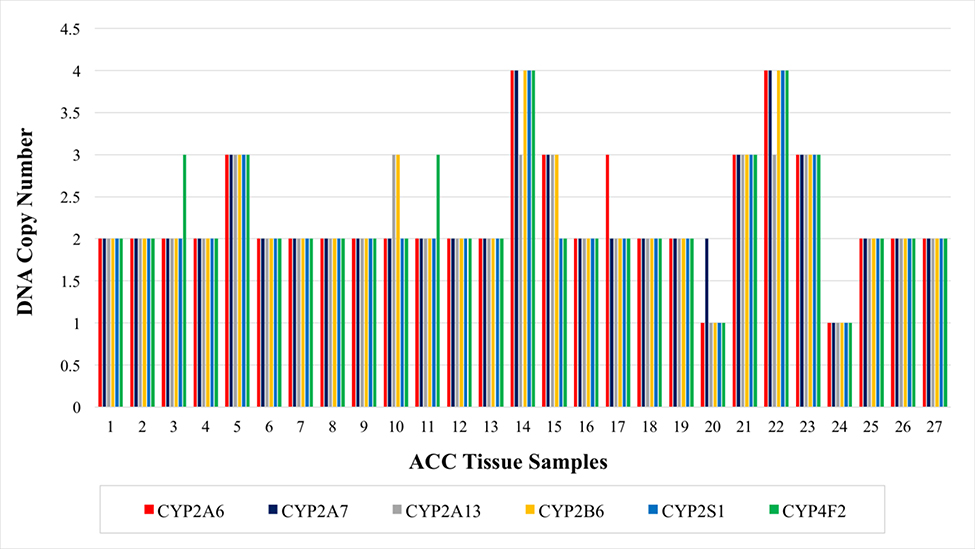
As noted previously, recent ACC research has demonstrated a broad distribution of CNVs across the genome. However, certain chromosome 19 CYP genes, particularly within the 19q13 region appeared to be significantly amplified.4 To verify these findings, TaqMan Copy Number Assays were conducted targeting CYP genes predicted by sequencing to have copy number gains (CYP2A6, CYP2A7, CYP2A13, CYP2B6, CYP2S1, & CYP4F2). Ten tumors (37.0%) had three or more CYP copies while two tumors had one copy (7.4%). All genes tested were more often gained than lost. In regards to CYP2A6 specifically, 7/27 samples (25.9%) were amplified while two samples harbored single copy deletions (Fig 1). The three normal adrenal controls were copy number neutral.
Fig 1.
Cytochrome P450 (CYP) gene copy number analysis in individual adrenocortical carcinoma (ACC) samples (n = 27). Ten patients (37.0%) had one or more CYP gene copy gains while two patients had copy deletions (7.4%). All genes tested were more often amplified than deleted. Of the 27 samples tested, 25.9% (7/27) harbored CYP2A6 copy gains.
Overexpression of CYP2A6
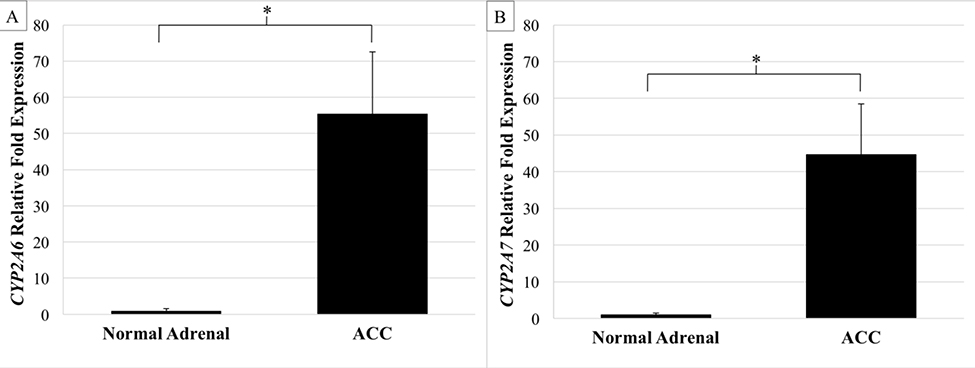
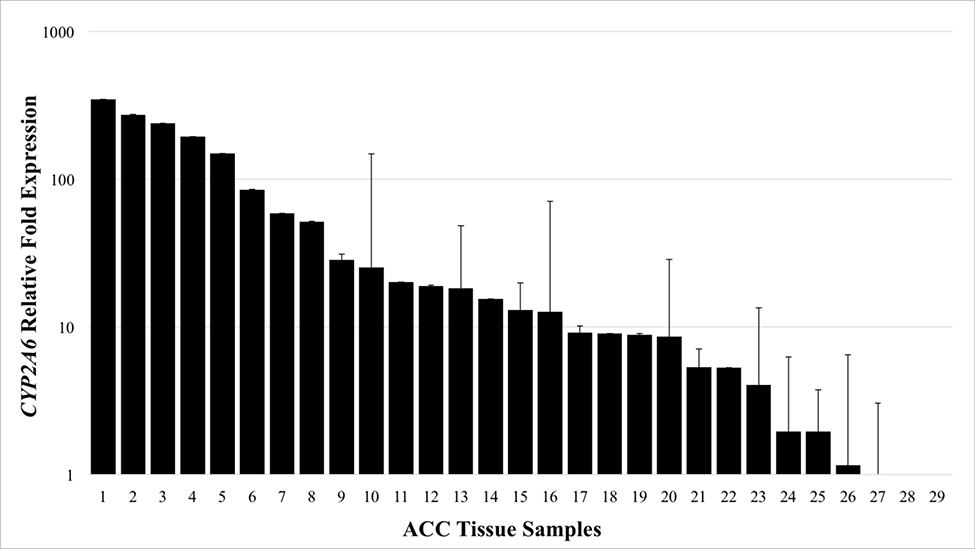
To determine if the observed copy number gains resulted in changes in transcriptional amplification, expression of the CYP genes was tested. Only the expression of CYP2A6 and CYP2A7 were found to be significantly increased with respective mean expression levels 55.4 and 44.7-fold greater than normal