Abstract
Water quality monitoring is becoming an essential part of our lives as increasing human activities continue to spill unknown and unexpected contaminants into our water systems. To ensure the provision of safe and clean water to the public and the ecosystem, the development of rapid and sensitive in situ early warning systems for water toxicity monitoring is crucial. In this work, an entirely paper-based microbial fuel cell sensor utilizing freeze-dried bacteria is demonstrated as a portable and disposable water toxicity sensor. The bacterial cells were preinoculated on the anode reservoir of the device, and they were freeze-dried, making their on-site and on-demand applications possible. Upon rehydration of the bacteria with the water samples, current readings were obtained, and inhibition ratios (IRs) were calculated for different concentrations of formaldehyde as a model toxin. For 0.001, 0.01, and 0.02% of formaldehyde, IRs of 7.88, 16.08, and 23.14% were obtained, respectively. These IRs showed a very good linearity with the formaldehyde concentrations at R2 = 0.995. Additionally, the shelf life of the freeze-dried microbial fuel cell sensor was investigated. Even after 14 days of storage in the desiccator, at 4, and at −20 °C, the performance outputs compared to the new device were all at 96%.
1. Introduction
Over the past few decades, the need to monitor water quality has become essential worldwide as more and more toxic contaminants are spilled into our water systems. While many people take clean water for granted, the provision of clean and safe drinking water is not universal. There are still more than 844 million people in the world who do not have access to safe water.1 As such, providing access to clean water has become one of the primary goals of the National Academy of Engineering and the United Nations in the 21st century.1,2 While a lot of efforts have been made under their guidance, water toxicity is still of great concern because of the large increase in human activities. Rapid advances in global industrialization, agriculture, human migration, and climate change have continued to contribute to water pollution,3,4 especially in developing countries where adequate sanitation and efficient water treatment plans and infrastructures are lacking.5 Therefore, to ensure the safety of public health and the ecosystem, the development of early warning devices to monitor water quality is essential.
Over the years, various methods to monitor toxic compounds in water have been developed. Conventional techniques include the use of physiochemical methods such as gas/liquid chromatography and mass spectrometry which are very accurate and sensitive to specific compounds. However, these methods suffer from the facts that they need to be done off-site, need expensive instruments, and are time-consuming.6 Moreover, with an increasing number of contaminants mixing and interacting with each other, more complicated contaminations called “combined pollutants” or “cocontaminants” may not accurately reflect the toxicity to humans and/or other organisms.3,7 To address this issue, biological early warning systems utilizing living organisms such as bivalves, protozoans, fish, and algae have been developed to evaluate the biological effects of the joint toxicity.8,9 Nevertheless, these methods also require a long time to process and analyze the data in laboratories as the organisms need to be continuously monitored for the changes in their growth and behavior.10
Recently, microbial fuel cells (MFCs) have gained a lot of interest as a water toxicity sensor as they have shown great potential to rapidly detect toxins in water in a cost-effective way.2,11,12 MFCs utilize electrochemically active microorganisms (exoelectrogens) as biocatalysts to oxidize organic matter to convert chemical energy into electrical energy, generating electricity. For these exoelectrogens, organic matter act as electron donors and the electrodes as electron acceptors, and as a result, a direct linear relationship between the electrical output of a MFC and the metabolic activity of the microorganisms can be measured.13,14 When the exoelectrogens are exposed to toxic compounds, their metabolic activities can be inhibited, thus decreasing their electron transfer rates to the electrodes. Therefore, the electrical outputs from the MFCs can be used as a measurement to monitor the presence and the intensity of toxins in water.15,16 Additionally, MFC sensors have several distinct advantages in that the water samples can provide organic substrates necessary for microorganisms and that there is no need for additional transducer or power source as the microorganisms generate electric signals directly.17
Despite the latest advances in MFC sensors, the use of these devices in practical applications have been limited because of the use of expensive materials and external equipment such Nafion in proton exchange membranes (PEMs) and pumps and tubing.2,11 Recently, paper electronics, or “papertronics”, have gained a great deal of attention as a new platform for applications in healthcare, flexible electronics, and environmental monitoring as both fluidic and electronic components can be incorporated onto a paper substrate.18 Papers have several advantages as a platform in that they are low-cost, disposable, lightweight, flexible, biocompatible, and biodegradable.19 Previously, given the unique characteristics, papers have also been used in the fabrication of MFCs.20−22 The paper-based MFCs offered additional improvements on the devices, given the intrinsic feature of paper to rapidly absorb the bacterial inoculum via capillary action, which also promoted immediate attachment of bacterial cells to the electrode.18,23 In this work, to make a disposable, single-use MFC sensor for rapid detection of toxins in water, an entirely paper-based MFC sensor was fabricated which did not require expensive manufacturing materials or other external equipment.
Additionally, to develop a portable, truly stand-alone device that could be used in resource-limited environments for real-time measurement of toxin levels, the bacterial cells were preinoculated onto the paper device using the freeze-drying (lyophilization) technique. Some of the most important characteristics that portable biosensors should have are as follows: (i) the ability to maintain the cells’ activity/viability during storage, (ii) the convenience of transport, (iii) the accessibility at any time, and (iv) the simple operational methods.24,25 In an attempt to satisfy these requirements, our previous work utilized air-drying of bacterial cells to preinoculate the bacteria on the MFC sensors. However, the practical use of these sensors was very limited because of the complicated bacterial sample handling and short shelf life.26 On the other hand, freeze-drying is one of the most commonly used methods for preservation and long-term storage of biological samples.27,28 Recently, our group, for the first time, demonstrated the idea of freeze-drying exoelectrogens on paper, and these exoelectrogens were readily rehydrated for on-demand power generation.29 Therefore, freeze-drying was chosen to be the most suitable method for preinoculation of bacterial cells because it allows maintenance of cells’ activity and viability during long-term storage and transport. Additionally, the freeze-dried bacterial cells on MFC sensors can be easily rehydrated with the sample waters and the readout process only requires a simple, widely available, and inexpensive digital multimeter which allows immediate usage and no additional sample preparation.
