Abstract
The aim of this study was to determine the effects of different olive processing methods on deltamethrin (DEL), dimethoate (DIM), and imidacloprid (IMI), the most commonly preferred synthetic insecticides for controlling olive pests such as the olive fruit fly. The hypothesis is that the fermentation could accelerate the degradation process of the insecticides. For this purpose, olives were left for fermentation (natural black olives) without and with starter addition (two Lactobacillus plantarum strains 112 and 123) and processed as dehydrated black olives. To monitor the degradation rate of insecticides, olives were first polluted with the insecticides and then the residues were detected periodically during the processes. The insecticide degradation rates were found to be significantly higher in natural black olives and natural black olives inoculated with both starters compared with those of crude olives and dehydrated black olives. At the end of fermentation (after 60 d), 53–61% of deltamethrin, 66–68% of dimethoate, and 42–50% of imidacloprid were removed in natural black olives and natural black olives inoculated with both starters. In dehydrated olives, the degradation of deltamethrin, dimethoate, and imidacloprid was lower with rates of 9.7, 40, and 13.4%, respectively. The current study demonstrated that natural and starter-added natural black olive processing accelerated the degradation of deltamethrin, dimethoate, and imidacloprid.
1. Introduction
Pesticides are one of the major inputs used for increasing the yield of agricultural commodities. Nevertheless, the presence of pesticide residues on processed food products is a crucial problem causing safety and health problems.1 Table olives have great economic importance especially for the Mediterranean countries and other olive-producing areas because of high production and consumption rates. The intensive use of insecticides for the main pests of olive trees leads to increased residues on olive fruits.2 Although alternative control methods have been implemented in many countries, unfortunately the broad-spectrum synthetic insecticides, organophosphorus (OPs), synthetic pyrethroids (SPs), and neonicotinoids (NEOs) are still the most commonly preferred insecticides for the control of the pests.3−5 However, improper use of these compounds can cause residue problems on agricultural products if the necessary precautions are not taken.
Deltamethrin (DEL), dimethoate (DIM), and imidacloprid (IMI) are the most common substances used during olive growing.6 Although numerous OP substances have been restricted in European Union countries, the use of DIM is still permitted in large parts of the world.4,5 DIM has been registered since 1951 as a systemic acaricide and insecticide with contact and stomach action. The compound is moderately toxic with an acute oral LD50 of 245 mg/kg for rats and a relative risk as a cholinesterase inhibitor in humans. A SP compound, DEL has been widely used since the 1980s on various crops and human-disease vectors. The acute toxicity of DEL is oral LD50 of 114–168 mg/kg for rats. A NEO substance, IMI was first used in 1991 as a systemic acetylcholine receptor agonist insecticide with contact and stomach action. The compound is moderately toxic, having an acute oral LD50 of 131 mg/kg for rats and causing side effects on the reproduction and development in humans.7 The half-lives of DIM, DEL, and IMI are 7.2–15.5, 11–19, and 174–191 days, respectively, depending on the hydrolysis activities and metabolism.8−10
Pesticides can be degraded by photolysis, hydrolysis, oxidation and reduction, and metabolism (plants, animals, or microorganisms) and affected by temperature and pH. Different food processing and preservation techniques, postharvest treatments, and cold storage have also been found to be effective. Techniques based on concentration (drying/dehydration and concentration) increased the pesticide residue levels in the end products, whereas milling, baking, winemaking, malting, and brewing lowered their levels in these. Refining, fermentation, and curing have been reported to affect the pesticide level in foods to a varied extent.11
Fermentation is a microbiological process in which enzymes transform carbohydrates, typically starch or sugar, into simpler components such as alcohols, acids, and gases and most of the proteins to amino acids and low-molecular-weight peptides. The most common groups of microorganisms involved in the fermentation of food, include yeasts, bacteria, and moulds, which produce enzymes that catalyze the fermentation process.12 The biological degradation of the pesticides by microorganisms is dependent on the structure of the chemical (volatility, insolubility in water, and adsorption ability to matrix compounds) and some environmental parameters (temperature, pH, moisture, and light).13
Some lactic acid bacteria (LAB), belonging to Lactobacillus and Leuconostoc genera, can metabolize broad-spectrum synthetic insecticides, i.e., OPs, SPs, NEOs, by their esterase enzymes and/or using the insecticides as carbon and energy sources.14−21 Besides, LAB have gained a lot of interest due to their health benefits and are widely used as probiotics and starter cultures for fermented products because of their generally recognized as safe (GRAS) status.22 Biodegradation of pesticides is a promising technology because of its potential for the removal of residues from food and agricultural products.13
There are several trade preparations for table olives such as alkali-treated olives, natural olives, dehydrated/shrivelled olives, and olives darkened by oxidation.23,24 There is no scientific information about the effect of different handling and processing methods on pesticide residues in olives. This research was focused on natural black olives and dehydrated black olives techniques because of the increasing demand of consumers to chemical-free, natural, and minimally processed food products. Natural black olives (NBOs) and dehydrated black olives (DBOs) are popular black table olive types. NBOs are obtained by placing fruits directly in brine with 8–9% salt, where they undergo complete or partial fermentation, and preserved or not by the addition of acidifying agents.24 Traditional NBO production is a spontaneous fermentation process that relies upon microorganisms present in fresh fruits and the processing environment. It is reported that the use of suitable starter cultures in NBO processing may help to standardize the fermentation, improve the microbiological quality, and increase the lactic acid yield to provide table olives of higher quality.25−28 On the other hand, DBOs are generally obtained by partial dehydration in coarse salt.24
The aim of this simulation study was to compare the effects of different olive processing methods (DBO, NBO) and LAB [NBO inoculated with lactic starter (NBOS)] on insecticide residues in the table olives polluted with DIM, DEL, and IMI.