adrenal tissue (P < .05; Fig 2). CYP2A6 was overexpressed in 89.7% of tumors (26/29; Range 0.08–347.3; Fig 3) while CYP2A7 was amplified in all samples (29/29; Range 1.04–373.7). Analysis of the two established ACC cell lines NCI-H295R and SW-13 revealed that CYP2A6 expression in NCI-H295R cells was 5.26-fold greater than normal adrenal tissue and poorly expressed in SW-13 (0.45-fold).
Fig 2.
A) Mean CYP2A6 expression is 55-fold greater in adrenocortical carcinoma samples (n = 29) than normal adrenal tissue (n = 10). B) CYP2A7 is expressed 44-fold greater in ACC than normal. Error bars, SEM. (*P < .05; 2-tailed t test).
Fig 3.
CYP2A6 expression in individual adrenocortical carcinoma (ACC) samples. mRNA expression in normal tissue (n = 10) is normalized to 1 and relative expressions in ACC samples (n = 29) are shown. X axis: ACC samples (numbered 1–29). Error bars, SEM.
Immunohistochemical detection of protein expression
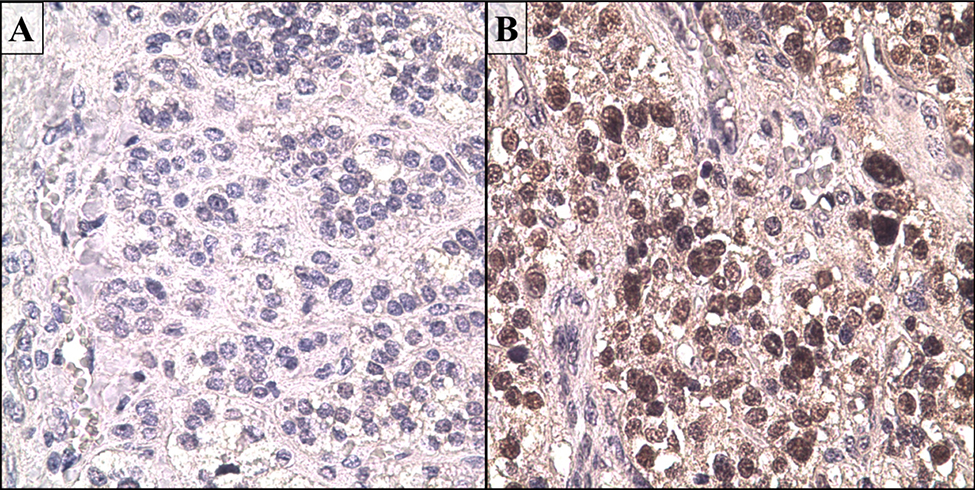
CYP2A6 protein expression in ACC samples was assessed through standard immunohistochemistry techniques. The level of expression in ACC was compared to immunostaining in normal adrenal cortical tissue. CYP2A6 protein expression was markedly greater in the ACC samples than normal adrenal tissue which had little to no detectable CYP2A6 expression (Fig 4). Staining intensity in ACC was greater in the nucleus compared to the cytoplasm. The lack of protein expression in the normal adrenal cortex is consistent with independently performed experiements.20
Fig. 4.