Herein, a novel paper-based, single-use MFC sensor utilizing freeze-dried bacteria is designed and fabricated as a portable water quality sensor for the first time (Figure 1). The MFC sensor is created by combining two paper layers for low-cost and simple design. Water samples to be monitored are directly dropped on the anodic reservoir, which rehydrates the freeze-dried bacteria on the device. After the complete rehydration, maximum current outputs are obtained and to compensate for any external factors and variations among different devices, the inhibition ratios (IRs) are calculated and compared. Then, the shelf life of the freeze-dried MFC sensor is evaluated by looking at the sensor’s performance for up to 2 weeks. Lastly, we investigate the disposability of the paper device by safely disposing it by incineration.
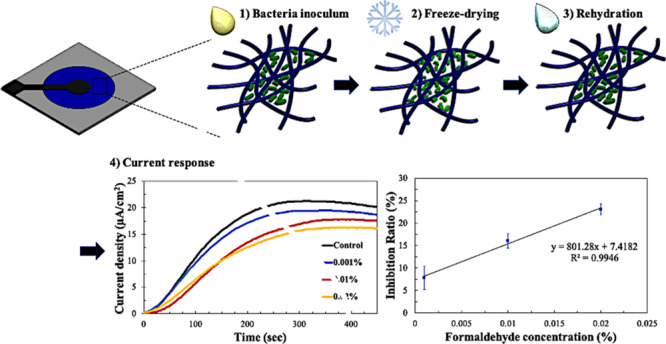
Figure 1.
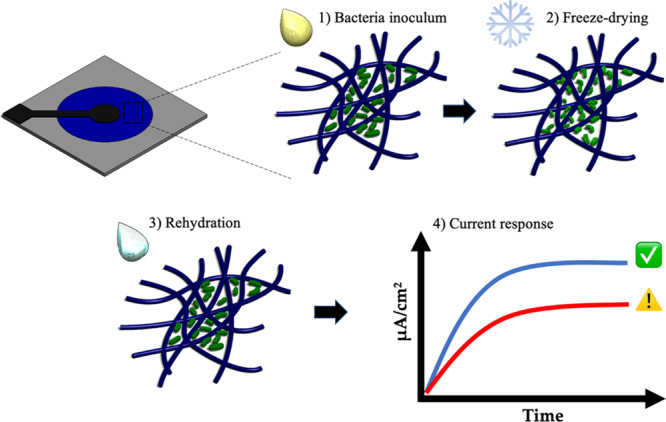
Schematic diagram showing the overall principle of the paper-based MFC sensor. Bacterial cells were inoculated on the paper device, freeze-dried, and rehydrated with sample water (steps 1–3). Then, the current responses to water with and without toxins were measured and compared (step 4).
2. Experimental Section
2.1. MFC Sensor Fabrication
For each MFC sensor, the anode and cathode layers were fabricated onto Whatman #1 filter papers. The layouts of the device were designed using the AutoCAD software and were printed on the papers using a solid-wax printer (Xerox ColorQube 8570). For each layer, the hydrophilic reservoir was defined by patterning wax around it to form a hydrophobic barrier. The wax pattern was printed on both sides of the paper and was heated at 120 °C for 2 min to allow complete penetration of the wax in the paper. For the cathode layer, additional wax was printed on one side of the paper to form a wax PEM. Adapting an already well-established method from our group, the cathode layer was heated at 120 °C for 40 s to only allow partial penetration of the wax.30
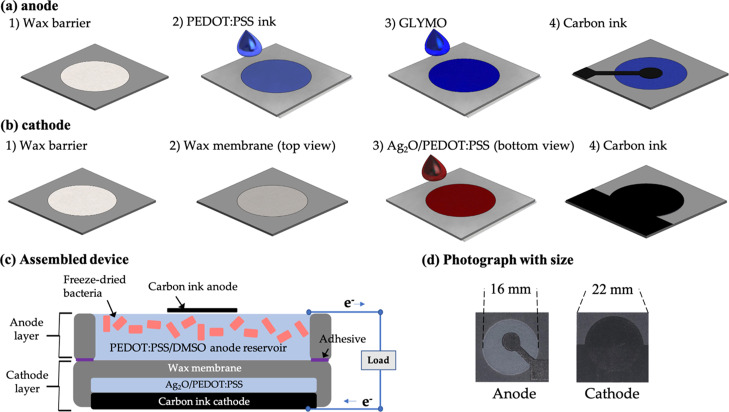
To make a functional anode, a conductive ink consisting of 1 wt % poly(3,4-ethylene dioxythiophene):polystyrene sulfonate (PEDOT:PSS, Sigma-Aldrich) and 5 wt % dimethyl sulfoxide (DMSO, Sigma-Aldrich) was injected on the anodic reservoir and air-dried. Then, 2 wt % 3-glycidyloxypropyl-trimethoxysilane (GLYMO) was pipetted on the reservoir to enhance the anodic hydrophilicity (Figure 2a). For the cathode, a silver-based mixture was prepared by mixing 2 mL of the conductive ink with 100 mg of Ag2O which acted as a solid electron acceptor. The mixture was sonicated for 1 h and thoroughly vortexed before being pipetted on the cathodic reservoir (Figure 2b). Then, for each anode and cathode layer, carbon ink was screen-printed on the top of the reservoir as a current collector (Figure 2c and d). After the two functional layers, the anode and cathode, were prepared, they were assembled together by spraying adhesives on the peripheries of the reservoirs (Figure 2c).
