A 50-year-old African American male never-smoker was diagnosed with stage IV non–small-cell lung cancer (NSCLC) in November 2007. His baseline computed tomography (CT) demonstrated bilateral lung nodules and biopsy confirmed a poorly differentiated tumor favoring large-cell carcinoma staining positive for cytokeratin 7 and thyroid transcription factor-1. He was originally treated with eight cycles of standard first-line chemotherapy consisting of carboplatin, paclitaxel, and bevacizumab from January 2008 to May 2008, achieving a partial response. Unfortunately, disease progression was documented in June 2008, and the patient commenced second-line erlotinib. Tumor analysis at that time demonstrated immunohistochemistry (IHC) +3 for epidermal growth factor receptor (EGFR) protein overexpression and fluorescent in situ hybridization (FISH) revealed a nonamplified but high polysomy count of ≥ 4 EGFR copies in ≥ 40% of cells (Fig 1). Erlotinib was discontinued in July 2008 due to disease progression after only 6 weeks of therapy.
Fig 1.
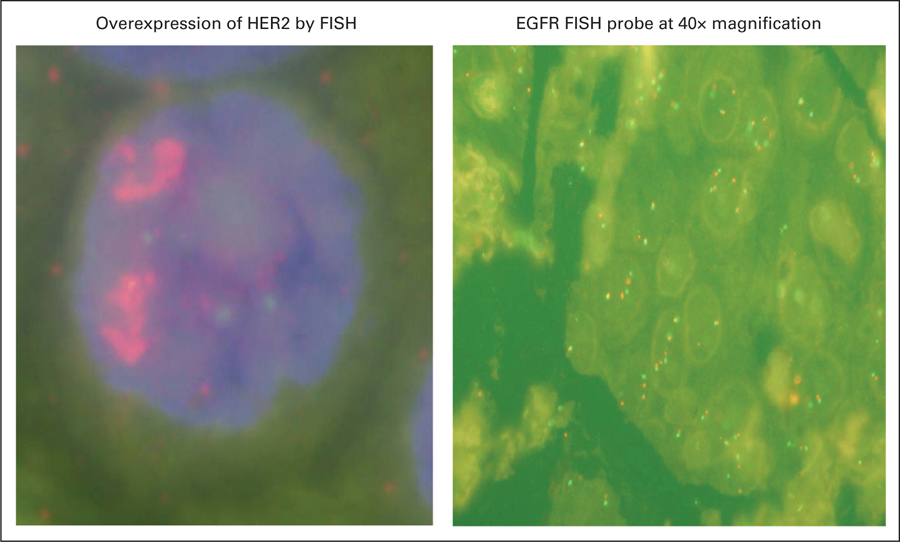
He presented to the National Cancer Institute in October 2008 for enrollment in a clinical trial involving a second generation irreversible pan–human epidermal growth factor receptor (HER) tyrosine kinase inhibitor (TKI; PF-00299804). He met all the eligibility criteria and had an excellent Eastern Cooperative Oncology Group (ECOG) performance status of 1. Molecular analysis revealed a K-Ras wild type, and HER2 (IHC +2 and FISH)–positive tumor (Table 1, Fig 1). No EGFR or HER2 mutations were detected. He was commenced on PF-00299804 in December 2008 and had a partial response (70% measurable response on CT scan) after 4 weeks of 45 mg orally once daily with 21 days per cycle (Fig 2). Of particular interest was a notable reduction in the patient’s soluble extracellular domain HER2 levels (Fig 3). The patient subsequently progressed after five cycles of PF-00299804 and was taken off study in April 2009. Radiological progression also correlated with a rise in serum HER2 levels (Fig 3).
Table 1.
Molecular Profiling of the Patient’s Tumor
| Profile | EGFR | HER2/neu | KRAS |
|---|---|---|---|
| IHC | +3 | +2 | |
| Increase in DNA copy number | High polysomy (≥ 4 EGFR copies in ≥ 40% of cells) | ||
| FISH | Not amplified | Amplified (6.1) | |
| Mutation (EGFR/HER2) | Wild type | Wild type | |
| KRAS mutation | Wild type |
Abbreviations: IHC, immunohistochemistry; EGFR, epidermal growth factor receptor; FISH, fluorescent in situ hybridization; HER2, human epidermal growth factor receptor 2.