Immunohistochemical analysis demonstrates increased expression of CYP2A6 in adrenocortical carcinoma (ACC). A) Normal adrenal cortex. B) ADD. Original magnification – 400X. Brown staining indicates CYP2A6 protein and dark blue indicates nuclear staining by hematoxylin.
Silencing of CYP2A6 in ACC cells
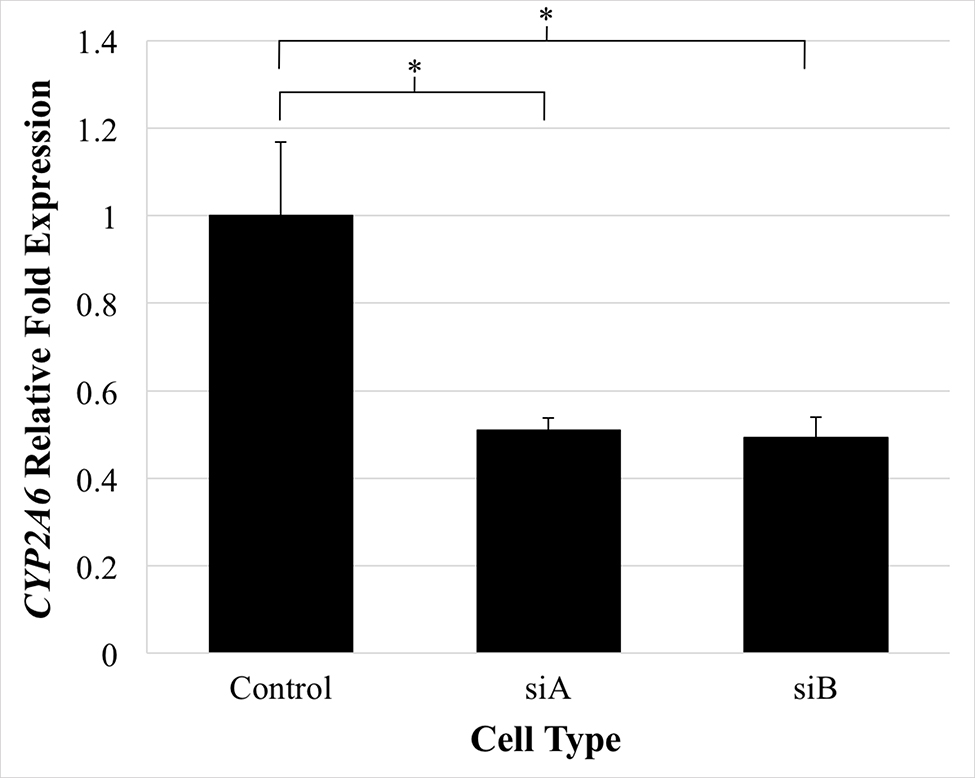
An analysis of CYP2A6 expression in two ACC cell lines revealed that expression was increased in NCI-H295R cells (5.26-fold) and reduced in SW-13 cells (0.45-fold) when compared to normal adrenal tissue. Since NCI-H295R cells have relatively high CYP2A6 expression, they were thought to better represent in vivo ACC cells which also overexpress CYP2A6 (Fig 3). Thus, this cell line was utilized for all silencing experiments. Silencing studies were not performed in SW-13 cells as reducing the low basal CYP2A6 expression was not anticipated to yield appreciable phenotypic changes. Silencing was performed in both the siA and siB groups at a concentration of 40 nM. SiB was used for functional studies as it had slightly more effective gene silencing (Fig 5).