Figure 2.
Fabrication steps of (a) the anode layer and (b) the cathode layer of the MFC sensor; (c) the cross-section of the assembled device; and (d) photo image of the MFC sensor with size.
2.2. Inoculum Preparation
Wild-type Shewanella oneidensis MR1 from −80 °C glycerol stock was inoculated in 15 mL of Luria-broth (LB) medium with gentle shaking in air for 24 h at 30 °C. The LB medium consisted of 10.0 g of tryptone, 5.0 g of yeast extract, and 5.0 g of NaCl per liter. The culture was then centrifuged to remove the supernatant, and the bacterial cells were resuspended in a fresh LB medium containing 10% sucrose as a cryoprotectant for freeze-drying. The concentration of bacteria was determined by measuring the optical density of bacteria at 600 nm (OD600) using a spectrophotometer. Herein, the bacterial concentration of an OD600 value of 3 was used to fully saturate the anodic reservoir. To improve the electricity generation and sensitivity of the MFC sensor, 10 μM of riboflavin was added to the culture.31
2.3. Freeze-Drying (Lyophilization) Procedures
To preinoculate the bacterial cells on the devices using the freeze-drying method, 150 μL of the S. oneidensis MR1 inoculum was pipetted onto the anodic reservoirs. The inoculated devices were then frozen at −80 °C and placed in a freeze-dryer (FreeZone Plus 2.5 Liter Cascade Benchtop Freeze Dry System, Labconco, USA) to undergo the lyophilization process for 12 h at 0.025 mbar pressure. After the process was complete, the devices were sealed in Ziploc bags and stored under different conditions: in the desiccator, at 4, and at −20 °C.
2.4. Test Setup and Analysis
The electric potentials between the anodes and cathodes were measured using a data acquisition system (NI, USB-6212) and were recorded via the customized LabView interface. An external resistor was used to close the circuit between the anode and the cathode. The current and power generations were calculated via Ohm’s law and Joule’s law, respectively. The current and power densities were normalized to the anode area. To assess the sensitivity of the devices, the changes in the current with different formaldehyde concentrations were calculated as the IR according to the following equation17
| 1 |
where Inor is current density when the MFC sensor is fed with normal water, and Itox is the current density when the sensor is fed with a toxic solution.
To test the MFC sensors’ response to toxins, formaldehyde was used as a model toxin as it has been widely used for testing in other MFC sensors.7,15,32 The EPA (U.S. Environmental Protection Agency) has set the threshold limit of formaldehyde in water to be 10 ppm (equal to 0.001% v/v).32 Therefore, to demonstrate the toxic events, different concentrations of formaldehyde (0.001, 0.01, and 0.02% v/v) were prepared in LB media and were tested with our paper-based MFC sensors. All experiments were performed in triplicate, and the error bars represent standard deviations of the tests.
2.5. Bacterial Fixation and Scanning Electron Microscopy Imaging
The freeze-dried MFC sensors were disassembled, and the anodes were immersed in 5% glutaraldehyde for 2 h at 4 °C for bacterial fixation. Then, the samples were dehydrated by serial 10 min transfers through 35, 50, 75, 95, and 100% ethanol, followed by another 10 min in hexamethyldisilazane. The 95 and 100% ethanol steps were performed twice. The samples were then air-dried overnight and sputter-coated with carbon. The samples were examined with a field emission scanning electron microscopy (SEM; Supra 55 VP, Zeiss).
3. Results and Discussion
3.1. Device Fabrication and Operation
Typically, MFC is composed of an anode, cathode, and a PEM. In the anodic chamber, exoelectrogens oxidize and breakdown the organic matter, producing electrons and protons. The electrons are then transferred to the anode and move through an external circuit to the cathode to form a current, while the protons are transferred to the cathodic chamber through the PEM. At the cathode, electrons and protons recombine to reduce an oxidant, forming water.12,33
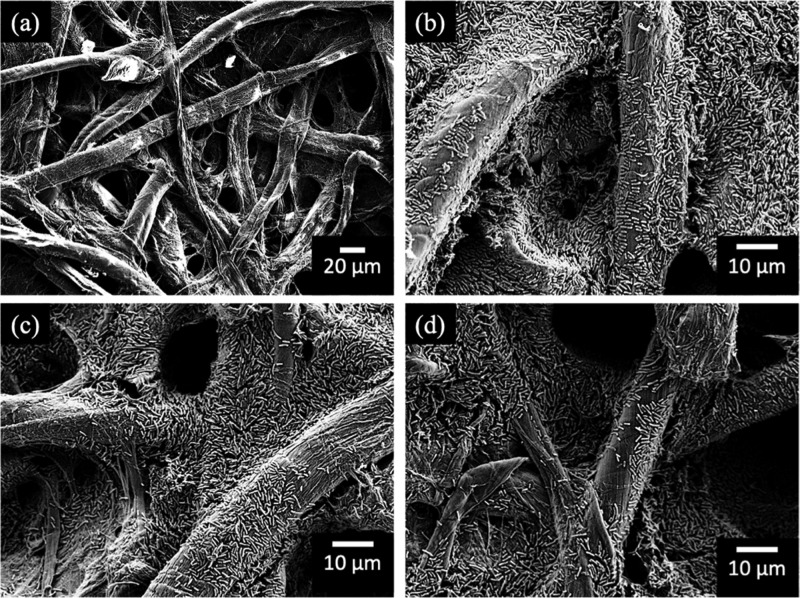
In this work, a paper-based MFC sensor was fabricated using two functional layers, the anode and the cathode. Paper was chosen as the platform for the device because on top of its low cost, flexibility, and disposability, papers offer microporous structures with large surface area to support internal bacterial biofilm growth and hydrophilic properties enabling easy liquid introduction via capillary action.30 However, paper by itself is nonconductive, making stable and consistent electron transfers a challenge. Recently, the Whiteside group developed a novel method that could cofabricate electronic and microfluidic properties on the paper using a water-dispersed conducting polymer mixture, PEDOT:PSS.34 Taking this idea forward, our group has previously demonstrated the practicability of using this technique to develop a conductive anodic reservoir for bacterial electron transfers.30 To improve the polymer’s conductivity and hydrophilicity, 5% DMSO and 2% GLYMO were added, respectively. On adding and drying of this conductive ink on the anodic reservoir, a conductive reservoir was formed without blocking the pores of the paper while also maintaining the hydrophilicity (Figure 3a). Once the bacterial inoculum was introduced to the reservoir, the liquid was quickly absorbed through the paper via capillary action, and within 30 min, very densely packed bacterial cells could be observed on the anodic reservoir (Figure 3b).