2. Results
2.1. Insecticide Residues
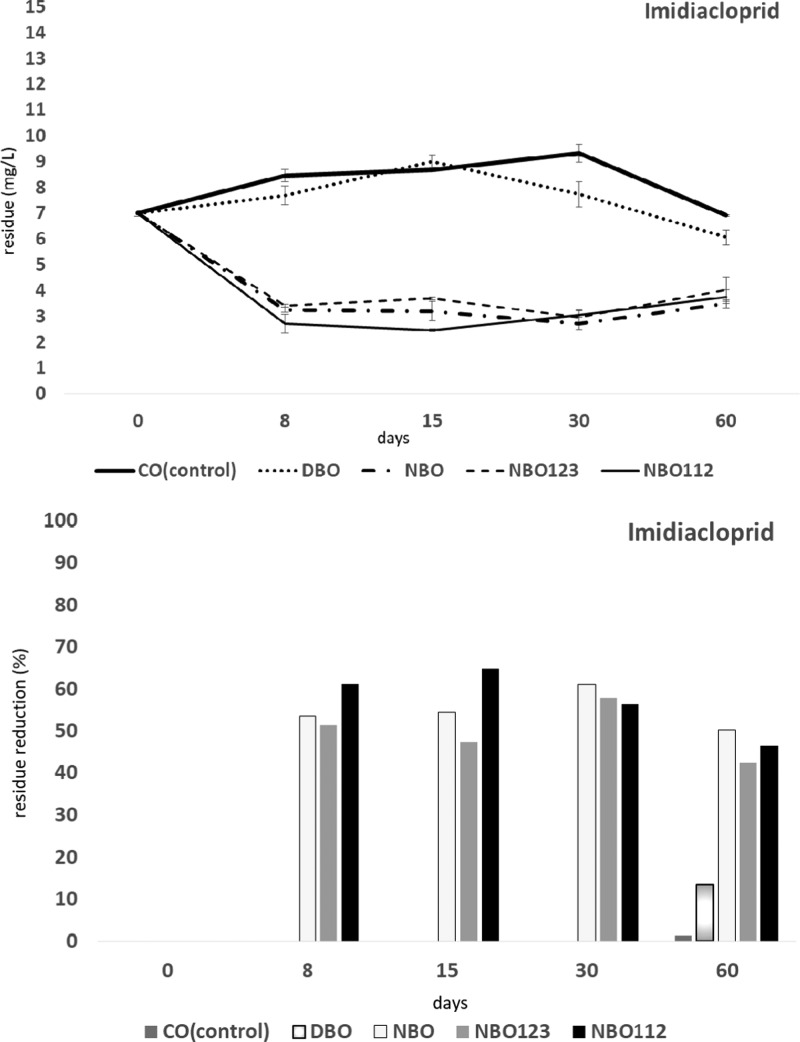
Changes in the IMI, DEL, and DIM levels in crude olives (CO, control), DBO, NBO, NBO with L. plantarum 112 (NBO112) and NBO with L. plantarum 123 (NBO123) are demonstrated in Figures 1−3. Eight days after the pollution with IMI, the insecticide amount was significantly decreased in NBO, NBO112, and NBO123 treatments (54, 51, and 61% reduction, respectively, F4,4 = 335.26, P < 0.01). The degradation rates in DBO were not significantly different from those in CO. The highest IMI residue was detected in CO, followed by DBO on day 8. A significant lower IMI residue was measured in NBO112 on the same detection day. In NBO, NBO112, and NBO123 treatments, there was no significant change from day 8 to day 60. But, in CO and DBO, a significant decrease in IMI amounts was found on day 60 (F4,4 = 86.2, P < 0.01). Nevertheless, these degradation rates in both CO and DBO did not reach the rates of all NBO treatments. In addition, LAB inoculation into NBO did not affect the IMI amount (F16,16 = 29.2, P < 0.01) (Figure 1).
Figure 1.
Effects on degradation of imidiacloprid (IMI) in olive fruits processed by different methods: crude olives (CO, control); dehydrated and/or shrivelled black olives (DBO); natural black olives (NBO); natural black olives with Lactobacillus plantarum 112 (NBO112); and natural black olives with L. plantarum 123 with L. plantarum 123 (NBO123).
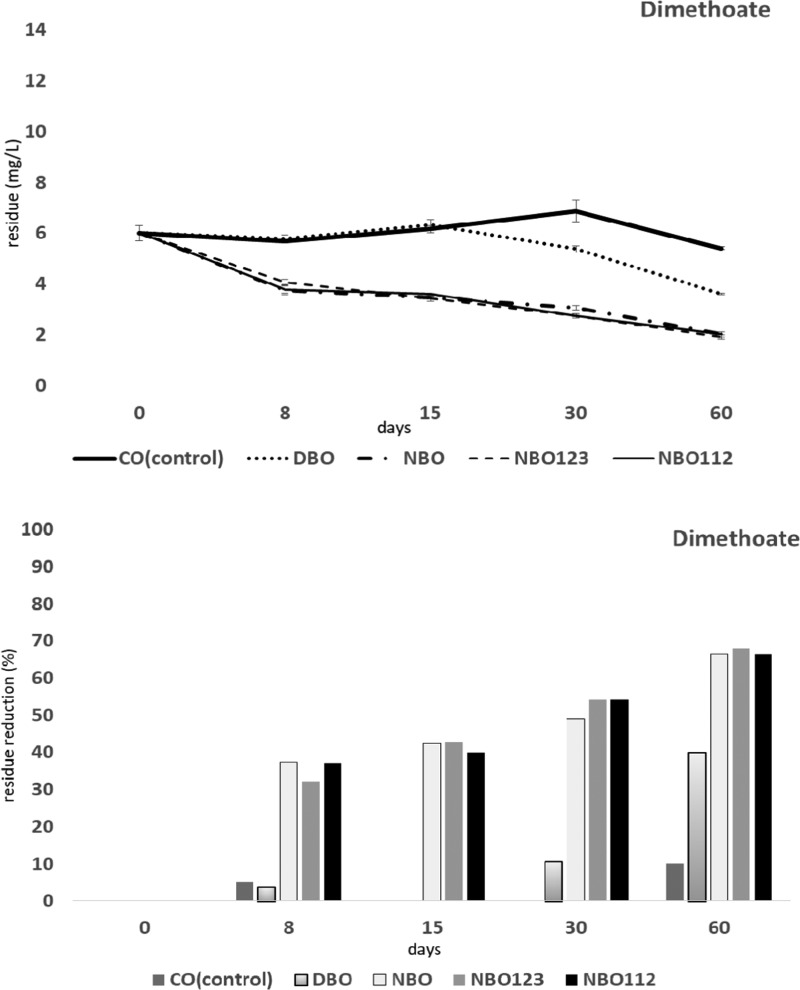
Figure 3.
Effects on degradation of dimethoate (DIM) in olive fruits processed by different methods: crude olives (CO, control); dehydrated and/or shrivelled black olives (DBO); natural black olives (NBO); natural black olives with L. plantarum 112 (NBO112); and natural black olives with L. plantarum 123 (NBO123).