Fig. 5.
SiRNA-induced silencing of CYP2A6 gene expression in NCI-H295R adrenocortical carcinoma cells. SiA and siB are unique, CYP2A6-targeted, siRNAs transfected at 40 nM and are shown compared with the control (scrambled siRNA) which has been normalized to a value of 1. Error bars, SEM. (*P < .05; 2-tailed t test).
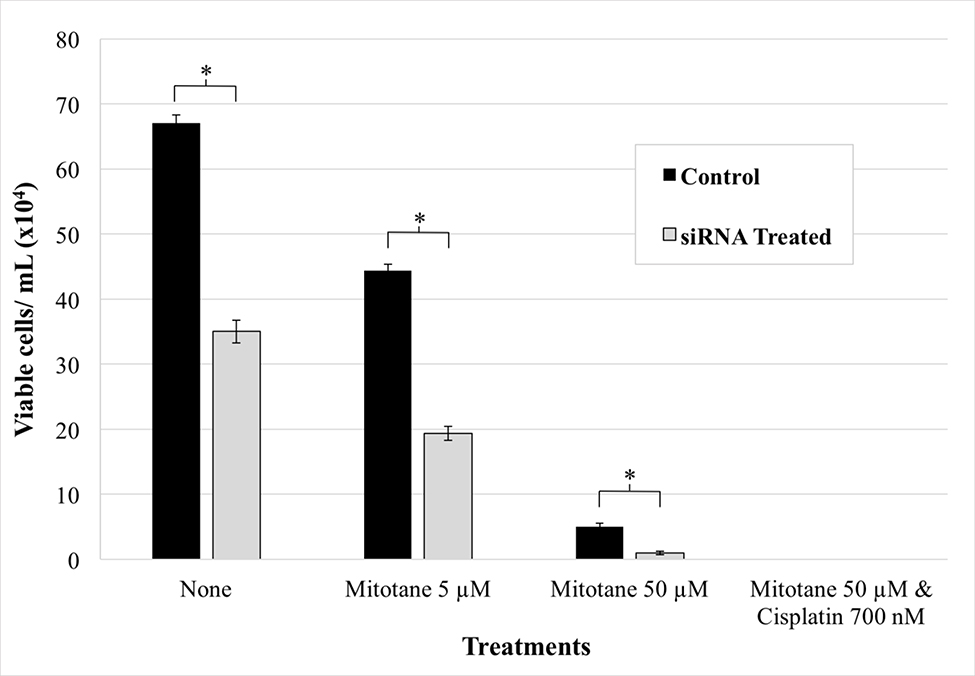
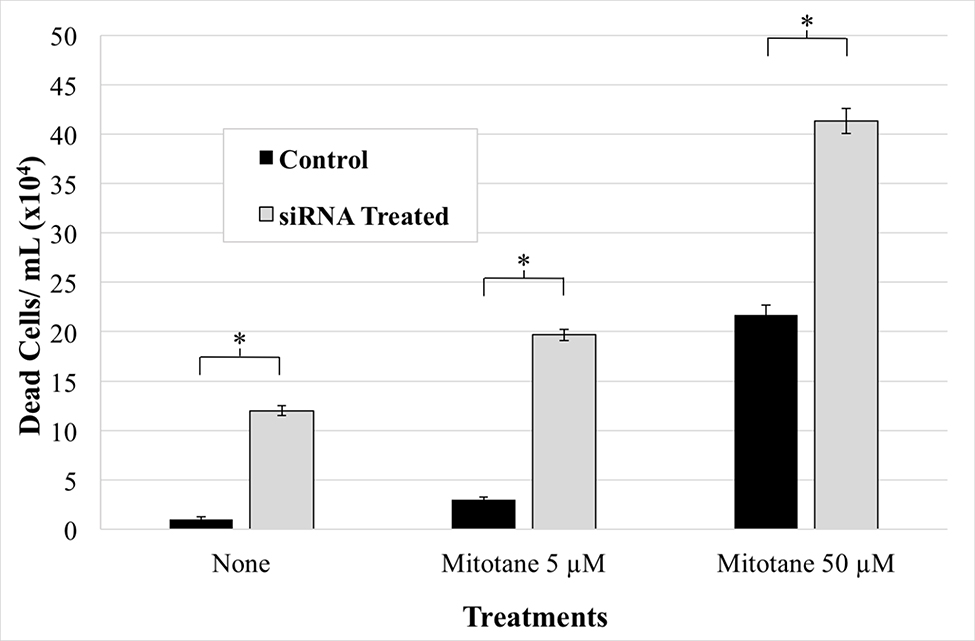
NCI-H295R cells with reduced CYP2A6 expression were compared to control cells with (1) no treatment, (2) 5 μM mitotane, (3) 50 μM mitotane, (4) 50 μM mitotane and 700 nM cisplatin, or (5) mock treatment. The concentrations were selected based on the previously demonstrated median lethal doses of the tested chemotherapy agents for NCI-H295R cells.21 As could be predicted, increasing the drug concentration individually or in combination resulted in decreased cell viability. Following combined treatment with cisplatin and mitotane, there were no remaining viable cells. SiRNA-treated cells exhibited accentuated loss of viability. Interestingly, reducing CYP2A6 expression alone appeared to be sufficient to decrease cell viability compared to control groups (P < .05; Fig 6, 7). These results suggest that the observed overexpression of CYP2A6 may impart a survival advantage to ACC.
Fig. 6.
Effect of CYP2A6 silencing on NCI-H295R adrenocortical carcinoma cell survival. Twenty-four hours post-transfection, cells were treated with or without chemotherapeutic agents and manually counted one day later. Cells with reduced CYP2A6 expression had significantly decreased viability. Control cells were treated with scrambled siRNA. Error bars, SEM. (*P < .05; 2-tailed t test).
Fig. 7.