Figure 3.
SEM images of the anode reservoirs under different conditions: (a) PEDOT:PSS coated anode, (b) bacterial inoculation before freeze-drying, (c) right after freeze-drying, and (d) 2 weeks after freeze-drying.
In MFCs, the cathode is an important factor limiting the overall device performance as electron acceptors are used at the cathode for the reduction process. Previously, electron acceptors, such as potassium ferricyanide and potassium permanganate, have often been used, but these chemicals are not suitable for disposable applications because of their toxicity and cost. Recently, air cathodes and solid-state cathodes have been gaining popularity because of their sustainable and environmentally friendly properties.35 In this work, a solid-state electron acceptor, Ag2O, was used on the cathode because our previous studies have shown that compared to the air cathode, Ag2O cathode generated greater power performance and more compact, versatile device designs. As a result, the electrons and protons generated by the exoelectrogens in the anode travelled to the cathode to reduce Ag2O to Ag and to form water.30
Finally, to improve the electricity generation and sensitivity of the MFC sensor, 10 μM of riboflavin was added to the bacterial inoculum right before freeze-drying. Typically, S. oneidensis MR-1 conduct extracellular electron transfer via two mechanisms. The first mechanism is by direct contact where the cells are physically attached to the anode surface, and the second is via indirect shuttles that transfer electrons to the anode. However, our works have shown that during the centrifugation process to collect the bacterial cells, all the shuttling chemical compounds were likely removed.21,36 But from the earlier findings, it is well-known that one of the main extracellular electron shuttles for S. oneidensis MR-1 is riboflavin, and the supplementation of this shuttling compound have shown to decrease the internal resistance and enhance the power/current output.31,37 Therefore, to increase the performance of our MFC sensor, riboflavin was added to the bacterial inoculum before freeze-drying.
3.2. Freeze-Drying
To construct a portable, in situ water toxicity sensor for on-demand applications, this work, for the first time, used freeze-dried exoelectrogens on paper as sensing microorganisms. Freeze-drying is one of the most commonly used methods for preservation of biological samples, allowing convenient storage and transport. During the freeze-drying process, the samples are rapidly frozen, usually at −80 °C, and the frozen water is subsequently removed by sublimation of ice under vacuum. This dehydration technique allows for minimal shrinkage of the cells and results in a completely soluble product that is easily rehydrated.29,38 While extensive studies on freeze-drying different proteins and microorganisms have been done previously,27,28 our group first demonstrated the idea of freeze-drying exoelectrogens to evaluate their electron transfer activities after rehydration.29 In this work, freeze-dried exoelectrogens were furthered evaluated to see their sensitivity to toxins in water.
To ensure maximum bacterial cell survival during freeze-drying, the amounts and sizes of ice crystals need to be kept minimum during the initial freezing process, which is usually accomplished by the use of cryoprotectants. A good cryoprotectant should be able to provide protection to the cells during freezing, be easily dried, and provide ease of rehydration.39 Regarding the cryoprotectant, several studies have shown that sugars such as trehalose and sucrose can increase the tolerance to desiccation in numerous bacterial cells due to the stabilization of membranes and proteins.27,40 As such, we chose 10% sucrose as the cryoprotectant as it is one of the most commonly used protectants during freeze-drying. The bacterial cells were prepared in LB medium containing 10% sucrose and were preloaded on the anodic reservoirs (Figure 3b). Then, the devices were freeze-dried, and the number of bacterial cells still intact on the anode were observed through SEM images. Figure 3c shows the image of bacterial cells taken right after freeze-drying, and Figure 3d shows the image after two weeks of freeze-drying. Both images were comparable to the nonfreeze-dried cells in Figure 3b, showing densely packed bacterial cells spread out on paper fibers.
3.3. MFC Sensor Performance and Toxicity Detection
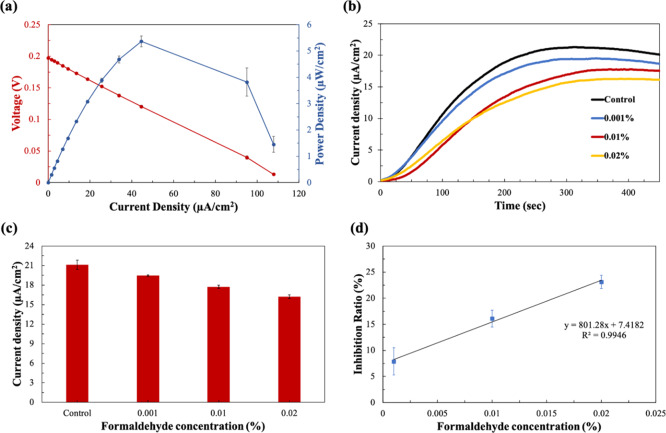
After freeze-drying the bacterial cells on the MFC sensor, the polarization curve and power output were derived and calculated from the saturated current values at different external resistors (Figure 4a). The polarization curves can provide important information on the major electrochemical losses in the device, including the activation loss, ohmic loss, and the mass transfer loss. From the graph, we were able to estimate the internal resistance of the device from the ohmic loss region, as it is in good agreement that the internal resistance of a device corresponds to the external resistor value, where the maximum power density is obtained.41 For our devices, a maximum power density of 5.36 μW/cm2 was obtained from a 10 kΩ external resistor. Therefore, 10 kΩ was chosen as the optimal external load for all other experiments.