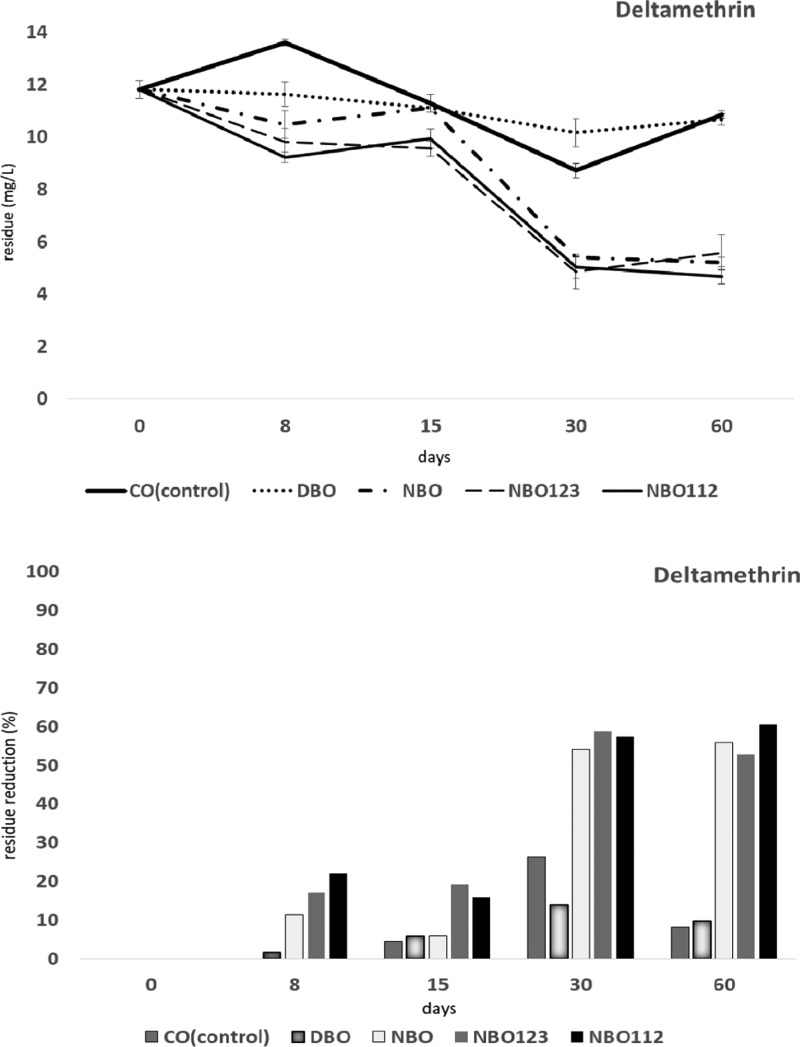
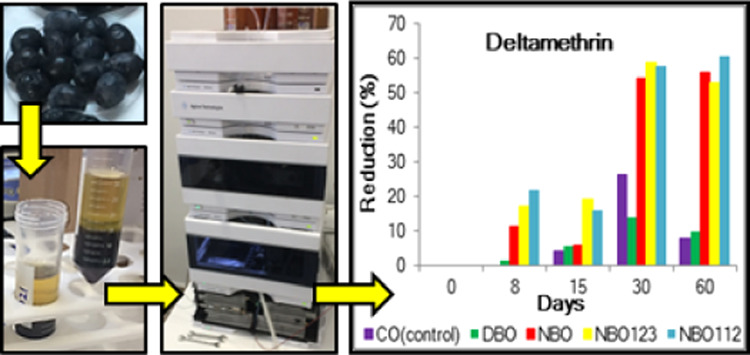
Eight and 30 days after pollution with DEL, the insecticide amount was significantly decreased in NBO, NBO112, and NBO123 (11–22% on day 8 and 54–57% on day 30) (F4,4 = 82.9, P < 0.01). On days 8, 30, and 60, the DEL amounts in NBO, NBO112, and NBO123 treatments were significantly lower compared to those in both CO and DBO treatment (F4,4 = 180.2, P < 0.01). The reduction rates in NBO112 and NBO123 treatments were higher than those in NBO from day 8 to 30 (F16,16 = 13.4, P < 0.01) (Figure 2).
Figure 2.
Effects on degradation of deltamethrin (DEL) in olive fruits processed by different methods: crude olives (CO, control); dehydrated and/or shrivelled black olives (DBO); natural black olives (NBO); natural black olives with L. plantarum 112 (NBO112); and natural black olives with L. plantarum 123 (NBO123).
A significant amount of DIM was decomposed rapidly in NBO, NBO112, and NBO123 treatments after 8 days (32–37% reduction). After day 8, the reduction rates in NBO were significantly higher compared with those in CO and DBO treatment. The differences among DIM amounts in NBO, NBO112, and NBO123 treatments were not significant (F4,4 = 197.9, P < 0.01). On day 60, a significantly large portion of the DIM amount (66–68%) was degraded in all NBO treatments (F4,4 = 171.2, P < 0.01). However, time–treatment interactions were found to be significant. Residues in CO and DBO at different time intervals were higher than those in all NBO treatments (F16,16 = 17.8, P < 0.01). Addition of starters (both L. plantarum 112 and 123) to NBOs was not affected the DIM residues (Figure 3).
2.2. Microbiological Changes
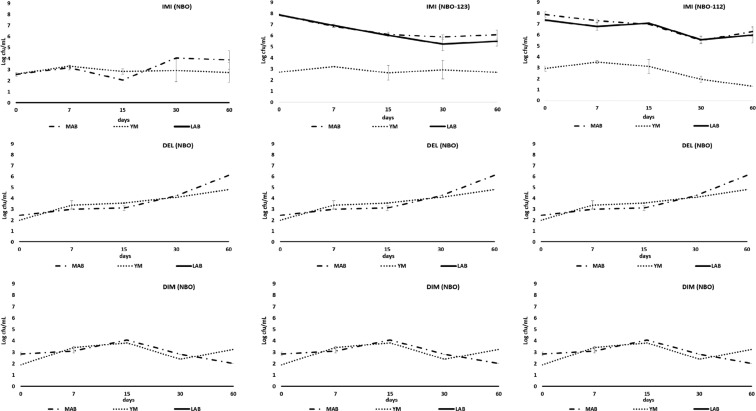
The growth of mesophilic aerobic bacteria (MAB), yeasts and moulds (YM), enterobacteria (ENB) and LAB during NBO treatments in the presence of DEL, DIM, and IMI are shown in Figure 4. ENB growth was not detected in all treatments. For all insecticide trials, a significant LAB growth (Figure 4) was detected in NBO treatments, which were inoculated with starters (NBO112 and NBO123), compared with their corresponding spontaneously fermented NBO treatments (Figure 4; DEL F2,2 = 700.49, P < 0.01; DIM F2,2 = 870.8, P < 0.01, IMI F2,2 = 741.11, P < 0.01). The initial LAB concentration was between 6.89 and 7.86 cfu/mL, and there was a continuous LAB existence during the whole process. No significant difference was detected between NBO123 and NBO112 trials for all insecticides. The growth of LAB was decreased significantly from day 0 to 7, but no difference was found between days 7 and 60, except for the NBO123 treatments of DIM and IMI (DEL F4,4 = 4.74, P < 0.01; DIM F4,4 = 10.36, P < 0.01, IMI F4,4 = 11.02, P < 0.01).