Effect of CYP2A6 silencing on NCI-H295R adrenocortical carcinoma cell survival. Twenty-four hours post-transfection, cells were treated with or without chemotherapeutic agents and manually counted one day later. Cells with reduced CYP2A6 expression had significantly decreased viability. Control cells were treated with scrambled siRNA. Error bars, SEM. (*P < .05; 2-tailed t test).
Clinical characteristics and CYP gene expression
An analysis of patient and tumor characteristics (age, gender, tumor diameter, pathological stage, metastatic status, and hormonal secretion) did not demonstrate a statistically significant relationship between CYP gene expression and any clinical parameter.
DISCUSSION
The current study investigates the role of specific members of the CYP superfamily, a critical facet in drug biotransformation and cell metabolism, and their potential influence on ACC progression and chemoresistance. CYP mediated drug metabolism is of special interest in a cancer known for resistance to oncologic therapy. Evaluation of the studied CYP genes, revealed CYP2A6 copy number amplification, mRNA overexpression, and increased protein production in ACC samples (Fig 1, 2A, 3 and 4). The difference in the degree of copy number amplification and gene expression indicates alternate regulatory mechanisms such as epigenetic activation of gene expression or deregulation of epigenetic silencing in normal cortex, both possibilities that require further investigation.
While CYP2A7 does not produce a functional protein due to a defective heme-binding domain, it is an enticing secondary genetic target when viewed in relation to CYP2A6.22 Despite its status as a pseudogene, there is evidence to suggest that CYP2A7 may act as a decoy for microRNA (miRNA) induced CYP2A6 inhibition.23 Therefore, increases in CYP2A7 expression, as found in this study (Fig 2B), would proportionally increase the availability of CYP2A6 by mitigating miRNA-promoted downregulation. The concurrent suppression of these CYP genes may be an interesting avenue for future research.