Fig 2.
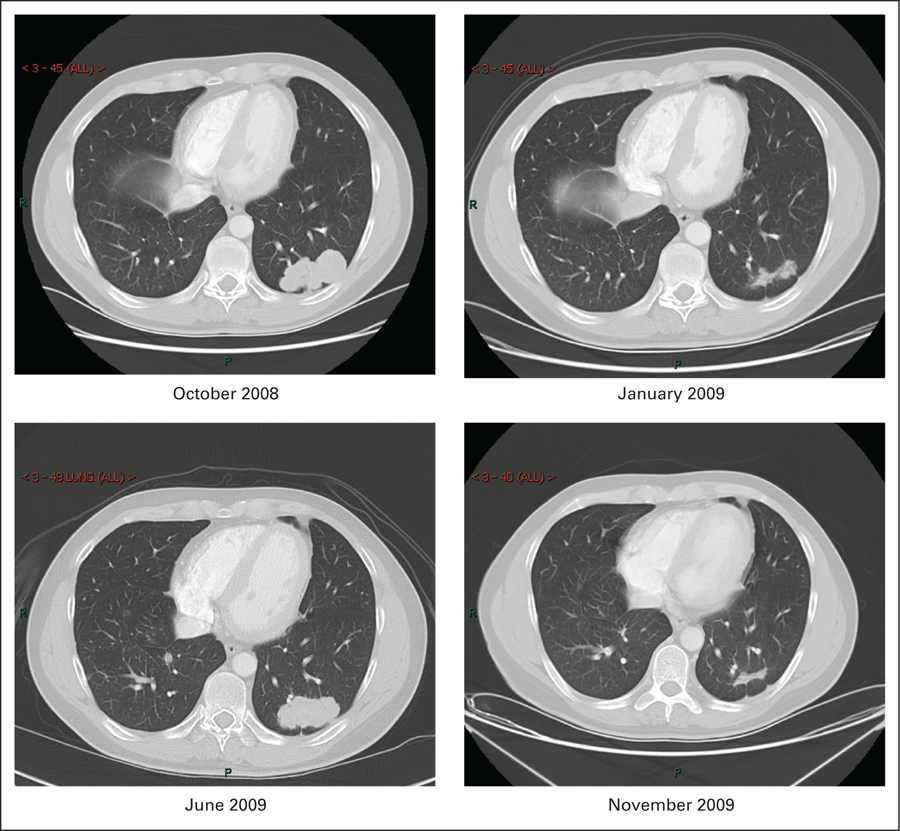
Fig 3.
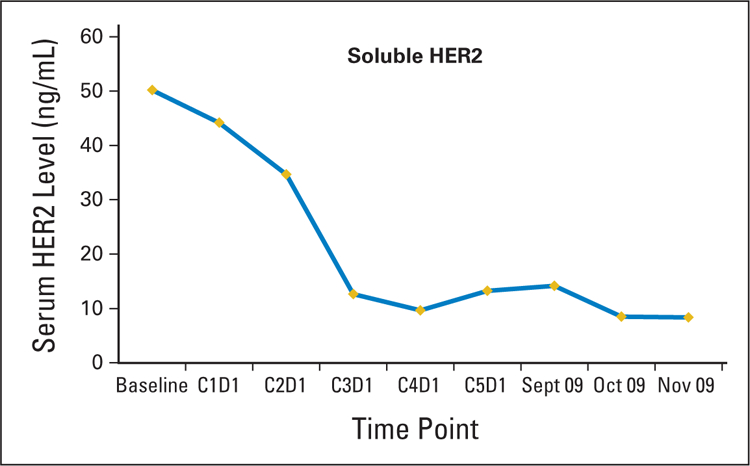
Based on the tumors molecular pattern (Table 1) and his excellent performance status the patient was started on fourth-line single agent trastuzumab in June 2009. After 6 weeks of weekly therapy, vinorelbine was added to trastuzumab (August 2009) after radiological progression on the targeted agent. After an additional 6 weeks of vinorelbine/trastuzumab, the patient developed a second partial response of approximately 70%, and a subsequent decline in serum HER2 levels was documented (Figs 2 and 3). Currently he continues to have disease response on every 3 weeks trastuzumab and weekly vinorelbine and is being followed expectantly.