Figure 4.
Mean microbial growth [mesophilic aerobic bacteria (MAB), lactic acid bacteria (LAB), yeast and moulds (YM)] in the presence of imidiacloprid (IMI), deltamethrin (DEL), and dimethoate (DIM) during different olive processing methods: natural black olives (NBO); natural black olives with L. plantarum 112 (NBO112); and natural black olives with L. plantarum 123 (NBO123).
There was evident yeast growth in all treatments during trials (Figure 4), with initial cell numbers between 1.84 and 2.93 log cfu/mL. The yeast growth displayed fluctuations; however, at the end of 60 days, the number of yeasts reached higher levels (2.30–4.79 log cfu/mL) than their initial numbers in all treatments, except in NBO112 polluted with IMI. The results of the MANOVA test showed that the growth of the yeast cells was not affected by the starter addition in all treatments with DIM (DIM F2,2 = 1.97, P = 0.16). The yeast numbers were significantly varied depending on the observation time (DEL F4,4 = 114.38, P < 0.01; DIM F4,4 = 26.62, P < 0.01, IMI F4,4 = 7.27, P < 0.01) in all trials. Although the highest yeast numbers were seen on the 15th day in all trials, no significant difference was detected between the treatments on this day.
The growth of total MAB showed differences between NBO and NBOs with the addition of starter (Figure 4). Based on the MANOVA test, the addition of both starters significantly increased the number of MAB cells (DEL F2,2 = 197.49, P < 0.01; DIM F2,2 = 189.53, P < 0.01, IMI F2,2 = 125.82, P < 0.01). The cell numbers in different observation times were found to be significantly different (DEL F4,4 = 31.83, P < 0.01; DIM F4,4 = 10.19, P < 0.01, IMI F4,4 = 7.43, P < 0.01) in all treatments. Time–treatment interactions were found to be significant in all treatments (DEL F8,8 = 16.44, P < 0.01; DIM F8,8 = 4.17, P < 0.01, IMI F8,8 = 2.73, P = 0.02).
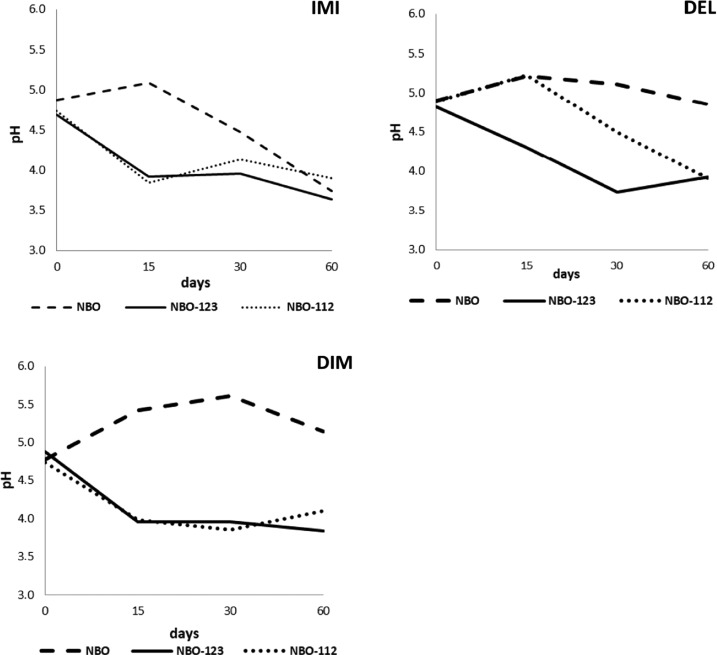
2.3. pH Changes
The pH changes of NBO brines are demonstrated in Figure 5. Marked differences were observed between the NBO and the starter-inoculated NBO in all pesticide-containing treatments. The pH decline (below 4) was significant in all starter-added treatments. In NBOs polluted with DEL and DIM, the pH was constantly higher than 4 during the whole process, but in NBOs polluted with IM, the pH started to decrease after the 15th day and finally reached below pH 4.
Figure 5.
pH changes in the presence of imidiacloprid (IMI), deltamethrin (DEL), and dimethoate (DIM) during different olive processing methods: natural black olives (NBO); natural black olives with L. plantarum 112 (NBO112); and natural black olives with L. plantarum 123 (NBO123).