In isolation, it could be argued that increased CYP2A6 expression is secondary to the ubiquitous genetic dysregulation inherent to ACC or perhaps, simply reflects the increased metabolic activity of malignant cells. However, the apparent absence of overexpression of other CYP genes co-amplified via copy gains and the near global increase in expression in tumors without copy gains suggest a more pointed or directional upregulation evolved for metabolic support of survival networks. Further, the functional data suggest that CYP2A6 expression may promote cell viability during mitotane treatment (Fig 6, 7). The demonstrated sensitization of ACC to low dose mitotane could have important clinical implications as even a marginal expansion in its therapeutic index would allow the treatment of patients that previously could not tolerate therapy due to the commonly encountered side effects. The heightened loss of viability demonstrated with mitotane/cisplatin treatment is also noteworthy. While mitotane is a strong inducer of CYP3A4, cisplatin is a minor CYP inhibitor.15,24 Additionally, as CYP2A6 expression was only reduced by 50%, it is arguable that the current silencing experiments may underestimate its true impact on cell proliferation (Fig 5). Future studies may be best directed toward developing a CYP2A6 knock-out model.
Intriguingly, CYP2A6 silencing reduced cell viability, an effect that has not been previously described in the literature. Instead, this enzyme is best known for its ability to catalyze the metabolism of nicotine. Loss of its function, such as in the CYP2A6*4 allelic variant, decreases the rate of nicotine metabolism, leading to decreased total cigarette consumption with a corresponding risk reduction of lung cancer.25 The enzyme has also been shown to hydroxylate coumarin, a precursor reagent in the production of warfarin. Despite numerous publications concerning these established actions, the role of CYP2A6 in ACC is unknown. Hypothetically, CYP2A6 has the potential to inactivate cytotoxic substrates in ACC; however, theories concerning its mechanism of action can only be considered speculative without further research.
The current study demonstrates overexpression of CYP2A6 in ACC with resultant increases in protein expression. Selection biases toward CYP2A6 expression, may support tumor viability and potentially increase chemoresistance in ACC. While further research will be essential in defining the underpinnings of the observed phenotypic changes, the data presented here expands the knowledge of ACC and may explain a component of its recurrent and aggressive nature.
ACKNOWLEDGMENTS
The authors would like to thank the Ohse Research Foundation at the Yale School of Medicine, the Stockholm County Council, the Swedish Cancer Society, and the Swedish Research Council for their support in funding this research.
REFERENCES
- 1.Lebastchi AH, Kunstman JW, Carling T. Adrenocortical Carcinoma: Current Therapeutic State-of-the-Art. J Oncol. 2012;2012:234726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juhlin CC, Goh G, Healy JM, Fonseca AL, Scholl UI, Stenman A, et al. Whole-exome sequencing characterizes the landscape of somatic mutations and copy number alterations in adrenocortical carcinoma. J Clin Endocrinol Metab. 2015;100(3):E493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assie G, Letouze E, Fassnacht M, Jouinot A, Luscap W, Barreau O, et al. Integrated genomic characterization of adrenocortical carcinoma. Nat Genet. 2014;46(6):607–12. [DOI] [PubMed] [Google Scholar]
- 4.Rubinstein JC, Brown TC, Goh G, Juhlin CC, Stenman A, Korah R, et al. Chromosome 19 amplification correlates with advanced disease in adrenocortical carcinoma. Surgery. 2016;159(1):296–301. [DOI] [PubMed] [Google Scholar]
- 5.Purnapatre K, Khattar SK, Saini KS. Cytochrome P450s in the development of target-based anticancer drugs. Cancer Lett. 2008;259(1):1–15. [DOI] [PubMed] [Google Scholar]
- 6.Daly AK. Advances in Pharmacology. Cytochrome P450 Function and Pharmacological Roles in Inflammation and Cancer. 2015;74:85–111. [DOI] [PubMed] [Google Scholar]