HER2 receptor expression is detectable by IHC in approximately 30% of patients with untreated NSCLC.1,2 IHC staining for HER2 is scored as 1+ in 20%, 2+ in 15%, and 3+ in 5% of patients with NSCLC.1,3,4 Gene amplification detected by FISH and IHC 3+ staining is present in only 2% to 5% of NSCLC.5 Positivity for HER2 varies according to histology, with the highest frequency seen in adenocarcinomas (17% to 42%), followed by large-cell carcinomas (2% to 40%), and a low frequency in squamous cell carcinomas (0% to 5%).6
Trastuzumab, the humanized monoclonal antibody developed against HER2, has been tested as a single agent and in combination with cytotoxic chemotherapy in patients with NSCLC.1,3,7–9 A phase II study, ECOG 2598, evaluated carboplatin, paclitaxel, and trastuzumab in HER2-positive (+1 to 3+ by IHC) patients with advanced lung cancer.1 Of 53 eligible patients, 85% were IHC +1/+2 and 15% were IHC +3. A second phase II trial in a similar patient population combined trastuzumab with gemcitabine and cisplatin.9 Unfortunately, both these trials failed to produce either an improved response rate or overall survival with the addition of trastuzumab to these commonly used platinum-based doublets. Subset analyses did demonstrate a trend towards a higher response rate in HER2 FISH–positive or IHC +3 patients. Pertuzumab is a HER2 dimerization inhibitor preventing homodimerization and heterodimerization of HER2 with other ErbB family members. A phase II study investigated pertuzumab as single agent in previously treated patients with locally advanced or metastatic NSCLC.10 No responses were seen in the 43 patients that were treated. Lapatinib, an oral reversible small molecule inhibitor of EGFR and HER2, has been tested in a phase II trial in patients with advanced or metastatic NSCLC with either bronchioloalveolar carcinoma or a never-smoking history. In total, 131 patients were randomly assigned, and limited activity was reported with a 2% partial response and 20% stable disease rate.11 A phase I study combined lapatinib with pemetrexed in the second-line setting for advanced NSCLC.12 Preliminary reports suggest promising activity. Ultimately, however, formal phase III randomized testing with preselection requirements limiting enrollment to 3+/FISH–positive patients are required to perform a critical assessment of the role of HER2-targeted agents in the treatment of advanced NSCLC.
Lung cancers that coexpress both EGFR and HER2 appear to have a more virulent behavior due to increased signaling potential.13 HER2 is the preferred partner for all of the HER family members, including EGFR.14 High synchronous coexpression of EGFR and HER2 is associated with an unfavorable prognosis in patients from early-stage to advanced-stage NSCLC.15,16 EGFR-HER2 heterodimers are associated with a stronger and more sustained proliferative signal of the EGFR tyrosine kinase than EGFR homodimerization, resulting in a more aggressive phenotype.15 HER2 gene amplification may improve tumor response to the first-generation TKIs with one study demonstrating a higher response rate to gefitinib in patients with EGFR mutations and increased HER2 copy number than in those without HER2 overexpression (response rate 87.5% v 14.2%, P = .01).17
Ultimately, agents that target the coexpression of both EGFR and HER2 may result in improved outcomes. Interestingly, the patient in this case report had a dramatic response to the second-generation, quinazalone-based irreversible pan-HER family TKI (PF-00299804) but showed no response to erlotinib. The patients tumor did overexpress EGFR by DNA copy number and HER2 (FISH+) but no mutations were detected (Table 1). Overexpression of HER2 has been proven to be an independent unfavorable prognostic factor in resected NSCLC.18 In NSCLC, somatic mutations in HER2 (2% to 3%) or an amplification of wild-type HER 2 may be associated with resistance to the first generation TKIs by maintaining phosphorylation of EGFR, HER3, and Akt.19 The majority of HER2 mutations in lung cancer, as per EGFR-activating mutations, occur predominately in adenocarcinomas of female Asian never smokers.20 These mutations may circumvent EGFR-mediated signaling in NSCLC. PF-00299804 has demonstrated activity both in the presence of EGFR activating mutations and inactivating resistance mutations (T790M or exon 20 insertions) in preclinical and clinical studies.19,21,22 PF-00299804 also effectively inhibits HER2-mutant and NSCLC cell lines that harbor amplifications of wild-type HER2 (as per this patient). PF-00299804 is currently being investigated in ongoing phase II and phase III clinical trials.