3. Discussion
In the present study, it has been demonstrated that insecticide degradation rates were found to be significantly higher in NBO, NBO112, and NBO123 treatments compared with those in DBO and CO. When olive fruits were polluted with DEL, DIM, and IMI, they were degraded by 11–22%, 32–37%, and 51–61% after a fermentation period of 8 days and 53–61%, 66–68%, and 42–50% after a fermentation period of 60 days (optimum fermentation time), respectively, in NBO, NBO112, and NBO123 treatments. In accordance with our results, some researchers reported that fermentation generally causes a decrease in OPs and SPs levels in processed foods such as cider, vinegar, kimchi (fermented Chinese cabbage), wheat, and skimmed milk.14,16,22,29−31 Similarly, DIM and DEL were degraded during the fermentations of wine, bread, and yoghurt.32−34 It has been previously demonstrated that some OPs and SPs, namely, chlorpyrifos, malathion, methidathion, parathion, dimethoate, bifenthrin, deltametrin, permethrin, and fenvalerate, degraded during the fermentation of some foods containing LAB and yeasts.16,22,34−37 Kawar et al. showed that methidathion (OPs) and dimethoate levels were degraded about 46 and 85%, respectively, in fermented wine after a fermentation period of 57 days.35 Moreover, Banna and Kawar demonstrated that the parathion (OPs) levels in cider and vinegar decreased about 70 and 80%, after a fermentation period of 12 and 57 days, respectively.29 Fatichenti et al. found that some SPs (deltamethrin, permethrin, and fenvalerate) were almost totally degraded with yeast (Saccharomyces cerevisiae) activity after a fermentation period of 9 days.36 Cho et al. reported that chlorpyrifos was degraded quickly with LAB activity within 3 days (83.3%) during Kimchi fermentation.16
On the other hand, our study demonstrated that the degradation rates in DBO were 1.5, 3.8, and 0% after 8 days and 14, 1.5, and 0% after 30 days (optimum consumption time for DBO), respectively, for DEL, DIM, and IMI. These unfavorable insecticide residues and their stabilities in DBO processing may be caused by water loss during the dehydration of olives under dark-room conditions.38 El Beit et al. revealed that the pesticide levels did not change under acid conditions or high salt concentrations.38
Pesticide degradations are dependent on several processing and environmental conditions such as temperature, light, moisture, and pH.39−41 In general, OP, SP, and NEO insecticides are stable in acidic pH and easily degraded in alkali pH.38,42−44 Some researchers found that IMI was slowly hydrolyzed and was stable between pH 4 and 9 when protected from light under sterile conditions. Hydrolysis was more rapid under alkaline (up to pH 9) conditions.42,44 Generally, the pH becomes lower (≤4) during olive fermentations, which is similar to our results (Figure 5). Previous soil studies showed that the reason of insecticide degradation is both chemical hydrolysis in alkaline pH and microbial activity.39,45,46 Therefore, the degradation in the present study could be caused by microbial activity rather than hydrolysis due to the acidic conditions in the medium. It was reported that some microbial agents can metabolize insecticides by their esterase enzymes and using these compounds as carbon and energy sources.39,47 It is well known that some bacteria can metabolize insecticides by their specific enzymes such as esterase.48 Several studies have previously shown that many OPs, including esters of phosphoric acid, could be hydrolyzed by carboxylesterase and phosphotriesterase.11,18 In addition, some microbial agents (e.g., S. cerevisiae, L. plantarum, Lactobacillus bulgaricus, Lactobacillus paracasei, Leuconostoc mesenteroides, Lactobacillus brevis, Lactobacillus sakei, and Lactobacillus casei) used OPs and SPs insecticides as carbon and energy sources in some processed food media.15−17,20,21,49 In the current study, activities of yeasts and bacteria were determined in NBO treatments. Although significant LAB cell growth was found in black table olives inoculated with the two L. plantarum strains compared with those without inoculation (NBO), the presence of L. plantarum 112 and L. plantarum 123 did not affect the insecticide degradation. Additionally, the trend of yeast cell growth was related to insecticide degradation. The yeast cell growth increased during the first 15 days, when the insecticides were degraded rapidly. In a research by Randazzo et al. on fermented green olives, LAB and yeast populations were affected by the presence of copper.50 The yeast growth was detected at the beginning of fermentation and was constant till the end of the process. This was in accordance with results of Ohshiro et al., who reported that the degradative role of the microorganisms accelerates in association with yeasts present in the medium.51
4. Conclusions
The degradation rates of three different insecticides did not exceed 14% after 30 days in dry salted olive process. In addition, the insecticide residues in the dry salted olives were similar to those in the crude olive samples stored in the dark at room temperature. This can lead to a risk of high chemical residues during the consumption of the product. More than half of the chemical residues were degraded after 30 days in olives polluted with three insecticides and processed in brine under the same conditions, despite the insecticides having different chemical structures. This shows that the brining process in black olives is a useful method for the reduction of insecticide residues. In fact, even if the LAB is inoculated artificially to the brines, the effect of the inoculation on the pesticide degradation often does not change much.
This research has shown that the consumption of dry salted olives may cause a great risk when no attention is paid to preharvest intervals and good agricultural practices. The fermentation is generally used for improving the nutritional quality and shelf life of foods, in addition to its positive effects on the decontamination of chemical contaminants, such as pesticides. Although black olive brining accelerates the degradation of the insecticides, this process, in which there is no ultraviolet light from the sun, is not fully successful in removing all insecticide residues by the end of fermentation. Therefore, good agricultural practices in olive orchards should be the first priority.
5. Experimental Section
5.1. Chemicals and Reagents
The analytical standard reagents, dimethoate [DIM; O,O-diethyl-O-3,5,6-trichloro-2-pyridyl phosphorothioate], deltamethrin [DEL; (S)-cyano-(3-phenoxyphenyl)methyl] (1R,3R)-3-(2,2-dibromoethenyl)-2,2-dimethylcyclopropane-1-carboxylate], and imidiacloprid [IMI; (E)-1-(6-chloro-3-pyridylmethyl)-N-nitroimidazolidin-2-ylideneamine], were purchased from Dr. Ehrenstorfer GmbH (Germany). Emulsifiable concentrate commercial substances DIM (40 g/L Poligor, Hektaş Company, Turkey), DEL (25 g/L Deltharin, Hektaş Company, Turkey), and IMI (350 g/L Confidor, Bayer Company, Germany) were also obtained from manufacturers. All other reagents were analytical grade. Salts used during olive processing were obtained from local markets.
5.2. Olive Fruits
Olive fruits of “Gemlik” cv. were harvested at the black-ripe stage suitable for NBO and DBO productions from an experimental olive orchard in Orhangazi district of Bursa, Turkey, in 2018. None of the pesticides were applied to olive trees in this orchard during the growing season, and the olive fruits were free from all pesticides.
5.3. Starter Microorganisms
Two L. plantarum strains (112 and 123) used in this research were previously isolated from the fermentation brines of NBO. Strains were identified by 16s rRNA technique and differentiated from other group members according to Torriani et al.52,53L. plantarum strains were propagated in De Man, Rogosa, and Sharpe (MRS) broth at 30 °C. Eighteen to 24 h old test strains at a concentration of 108–109 cfu/mL were centrifuged at 10 000g, washed twice in sterile saline, and resuspended in brine.15 NBOSs were inoculated with strains at a final concentration of 107–108 cfu/mL.