- 7.Lang NP, Butler MA, Massengill J, Lawson M, Stotts RC, Hauer-Jensen M, et al. Rapid metabolic phenotypes for acetyltransferase and cytochrome P4501A2 and putative exposure to food-borne heterocyclic amines increase the risk for colorectal cancer or polyps. Cancer Epidemiol Biomarkers Prev. 1994;3(8):675–82. [PubMed] [Google Scholar]
- 8.Gandhi AV, Saxena S, Relles D, Sarosiek K, Kang CY, Chipitsyna G, et al. Differential expression of cytochrome P450 omega-hydroxylase isoforms and their association with clinicopathological features in pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2013;20 Suppl 3:S636–43. [DOI] [PubMed] [Google Scholar]
- 9.Murtha T, Korah R, Carling T. Suppression of Cytochrome P450 4B1: An Early Event in Adrenocortical Tumorigenesis. Surgery (In Press). 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madhunapantula SV, Mosca P, Robertson GP. Steroid hormones drive cancer development. Cancer Biol Ther. 2010;10(8):765–6. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Antona C, Ingelman-Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene. 2006;25(11):1679–91. [DOI] [PubMed] [Google Scholar]
- 12.Bergenstal DM, Lipsett MB, Moy RH, Hertz R. Regression of adrenal cancer and suppression of adrenal function in man by o,p’DDD. Transactions of the Association of American Physicians. 1959:72–341. [Google Scholar]
- 13.Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356(23):2372–80. [DOI] [PubMed] [Google Scholar]
- 14.Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366(23):2189–97. [DOI] [PubMed] [Google Scholar]
- 15.Kroiss M, Quinkler M, Lutz WK, Allolio B, Fassnacht M. Drug interactions with mitotane by induction of CYP3A4 metabolism in the clinical management of adrenocortical carcinoma. Clin Endocrinol (Oxf). 2011;75(5):585–91. [DOI] [PubMed] [Google Scholar]
- 16.Sbiera S, Leich E, Liebisch G, Sbiera I, Schirbel A, Wiemer L, et al. Mitotane Inhibits Sterol-O-Acyl Transferase 1 Triggering Lipid-Mediated Endoplasmic Reticulum Stress and Apoptosis in Adrenocortical Carcinoma Cells. Endocrinology. 2015;156(11):3895–908. [DOI] [PubMed] [Google Scholar]
- 17.Juhlin CC, Stenman A, Haglund F, Clark VE, Brown TC, Baranoski J, et al. Whole-exome sequencing defines the mutational landscape of pheochromocytoma and identifies KMT2D as a recurrently mutated gene. Genes Chromosomes Cancer. 2015;54(9):542–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 19.Korah R, Healy JM, Kunstman JW, Fonseca AL, Ameri AH, Prasad ML, et al. Epigenetic silencing of RASSF1A deregulates cytoskeleton and promotes malignant behavior of adrenocortical carcinoma. Mol Cancer. 2013;12:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ponten F, Jirstrom K, Uhlen M. The Human Protein Atlas--a tool for pathology. J Pathol. 2008;216(4):387–93. [DOI] [PubMed] [Google Scholar]
- 21.Lehmann TP, Wrzesinski T, Jagodzinski PP. The effect of mitotane on viability, steroidogenesis and gene expression in NCIH295R adrenocortical cells. Mol Med Rep. 2013;7(3):893–900. [DOI] [PubMed] [Google Scholar]
- 22.Raunio H, Rautio A, Gullsten H, Pelkonen O. Polymorphisms of CYP2A6 and its practical consequences. Br J Clin Pharmacol. 2001;52(4):357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakano M, Fukushima Y, Yokota S, Fukami T, Takamiya M, Aoki Y, et al. CYP2A7 pseudogene transcript affects CYP2A6 expression in human liver by acting as a decoy for miR-126. Drug Metab Dispos. 2015;43(5):703–12. [DOI] [PubMed] [Google Scholar]
- 24.Masek V, Anzenbacherova E, Machova M, Brabec V, Anzenbacher P. Interaction of antitumor platinum complexes with human liver microsomal cytochromes P450. Anticancer Drugs. 2009;20(5):305–11. [DOI] [PubMed] [Google Scholar]
- 25.Ariyoshi N, Miyamoto M, Umetsu Y, Kunitoh H, Dosaka-Akita H, Sawamura Y, et al. Genetic polymorphism of CYP2A6 gene and tobacco-induced lung cancer risk in male smokers. Cancer Epidemiol Biomarkers Prev. 2002;11(9):890–4. [PubMed] [Google Scholar]