HER2 gene amplification and protein overexpression are routinely assessed in breast cancer by evaluating tumor tissue. Circulating levels of serum HER2 can also be used to evaluate HER2 status. HER2-bearing epithelial cells shed the extracellular domain of the protein that is cleaved from the receptor into the serum as a protein of approximately 105 kkDa and can be detected by enzyme-linked immunosorbent assays.23 Circulating HER2 protein is an independent prognostic factor in patients with advanced NSCLC.5 Serum HER2 extracellular domain concentrations in NSCLC range from 0 to 47.4 ng/mL.24 FISH-positive patients tend to have higher serum HER2 levels compared with IHC-positive patients.23
This case report highlights the importance of individualizing patient therapies in NSCLC. Treatment outcomes for advanced NSCLC have to date been limited by the empiric administration of cytotoxic chemotherapy. The move towards personalized medicine represents a paradigm shift in the management of NSCLC. Molecular profiling of tumors can establish effective therapies to combat advanced or recurrent disease. We now have a number of targeted agents that are either approved or are undergoing clinical testing which may ultimately lead to improved response rates and overall survival. The irreversible pan-HER family TKIs (eg, PF-00299804) may prove to be effective in lung cancers without an activating EGFR mutation or in tumors with coexpression of HER2 and EGFR. Blockade of both signaling pathways may ultimately yield superior results to single pathway inhibition. Indeed, preclinical studies in a wide variety of malignant cell types, including NSCLC, have shown that EGFR/HER2 inhibition can induce superior antitumor activity compared with single receptor targeting.18 Trials of trastuzumab and other HER2-targeted agents have to date failed to demonstrate clinical benefit in NSCLC either as monotherapy or in combination with chemotherapy. These studies have been criticized for using IHC analysis to assess HER2 status, a method that is not optimal. Trastuzumab may have a role in preselected NCSLC patients with HER2 gene amplification or activating mutations.25 HER2 genomic gain and mutation play an integral role in tumor cell survival in some lung cancers. Serial measurement of serum HER2 may provide predictive information to help guide therapeutic decisions in the treatment of a subset of patients with advanced NSCLC.
Footnotes
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
Contributor Information
Ronan J. Kelly, National Cancer Institute, Mark O. Hatfield Clinical Research Center, Bethesda, MD
Corey Carter, National Navy Medical Center, Bethesda, MD.
Giuseppe Giaccone, National Cancer Institute, Mark O. Hatfield Clinical Research Center, Bethesda, MD.
REFERENCES
- 1.Langer CJ, Stephenson P, Thor A, et al. : Trastuzumab in the treatment of advanced non–small-cell lung cancer: Is there a role? Focus on Eastern Cooperative Oncology Group study 2598. J Clin Oncol 22:1180–1187, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Johnson BE, Janne PA: Rationale for a phase II trial of pertuzumab, a HER-2 dimerization inhibitor, in patients with non–small-cell lung cancer. Clin Cancer Res 12:4436s–4440s, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Zinner RG, Glisson BS, Fossella FV, et al. : Trastuzumab in combination with cisplatin and gemcitabine in patients with Her2-overexpressing, untreated, advanced non–small-cell lung cancer: Report of a phase II trial and findings regarding optimal identification of patients with Her2-overexpressing disease. Lung Cancer 44:99–110, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Hirsch FR, Varella-Garcia M, Franklin WA, et al. : Evaluation of HER-2/neu gene amplification and protein expression in non–small-cell lung carcinomas. Br J Cancer 86:1449–1456, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinmoller P, Gross C, Beyser K, et al. : HER2 status in non–small-cell lung cancer: Results from patient screening for enrollment to a phase II study of herceptin. Clin Cancer Res 9:5238–5243, 2003 [PubMed] [Google Scholar]