5.4. Insecticide Pollution of Table Olives and Experimental Design
Crude olives of all treatments were homogeneously sprayed with commercial formulations of DIM, DEL, and IMI, separately, using a Potter precision spray tower at the 10 bar and 3 s settings (Burkard Manufacturing Co. Ltd., Rickmansworth, UK). The fruits were polluted at the following doses (DEL: 14 mg/L, DIM: 8.5 mg/L, and IMI: 7 mg/L), which are quite above the Maximum Residue Limits (MRL, DEL: 1.0 mg/L, DIM: 3.0 mg/L, and IMI: 0.5 mg/L) for olive products in European countries.5 The insecticide degradation changes were investigated by five different treatments: (i) CO, (ii) DBO, (iii) NBO, (iv) NBO112 and (v) NBO123. All of the treatments and analyses were done in triplicate, and all jars and bottles were kept at room temperature (Table 1).
Table 1. Experimental Design, Treatments, and Conditions.
| insecticides | sample name | sample code | treatments & conditions |
|---|---|---|---|
| for each insecticide (DIM, DEL, and IMI) | crude olives, Control | CO | 60 g of crude olives were sprayed with insecticides, put into 105 mL volume glass jars without any treatment, and kept at room temperature |
| dehydrated black olives | DBO | 60 g of crude olives were sprayed with insecticides, put in 190 mL volume glass jars with 12 g of coarse salt (20%), mixed effectually, and kept at room temperature | |
| natural black olives | NBO | 60 g of crude olives were sprayed with insecticides, put into 105 mL volume glass jars in brines containing 6% salt immediately after harvest, and allowed to ferment spontaneously at room temperature | |
| natural black olives with L. plantarum 112 | NBO112 | 60 g of crude olives were sprayed with insecticides, put into 105 mL volume glass jars in brines containing 6% salt immediately after harvest, inoculated with L. plantarum 112 at a final concentration of 107–108 cfu/mL, and allowed to ferment at room temperature | |
| natural black olives with L. plantarum 123 | NBO123 | 60 g of crude olives were sprayed with insecticides, put into 105 mL volume glass jars in brines containing 6% salt immediately after harvest, inoculated with L. plantarum 123 at a final concentration of 107–108 cfu/mL, and allowed to ferment at room temperature |
5.5. Monitoring the Growth of Test Strains
Brine samples were microbiologically analyzed at regular intervals for MAB, LAB, ENB, and YM.54,55 Enumeration of microorganisms was carried out using a spiral plating system (Easy Spiral, Interscience, France). Appropriate dilutions were plated on Plate Count Agar, MRS Agar, and Oxytetracycline Glucose Yeast Extract Agar (Merck KGaA, Darmstadt, Germany) for MAB, LAB, and YM, respectively, and incubated at 30 °C for 48 h. Violet Red Bile Glucose Agar (Merck KGaA, Darmstadt, Germany) was used for the enumeration of ENB and incubated at 35 °C for 48 h. The curves of cell growth for each microbial group were plotted using Excel program of Windows.
5.6. Monitoring the pH
The pH of all NBO treatments was monitored periodically in brines with a pH 315i model (WTW, Germany) pHmeter.
5.7. Insecticide Extraction Procedure
Samples from all experiments (60 g) were crushed and homogenized, and aliquots of 15 g of each were used for extraction and analysis for pesticides. Extraction and partition of insecticides were done with the Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) method based on the manufacturer’s instructions.56 Briefly, each homogenized olive sample was put in a 50 mL polypropylene centrifuge tube and then added with 10 mL of acetonitrile (containing 1% acetic acid). The tubes were hand-shaken vigorously for 1 min. Then, the tubes were added with 6 g of anhydrous MgSO4 and 1.5 g of sodium acetate (NaAc), shaken vigorously for 1 min, and centrifuged at 5000 rpm for 2 min. After that, 8 mL of the supernatant was collected from each tube and transferred into a 15 mL falcon tube containing 1200 mg of anhydrous MgSO4, 400 mg of PSA, and 400 mg of C18. The tubes were mixed with a vortex for 30 sn and then centrifuged at 5000 rpm for 2 min. Lastly, 1 mL of supernatant from each tube was transferred into glass autosampler vials for further liquid chromatography with tandem mass spectrometry (LC-MS-MS) analysis.
5.8. LC-MS-MS Analysis
Concentrations of DIM, DEL, and IMI were measured using Agilent Technologies 6470 Triple Quad Liquid-Mass Spectrometry (Agilent, Santa Clara, CA). Choromatographic separation was achieved by gradient elution using an Agilent Poroshell SB-C18 column (3 × 100 mm × 2.7 μm). One microliter of filtrate was injected into the LC-MS-MS. The mobile phase consisted of A, water at 0.1% formic acid with 1 mM of ammonium formate, and B, methanol. The gradient program was as follows: 0–0.5 min 70% A, 0.5–8 min 70% A, 8–12.5 min 5% A, 12.5–12.6 min 5% A, and 12.6–15.0 min 70% A. The mobile phase flow rate was 0.52 mL/min. The detection by mass spectrophotometer (MS) was carried out in multiple reactions monitoring (MRM) and the source was electrospray ionization (ESI) in a positive mode. The gas flow was 10 psi; gas capillary voltage was 3600 V, source temperature was set at 100 °C. The validation studies (linearity, mean recovery, precision, and specificity) were performed with pesticide-free olive samples according to the European Commission DG Health and Food Safety Guidelines SANTE/11813/2017.57 Calibration curves of the insecticides were prepared in triplicate at seven concentrations (from 0.02 to 2 mg/L). The correlation coefficient (R2) of the calibration curves obtained for all the compounds were ≥0.99. Mean recovery and precision were achieved by analyzing the spiked olive samples at 0.002 mg/L concentration. The limits of detection (LODs) and limits of quantitation (LOQs, in olive matrix) for DIM, DEL, and IMI were 0.0022, 0.0026, and 0.0024 and 0.0032, 0.0023, and 0.0029 mg/L, respectively. The average recoveries for DIM, DEL, and IMI at levels of 0.002–0.05 mg/kg were 70.5, 70.0, and 100.5%, respectively, with repeatability and reproducibility ≤5.81%.