Figure 4.
(a) Polarization curve and power output of the MFC sensor calculated from the saturated current outputs at different external resistors; (b) current responses to the formaldehyde solutions as a function of time under 10 kΩ; (c) current densities of different formaldehyde solutions at 6 min; and (d) calibration curve of the MFC sensor showing IR (%) versus formaldehyde concentration (%).
The freeze-dried MFC sensors were then subjected to toxicity testing using formaldehyde as a model toxin. A volume of 150 μL of sample solutions containing different concentrations of formaldehyde (control, 0.001, 0.01, and 0.02%) were directly dropped onto the anodic reservoir where the bacterial cells were pre-inoculated. As soon as the solution was introduced, the liquid quickly spread around the anode, rehydrating the bacterial cells, and generating current within seconds. Rapid current responses were detected over a concentration range of 0–0.02%, and in just about 360 s (6 min), the current densities of the MFC sensors reached the maximum value (Figure 4b). Our MFC sensor using freeze-dried bacteria showed a clear decrease in current over time with increasing formaldehyde concentration. Then, to limit any variations among the devices, current readings at 6 min were used to compare the toxicity of each formaldehyde concentrations. For the control sample that had no formaldehyde, the current reading was 21.12 μA/cm2, and for the formaldehyde concentrations 0.001, 0.01, and 0.02%, the current densities were 19.45, 17.72, and 16.23 μA/cm2, respectively (Figure 4c). The repeated three measurements from each formaldehyde concentrations had a relative standard deviation of less than 3.4%. To better understand the inhibitory effects of the toxin to the bacterial cells compared to the control, these current readings were calculated as the IR according to eq 1. Compared to the control, the IR for 0.001, 0.01, and 0.02% formaldehyde were 7.88, 16.08, and 23.14%, respectively. When these IR and formaldehyde concentration were compared, the calibration curve showed a very good linear relationship between 0.001 and 0.02%, with the R2 value of 0.9946 (Figure 4d). Our results have shown that the freeze-dried exoelectrogens are still metabolically active after rehydration, and they were very sensitive to formaldehyde in water. Therefore, when water with unknown toxicity needs to be analyzed, the corresponding formaldehyde equivalent concentration can be assessed from the IR obtained with the water. Although it is nonspecific, the sensor could be used as a rapid and on-site early warning system for toxic substances. Additionally, our paper-based MFC sensors utilizing freeze-dried bacteria have shown significant improvements compared to our previous works in terms of both sensitivity and portability in that these MFC sensors do not require external pumps, can be stored for long-term with ease of transport, and can detect formaldehyde concentrations as low as 0.001%.15,26
3.4. Storage Conditions and Long-Term Stability
In addition to the type of cryoprotectant being used, there are several other factors that determine the viability of the bacterial cells during and after the freeze-drying process, including the freezing temperature, the rehydration condition, and the storage condition. Especially for long-term storage, if the optimal conditions are met, then the freeze-dried samples can be kept for months and years without losing their activity or viability.42 However, one of the challenges freeze-dried samples face is that they are very sensitive to moisture which can lead to the loss of viability during storage.25,38 To investigate how the different storage conditions affect the MFC sensor’s performance over time, freeze-dried MFC sensors were sealed in Ziploc bags to prevent oxygen intrusion as much as possible and were stored under three different conditions: in the desiccator, at 4, and at −20 °C.
To test the long-term stability of our paper-based MFC sensors after freeze-drying, their performance after 7 and 14 days was measured and compared to that of a fresh freeze-dried sensor. Figure 5 shows the performance outputs (%) of the long-term stored devices. After 7 days, the performance levels of the devices from different storage conditions varied a little with the devices stored in the desiccator, at 4, and at −20 °C giving 98.43, 99.55, and 97.03% performance outputs, respectively. After 14 days, the performance outputs decreased very slightly again to 96.66, 96.58, and 96.86% for devices in the desiccator, at 4, and at −20 °C, respectively. Even after two weeks of storage, our MFC sensors showed very good performance under all the conditions, showing promise as a portable biosensor with long shelf life. Among the different conditions, the devices stored at −20 °C showed the least variation after 7 and 14 days. This may be due to the humidity available in the air, which decreases as the surrounding temperature gets lower.25 As such, the optimal temperature for storing freeze-dried MFC sensors might be at −20 °C. Our results were in consistence with other studies that have also stored freeze-dried bacteria at −20 °C for several months to years without losing much viability.38,40
Figure 5.
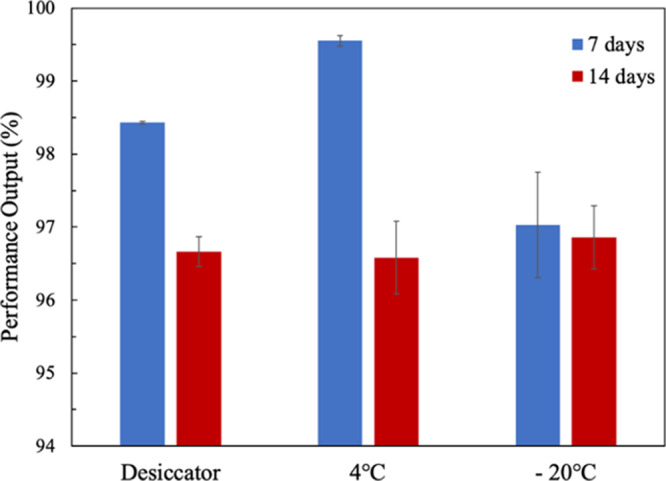
Long-term stability of freeze-dried MFC sensors under different storage conditions. The performance outputs were measured after 7 and 14 days.