- 6.Hirsch FR, Franklin WA, Veve R, et al. : HER2/neu expression in malignant lung tumors. Semin Oncol 29:51–58, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Clamon G, Herndon J, Kern J, et al. : Lack of trastuzumab activity in non–small-cell lung carcinoma with overexpression of erb-B2: 39810: A phase II trial of Cancer and Leukemia Group B. Cancer 103:1670–1675, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Krug LM, Miller VA, Patel J, et al. : Randomized phase II study of weekly docetaxel plus trastuzumab versus weekly paclitaxel plus trastuzumab in patients with previously untreated advanced non–small-cell lung carcinoma. Cancer 104:2149–2155, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Gatzemeier U, Groth G, Butts C, et al. : Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non–small-cell lung cancer. Ann Oncol 15:19–27, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Herbst RS, Davies AM, Natale RB, et al. : Efficacy and safety of single-agent pertuzumab, a human epidermal receptor dimerization inhibitor, in patients with non–small-cell lung cancer. Clin Cancer Res 13:6175–6181, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Smylie M, Blumenschein GR Jr, Dowlati A, et al. : A phase II multicenter trial comparing two schedules of lapatinib (LAP) as first or second line monotherapy in subjects with advanced or metastatic non–small-cell lung cancer (NSCLC) with either bronchioloalveolar carcinoma (BAC) or no smoking history. J Clin Oncol 25:7611, 2007. (suppl) [Google Scholar]
- 12.Ramlau R, Thomas M, Plummer R, et al. : Phase I study of lapatinib, a dual-tyrosine kinase inhibitor, and pemetrexed in the second-line treatment of advanced or metastatic non–small-cell lung cancer. J Clin Oncol 27:e19027, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Johnson DH, Arteaga CL: Gefitinib in recurrent non–small-cell lung cancer: An IDEAL trial? J Clin Oncol 21:2227–2229, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Graus-Porta D, Beerli RR, Daly JM, et al. : ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J 16:1647–1655, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brabender J, Danenberg KD, Metzger R, et al. : Epidermal growth factor receptor and HER2-neu mRNA expression in non–small-cell lung cancer Is correlated with survival. Clin Cancer Res 7:1850–1855, 2001 [PubMed] [Google Scholar]
- 16.Onn A, Correa AM, Gilcrease M, et al. : Synchronous overexpression of epidermal growth factor receptor and HER2-neu protein is a predictor of poor outcome in patients with stage I non–small-cell lung cancer. Clin Cancer Res 10:136–143, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Cappuzzo F, Varella-Garcia M, Shigematsu H, et al. : Increased HER2 gene copy number is associated with response to gefitinib therapy in epidermal growth factor receptor-positive non–small-cell lung cancer patients. J Clin Oncol 23: 5007–5018, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Nakamura H, Kawasaki N, Taguchi M, et al. : Association of HER-2 overexpression with prognosis in non–small-cell lung carcinoma: A metaanalysis. Cancer 103:1865–1873, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Engelman JA, Zejnullahu K, Gale CM, et al. : PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res 67:11924–11932, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Shigematsu H, Takahashi T, Nomura M, et al. : Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res 65:1642–1646, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Janne PA, Schellens JH, Engelman JA, et al. : Preliminary activity and safety results from a phase I clinical trial of PF-00299804, an irreversible pan-HER inhibitor, in patients (pts) with NSCLC. J Clin Oncol 26:8027, 2008 [Google Scholar]
- 22.Janne PA, Reckamp K, Koczywas M, et al. : Efficacy and safety of PF-00299804 (PF299) in patients (pt) with advanced NSCLC after failure of at least one prior chemotherapy regimen and prior treatment with erlotinib (E): A two-arm, phase II trial. J Clin Oncol 27:8063, 2009 [Google Scholar]
- 23.Papila C, Uzun H, Balci H, et al. : Clinical significance and prognostic value of serum sHER-2/neu levels in patients with solid tumors. Med Oncol 26:151–156, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Ardizzoni A, Cafferata MA, Paganuzzi M, et al. : Study of pretreatment serum levels of HER-2/neu oncoprotein as a prognostic and predictive factor in patients with advanced non–small-cell lung carcinoma. Cancer 92:1896–1904, 2001 [PubMed] [Google Scholar]
- 25.Cappuzzo F, Bemis L, Varella-Garcia M: HER2 mutation and response to trastuzumab therapy in non–small-cell lung cancer. N Engl J Med 354:2619–2621, 2006 [DOI] [PubMed] [Google Scholar]