5.9. Statistical Analysis
Repeated measured variance analysis (MANOVA) was performed on mean values for each observation time. The effects of time and processing method and interaction of both factors were analyzed with the fit model of SAS. Then, posthoc testing (P < 0.05) of multiple comparisons was performed by Tukey test.58
Acknowledgments
This project was supported by Bursa Uludag University, Scientific Research Unit, Bursa, Turkey, Grant Project No. OUAP(Z)-2015/9. Authors also thank Gulden Hazarhun for technical assistance during pesticide analyses. There is no conflict of interest about this research. Aysegul Yildirim Kumral designed and set up the experiments, Nabi Alper Kumral performed insecticide pollution and the data analyses, Aysegul Yildirim Kumral, Busra Maden, and Buse Artik performed the microbial tests, Nabi Alper Kumral and Aysenur Kolcu made pesticide analysis, and all authors contributed in writing, reading, and approving the manuscript.
The authors declare no competing financial interest.
References
- González-Rodríguez R. M.; Rial-Otero R.; Cancho-Grande B.; Gonzalez-Barreiro C.; Simal-Gándara J. A review on the fate of pesticides during the processes within the food-production chain. Crit. Rev. Food Sci. Nutr. 2011, 51, 99–114. 10.1080/10408390903432625. [DOI] [PubMed] [Google Scholar]
- Kovanci B.; Kumral N. A. In Insect Pests in Olive Groves of Bursa (Turkey), Presented at V. International Symposium on Olive Growing; Izmir, Turkey, Sep 27–Oct 2, 2004.
- Tzanakakis M. Seasonal development and dormancy of insects and mites feeding on olive: a review. Neth. J. Zool. 2003, 52, 87–224. 10.1163/156854203764817670. [DOI] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). Statistical Database. http://faostat.fao.org (accessed March 13, 2020).
- EU (European Commission). Pesticides Database. http://ec.europa.eu/food/plant/pesticides.en (accessed March 13, 2020).
- Republic of Turkey, Ministry of Food, Agriculture and Livestock, Plant Protection Products Database. https://bku.tarim.gov.tr/Arama/Index (accessed March 13, 2020).
- University of Hertfordshire, Agricultural Substances Databases: Background and Support Information. https://sitem.herts.ac.uk/aeru/ppdb/en/docs/2_5eco.pdf (accessed March 13, 2020).
- Simon J. Y.The Toxicology and Biochemistry of Insecticides; CRC Press: London, 2014; pp 231–250. [Google Scholar]
- Roberts T. R.; Hutson D. H.; Lee P. W.; Nicholls P. H.; Plimmer J. R.. Metabolic Pathways of Agrochemicals Part 2 Insecticides and Fungicides; Royal Society of Chemistry: Cambridge, UK, 1999; pp 1–1476. [Google Scholar]
- Cycoń M.; Zmijowska A.; Piotrowska-Seget Z. Enhancement of deltamethrin degradation by soil bioaugmentation with two different strains of Serratia marcescens. Int. J. Environ. Sci. Technol. 2014, 11, 1305–1316. 10.1007/s13762-013-0322-0. [DOI] [Google Scholar]
- Bajwa U.; Sandhu K. S. Effect of handling and processing on pesticide residues in food - a review. J. Food Sci. Technol. 2014, 51, 201–220. 10.1007/s13197-011-0499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes-López O.; Gonzalez-Casteneda J.; Carabenz-Trejo A. Influence of solid substrate fermentation on the chemical composition. J. Ferment. Bioeng. 1991, 71, 58–62. 10.1016/0922-338X(91)90304-Y. [DOI] [Google Scholar]
- Regueiro J.; Lopez-Fernandez O.; Rial-Otero R.; Cancho-Grande B.; Simal-Gándara J. A. Review on the fermentation of foods and the residues of pesticides - biotransformation of pesticides and effects on fermentation and food quality. Crit. Rev. Food Sci. Nutr. 2015, 55, 839–863. 10.1080/10408398.2012.677872. [DOI] [PubMed] [Google Scholar]
- Choi Y. J.; Miguez C. B.; Lee B. H. Characterization and heterologous gene expression of a novel esterase from Lactobacillus casei CL96. Appl. Environ. Microbiol. 2004, 70, 3213–3221. 10.1128/AEM.70.6.3213-3221.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. M.; Math R. K.; Islam S. M.; Lim W. J.; Hong S. Y.; Kim J. M.; Yun M. G.; Cho J. J.; Yun H. D. Biodegradation of chlorpyrifos by lactic acid bacteria during kimchi fermentation. J. Agric. Food Chem. 2009, 57, 1882–1889. 10.1021/jf803649z. [DOI] [PubMed] [Google Scholar]
- Islam S. M. A.; Math R. K.; Cho K. M.; Lim W. J.; Hong S. Y.; Kim J. M.; Yun M. G.; Cho J. J.; Yun H. D. Organophosphorus hydrolase (OpdB) of Lactobacillus brevis WCP902 from kimchi is able to degrade organophosphorus pesticides. J. Agric. Food Chem. 2010, 58, 5380–5386. 10.1021/jf903878e. [DOI] [PubMed] [Google Scholar]
- Kumral A. Y.; Kumral N. A. In Decontamination of Insecticides by Lactic Acid Bacteria, Presented at 24. International Scientific-Expert-Conference of Agriculture and Food Industry; Sarajevo, Bosnia and Herzegovina, Sep 25–28, 2013.
- Kumral A. Y.; Kumral N. A. In A Preliminary Study for the Survival of Different Lactobacillus plantarum Strains in Mineral Salt Medium with Chlorpyrifos and Deltamethrin, Presented at 25. International-Scientific-Expert Congress on Agriculture and Food Industry; Izmir, Turkey, 2014.
- Kumral A. Y.; Kumral N. A.; Gurbuz O. Chlorpyrifos and deltamethrin degradation potentials of two Lactobacillus plantarum strains. Turk. Entomol Derg. 2020, 44, 165–176. 10.16970/entoted.625156. [DOI] [Google Scholar]
- Zhao X.-H.; Wang J. A brief study on the degradation kinetics of seven organophosphorus pesticides in skimmed milk cultured with Lactobacillus spp. at 42 °C. Food Chem. 2012, 131, 300–304. 10.1016/j.foodchem.2011.08.046. [DOI] [Google Scholar]
- Dorđević T. M.; Siler-Marinkovic S. S.; Durovic R. D.; Dimitrijevic-Brankovic S. I.; Gajic Umiljendic J. S. Stability of the pyrethroid pesticide bifenthrin in milled wheat during thermal processing, yeast and lactic acid fermentation, and storage. J. Sci. Food Agric. 2013, 93, 3377–3383. 10.1002/jsfa.6188. [DOI] [PubMed] [Google Scholar]
- Maragkoudakis P. A.; Zoumpopoulou G.; Miaris C.; Kalantzopoulos G.; Pot B.; Tsakalidou E. Probiotic potential of Lactobacillus strains isolated from dairy products. Int. Dairy J. 2006, 16, 189–199. 10.1016/j.idairyj.2005.02.009. [DOI] [Google Scholar]
- Garcia E.; Luh B. S.; Martin H.. Olives. In Processing Fruits: Science and Technology; Barret Somogyi L. P.; Ramaswamy S. H., Eds.; CRC Press: Florida, 2005; pp 751–754. [Google Scholar]
- Codex Standard for Table Olives 66-1981. Codex Alimentarius. > input > download > standards www.fao.org (accessed March 13, 2020).