3.5. Disposability
One of the important requirements for single-use devices is the disposability. This is more emphasized in portable, single-use MFC sensors because if they are not safely and properly disposed of after use, the fabrication materials and microorganisms may potentially contaminate the environment or pose a risk of bacterial infection. Unlike many of the conventional plastic-based devices, one of the advantages of using paper as a platform is its easily disposable nature.43 Post use, paper-based MFC sensors can be easily disposed of by simply incinerating the device. Figure 6 shows the simple process of burning the MFC sensor with a flame, which took only about 15 s for the device to completely burn.
Figure 6.
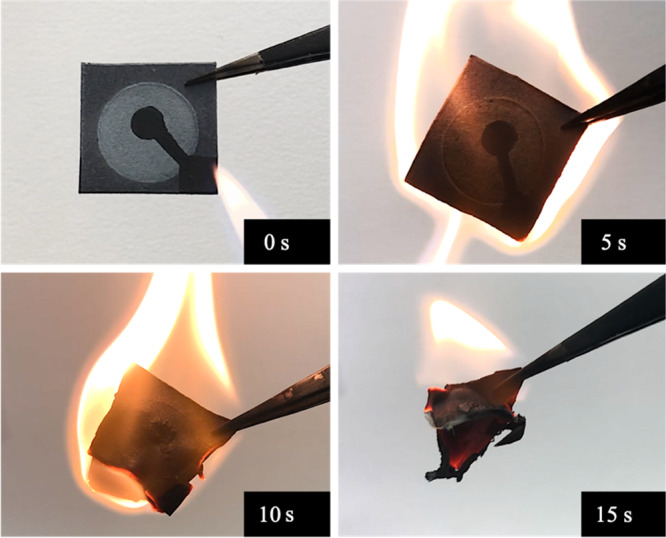
Disposal of the paper-based MFC sensor by incineration. The whole process took about 15 s.
4. Conclusions
In this work, we demonstrated a novel paper-based MFC sensor utilizing freeze-dried bacteria for in situ water quality monitoring. The MFC sensor was made entirely of paper coated with PEDOT:PSS to make the reservoirs conductive. S. oneidensis MR-1 were then pre-inoculated on the MFC sensor, and they were freeze-dried, making their portable, on-site, and on-demand applications possible. Our sensor confirmed that these exoelectrogens still maintain active metabolism even after rehydration, showing sensitivity to formaldehyde in water. The MFC sensors also showed good shelf life, up to 2 weeks, when they were stored at −20 °C. Future studies looking at longer shelf life and the ability to store these sensors at ambient temperatures without losing cell activity/viability will make these paper-based MFC sensors with freeze-dried bacterial cells an essential tool for water quality monitoring.
Acknowledgments
This work is supported by the Office of Naval Research (#N00014-81-1–2422), the National Science Foundation (ECCS #1703394 & ECCS #1920979), and the SUNY Binghamton Research Foundation (SE-TAE).
The authors declare no competing financial interest.
References
- Vikesland P. J. Nanosensors for Water Quality Monitoring. Nat. Nanotechnol. 2018, 13, 651–660. 10.1038/s41565-018-0209-9. [DOI] [PubMed] [Google Scholar]
- Yang W.; Wei X.; Fraiwan A.; Coogan C. G.; Lee H.; Choi S. Fast and Sensitive Water Quality Assessment: A Ml-Scale Microbial Fuel Cell-Based Biosensor Integrated with an Air-Bubble Trap and Electrochemical Sensing Functionality. Sens. Actuators, B 2016, 226, 191–195. 10.1016/j.snb.2015.12.002. [DOI] [Google Scholar]
- Yang Y.; Wang Y.-Z.; Fang Z.; Yu Y.-Y.; Yong Y.-C. Bioelectrochemical Biosensor for Water Toxicity Detection: Generation of Dual Signals for Electrochemical Assay Confirmation. Anal. Bioanal. Chem. 2018, 410, 1231–1236. 10.1007/s00216-017-0656-4. [DOI] [PubMed] [Google Scholar]
- Scognamiglio V.; Antonacci A.; Arduini F.; Moscone D.; Campos E. V. R.; Fraceto L. F.; Palleschi G. An Eco-Designed Paper-Based Algal Biosensor for Nanoformulated Herbicide Optical Detection. J. Hazard. Mater. 2019, 373, 483–492. 10.1016/j.jhazmat.2019.03.082. [DOI] [PubMed] [Google Scholar]
- Hunter P. R.; Zmirou-Navier D.; Hartemann P. Estimating the Impact on Health of Poor Reliability of Drinking Water Interventions in Developing Countries. Sci. Total Environ. 2009, 407, 2621–2624. 10.1016/j.scitotenv.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Cui Y.; Lai B.; Tang X. Microbial Fuel Cell-Based Biosensors. Biosensors 2019, 9, 92. 10.3390/bios9030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Gao N.; Zhou Q. Concentration Responses of Toxicity Sensor with Shewanella Oneidensis MR-1 Growing in Bioelectrochemical Systems. Biosens. Bioelectron. 2013, 43, 264–267. 10.1016/j.bios.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Xiao Y.; De Araujo C.; Sze C. C.; Stuckey D. C. Toxicity Measurement in Biological Wastewater Treatment Processes: A Review. J. Hazard. Mater. 2015, 286, 15–29. 10.1016/j.jhazmat.2014.12.033. [DOI] [PubMed] [Google Scholar]
- Hung K. W. Y.; Suen M. F. K.; Chen Y. F.; Cai H. B.; Mo Z. X.; Yung K. K. L. Detection of Water Toxicity Using Cytochrome P450 Transgenic Zebrafish as Live Biosensor: For Polychlorinated Biphenyls Toxicity. Biosens. Bioelectron. 2012, 31, 548–553. 10.1016/j.bios.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Bae M.-J.; Park Y.-S. Biological Early Warning System Based on the Responses of Aquatic Organisms to Disturbances: A Review. Sci. Total Environ. 2014, 466–467, 635–649. 10.1016/j.scitotenv.2013.07.075. [DOI] [PubMed] [Google Scholar]
- Chouler J.; Cruz-Izquierdo Á.; Rengaraj S.; Scott J. L.; Di Lorenzo M. A Screen-Printed Paper Microbial Fuel Cell Biosensor for Detection of Toxic Compounds in Water. Biosens. Bioelectron. 2018, 102, 49–56. 10.1016/j.bios.2017.11.018. [DOI] [PubMed] [Google Scholar]
- Dávila D.; Esquivel J. P.; Sabaté N.; Mas J. Silicon-Based Microfabricated Microbial Fuel Cell Toxicity Sensor. Biosens. Bioelectron. 2011, 26, 2426–2430. 10.1016/j.bios.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Stein N. E.; Hamelers H. V. M.; Buisman C. N. J. Stabilizing the Baseline Current of a Microbial Fuel Cell-Based Biosensor through Overpotential Control under Non-Toxic Conditions. Bioelectrochemistry 2010, 78, 87–91. 10.1016/j.bioelechem.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Jiang Y.; Liang P.; Zhang C.; Bian Y.; Yang X.; Huang X.; Girguis P. R. Enhancing the Response of Microbial Fuel Cell Based Toxicity Sensors to Cu(II) with the Applying of Flow-through Electrodes and Controlled Anode Potentials. Bioresour. Technol. 2015, 190, 367–372. 10.1016/j.biortech.2015.04.127. [DOI] [PubMed] [Google Scholar]
- Yang W.; Wei X.; Choi S. A Dual-Channel, Interference-Free, Bacteria-Based Biosensor for Highly Sensitive Water Quality Monitoring. IEEE Sens. J. 2016, 16, 8672–8677. 10.1109/JSEN.2016.2570423. [DOI] [Google Scholar]
- Stein N. E.; Hamelers H. V. M.; Buisman C. N. J. Influence of Membrane Type, Current and Potential on the Response to Chemical Toxicants of a Microbial Fuel Cell Based Biosensor. Sens. Actuators, B 2012, 163, 1–7. 10.1016/j.snb.2011.10.060. [DOI] [Google Scholar]
- Jiang Y.; Yang X.; Liang P.; Liu P.; Huang X. Microbial Fuel Cell Sensors for Water Quality Early Warning Systems: Fundamentals, Signal Resolution, Optimization and Future Challenges. Renew. Sustain. Energy Rev. 2018, 81, 292–305. 10.1016/j.rser.2017.06.099. [DOI] [Google Scholar]
- Gao Y.; Choi S. Stepping Toward Self-Powered Papertronics: Integrating Biobatteries into a Single Sheet of Paper. Adv. Mater. Technol. 2017, 2, 1600194. 10.1002/admt.201600194. [DOI] [Google Scholar]
- Nguyen T. H.; Fraiwan A.; Choi S. Paper-Based Batteries: A Review. Biosens. Bioelectron. 2014, 54, 640–649. 10.1016/j.bios.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Winfield J.; Chambers L. D.; Rossiter J.; Greenman J.; Ieropoulos I. Urine-Activated Origami Microbial Fuel Cells to Signal Proof of Life. J. Mater. Chem. A 2015, 3, 7058–7065. 10.1039/c5ta00687b. [DOI] [Google Scholar]
- Fraiwan A.; Choi S. A stackable, two-chambered, paper-based microbial fuel cell. Biosens. Bioelectron. 2016, 83, 27–32. 10.1016/j.bios.2016.04.025. [DOI] [PubMed] [Google Scholar]
- Lee H.; Choi S. An Origami Paper-Based Bacteria-Powered Battery. Nano Energy 2015, 15, 549–557. 10.1016/j.nanoen.2015.05.019. [DOI] [Google Scholar]
- Fraiwan A.; Kwan L.; Choi S. A Disposable Power Source in Resource-Limited Environments: A Paper-Based Biobattery Generating Electricity from Wastewater. Biosens. Bioelectron. 2016, 85, 190–197. 10.1016/j.bios.2016.05.022. [DOI] [PubMed] [Google Scholar]
- Choi S.; Gu M. B. A Portable Toxicity Biosensor Using Freeze-Dried Recombinant Bioluminescent Bacteria. Biosens. Bioelectron. 2002, 17, 433–440. 10.1016/S0956-5663(01)00303-7. [DOI] [PubMed] [Google Scholar]
- Bock Gu M.; Hyung Choi S.; Woo Kim S. Some Observations in Freeze-Drying of Recombinant Bioluminescent Escherichia Coli for Toxicity Monitoring. J. Biotechnol. 2001, 88, 95–105. 10.1016/S0168-1656(01)00268-1. [DOI] [PubMed] [Google Scholar]
- Cho J. H.; Gao Y.; Choi S.. A Portable, Single-Use, Paper-Based Microbial Fuel Cell Sensor for Rapid, on-Site Water Quality Monitoring. Sens. Switz. 2019, 19 (). DOI: 10.3390/s19245452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie S. B.; Israeli E.; Lighthart B.; Crowe J. H.; Crowe L. M. Trehalose and Sucrose Protect Both Membranes and Proteins in Intact Bacteria during Drying. Appl. Environ. Microbiol. 1995, 61, 3592–3597. 10.1128/aem.61.10.3592-3597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto-Shinohara Y.; Sukenobe J.; Imaizumi T.; Nakahara T. Survival of Freeze-Dried Bacteria. J. Gen. Appl. Microbiol. 2008, 54, 9–24. 10.2323/jgam.54.9. [DOI] [PubMed] [Google Scholar]
- Mohammadifar M.; Choi S. A Papertronic, On-Demand and Disposable Biobattery: Saliva-Activated Electricity Generation from Lyophilized Exoelectrogens Preinoculated on Paper. Adv. Mater. Technol. 2017, 2, 1700127. 10.1002/admt.201700127. [DOI] [Google Scholar]
- Gao Y.; Choi S. Merging Electric Bacteria with Paper. Adv. Mater. Technol. 2018, 3, 1800118. 10.1002/admt.201800118. [DOI] [Google Scholar]
- Covington E. D.; Gelbmann C. B.; Kotloski N. J.; Gralnick J. A. An Essential Role for UshA in Processing of Extracellular Flavin Electron Shuttles by Shewanella Oneidensis. Mol. Microbiol. 2010, 78, 519–532. 10.1111/j.1365-2958.2010.07353.x. [DOI] [PubMed] [Google Scholar]
- Chouler J.; Monti M. D.; Morgan W. J.; Cameron P. J.; Di Lorenzo M. A Photosynthetic Toxicity Biosensor for Water. Electrochim. Acta 2019, 309, 392–401. 10.1016/j.electacta.2019.04.061. [DOI] [Google Scholar]
- Chen Y.; Ji W.; Yan K.; Gao J.; Zhang J. Fuel Cell-Based Self-Powered Electrochemical Sensors for Biochemical Detection. Nano Energy 2019, 61, 173–193. 10.1016/j.nanoen.2019.04.056. [DOI] [Google Scholar]
- Hamedi M. M.; Ainla A.; Güder F.; Christodouleas D. C.; Fernández-Abedul M. T.; Whitesides G. M. Integrating Electronics and Microfluidics on Paper. Adv. Mater. 2016, 28, 5054–5063. 10.1002/adma.201505823. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Mohammadifar M.; Choi S. From Microbial Fuel Cells to Biobatteries: Moving toward On-Demand Micropower Generation for Small-Scale Single-Use Applications. Adv. Mater. Technol. 2019, 4, 1900079. 10.1002/admt.201900079. [DOI] [Google Scholar]
- Mohammadifar M.; Choi S. A Solid Phase Bacteria-Powered Biobattery for Low-Power, Low-Cost, Internet of Disposable Things. J. Power Sources 2019, 429, 105–110. 10.1016/j.jpowsour.2019.05.009. [DOI] [Google Scholar]
- Liu Y.; Ding M.; Ling W.; Yang Y.; Zhou X.; Li B.-Z.; Chen T.; Nie Y.; Wang M.; Zeng B.; Li X.; Liu H.; Sun B.; Xu H.; Zhang J.; Jiao Y.; Hou Y.; Yang H.; Xiao S.; Lin Q.; He X.; Liao W.; Jin Z.; Xie Y.; Zhang B.; Li T.; Lu X.; Li J.; Zhang F.; Wu X.-L.; Song H.; Yuan Y.-J. A Three-Species Microbial Consortium for Power Generation. Energy Environ. Sci. 2017, 10, 1600–1609. 10.1039/c6ee03705d. [DOI] [Google Scholar]
- Bjerketorp J.; Håkansson S.; Belkin S.; Jansson J. K. Advances in Preservation Methods: Keeping Biosensor Microorganisms Alive and Active. Curr. Opin. Biotechnol. 2006, 17, 43–49. 10.1016/j.copbio.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Zhao G.; Zhang G. Effect of Protective Agents, Freezing Temperature, Rehydration Media on Viability of Malolactic Bacteria Subjected to Freeze-Drying. J. Appl. Microbiol. 2005, 99, 333–338. 10.1111/j.1365-2672.2005.02587.x. [DOI] [PubMed] [Google Scholar]
- Martín-Betancor K.; Durand M.-J.; Thouand G.; Leganés F.; Fernández-Piñas F.; Rodea-Palomares I. Microplate Freeze-Dried Cyanobacterial Bioassay for Fresh-Waters Environmental Monitoring. Chemosphere 2017, 189, 373–381. 10.1016/j.chemosphere.2017.09.030. [DOI] [PubMed] [Google Scholar]
- Fan Y.; Sharbrough E.; Liu H. Quantification of the Internal Resistance Distribution of Microbial Fuel Cells. Environ. Sci. Technol. 2008, 42, 8101–8107. 10.1021/es801229j. [DOI] [PubMed] [Google Scholar]
- Shin H. J. Genetically Engineered Microbial Biosensors for in Situ Monitoring of Environmental Pollution. Appl. Microbiol. Biotechnol. 2011, 89, 867–877. 10.1007/s00253-010-2990-8. [DOI] [PubMed] [Google Scholar]
- Tahernia M.; Mohammadifar M.; Hassett D. J.; Choi S. A Fully Disposable 64-Well Papertronic Sensing Array for Screening Electroactive Microorganisms. Nano Energy 2019, 65, 104026. 10.1016/j.nanoen.2019.104026. [DOI] [Google Scholar]