- Panagou E. Z.; Schillinger U.; Franz C. M. A. P.; Nychas G. J. E. Microbiological and biochemical profile of cv. Conservolea naturally black olives during controlled fermentation with selected strains of lactic acid bacteria. Food Microbiol. 2008, 25, 348–358. 10.1016/j.fm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Gardner N. J.; Savard T.; Obermeier P.; Caldwell G.; Champagne C. P. Selection and characterization of mixed starter cultures for lactic acid fermentation of carrot, cabbage, beet and onion vegetable mixtures. Int. J. Food Microbiol. 2001, 64, 261–275. 10.1016/S0168-1605(00)00461-X. [DOI] [PubMed] [Google Scholar]
- Nychas G. J. E.; Panagou E. Z.; Parker M. L.; Waldron K. W.; Tassau C. C. Microbial colonization of naturally black olives during fermentation and associated biochemical activities in the cover brine. Lett. Appl. Microbiol. 2002, 34, 173–177. 10.1046/j.1472-765x.2002.01077.x. [DOI] [PubMed] [Google Scholar]
- Leal-Sánchez M. V.; Ruiz-Barba J. L.; Sánchez A. H.; Rejano L.; Jimenez-Diaz R.; Garrido A. Fermentation profile and optimization of green olive fermentation using Lactobacillus plantarum LPCO10 as a starter culture. Food Microbiol. 2003, 20, 421–430. 10.1016/S0740-0020(02)00147-8. [DOI] [Google Scholar]
- Banna A. A.; Kawar N. S. Behavior of parathion in apple juice processed into cider and vinegar. J. Environ. Sci. Health, Part B 1982, 17, 505–514. 10.1080/03601238209372337. [DOI] [PubMed] [Google Scholar]
- Yun S. J. The change of residual chlorpyrifos during fermentation of kimchi. Korean J. Food Sci. Technol. 1989, 21, 590–594. [Google Scholar]
- Zhou X. W.; Zhao X. H. Susceptibility of nine organophosphorus pesticides in skimmed milk towards inoculated lactic acid bacteria and yogurt starters. J. Sci. Food Agric. 2015, 95, 260–266. 10.1002/jsfa.6710. [DOI] [PubMed] [Google Scholar]
- Cabras P.; Angioni A. Pesticide residues in grapes, wine, and their processing products. J. Agric. Food Chem. 2000, 48, 967–972. 10.1021/jf990727a. [DOI] [PubMed] [Google Scholar]
- Sharma J.; Satya S.; Kumar V.; Tewary D. K. Dissipation of pesticides during bread-making. Chem. Health Saf. 2005, 12, 17–22. 10.1016/j.chs.2004.08.003. [DOI] [Google Scholar]
- Bo L. Y.; Zhang Y. H.; Zhao X. H. Degradation kinetics of seven organophosphorus pesticides in milk during yoghurt processing. J. Serb. Chem. Soc. 2011, 76, 353–362. 10.2298/JSC100615035B. [DOI] [Google Scholar]
- Kawar N. S.; Iwata Y.; Dusch M. E.; Gunther F. A. Behavior of dialifor, dimethoate and methidathion in artificially fortified grape juice processed into wine. J. Environ. Sci. Health, Part B 1979, 14, 505–513. 10.1080/03601237909372146. [DOI] [PubMed] [Google Scholar]
- Fatichenti F.; Farris G. A.; Deiana P.; Cabras P.; Meloni M.; Pirisi F. M. The effect of Saccharomyces cerevisiae on concentration of dicarboximide and acylamide fungicides and pyrethroid insecticides during fermentation. Appl. Microbiol. Biotechnol. 1984, 20, 419–421. 10.1007/BF00261946. [DOI] [Google Scholar]
- Ruediger G. A.; Pardon K. H.; Sas A. N.; Godden P. W.; Pollnitz A. P. Fate of pesticides during the winemaking process in relation to malolactic fermentation. J. Agric. Food Chem. 2005, 53, 3023–3026. 10.1021/jf048388v. [DOI] [PubMed] [Google Scholar]
- El Beit I. O. D.; Wheelock J. V.; Cotton D. E. Factors influencing the degradation of dimethoate in soils and solutions. Int. J. Environ. Stud. 1978, 11, 253–260. 10.1080/00207237808737361. [DOI] [Google Scholar]
- Aislabie J.; Lloyd-Jones G. A review of bacterial degradation of pesticides. Soil Res. 1995, 33, 925–942. 10.1071/SR9950925. [DOI] [Google Scholar]
- Oliva J.; Cermeno S.; Camara M. A.; Martínez G.; Barba A. Disappearance of six pesticides in fresh and processed zucchini, bioavailability and health risk assessment. Food Chem. 2017, 229, 172–177. 10.1016/j.foodchem.2017.02.076. [DOI] [PubMed] [Google Scholar]
- Oliva J.; Paya P.; Camara M. A.; Barba A. Removal of famoxadone, fluquinconazole and trifloxystrobin residues in red wines: effects of clarification and filtration processes. J. Environ. Sci. Health, Part B 2007, 42, 775–781. 10.1080/03601230701550964. [DOI] [PubMed] [Google Scholar]
- Zheng W.; Liu W. Kinetics and mechanisms of the hydrolysis of imidacloprid. Pestic. Sci. 1999, 55, 482–485. . [DOI] [Google Scholar]
- Dikshit A. K. Stability of deltamethrin on pulses during storage and the effect of processing. Pestic. Res. J. 2002, 14, 40–46. [Google Scholar]
- Krohn J.; Hellpointner E. Environmental fate of imidacloprid. Pflanzenschutz-Nachr. Bayer 2002, 55, 1–26. [Google Scholar]
- Lakshmi C. V.; Kumar M.; Khanna S. Biotransformation of chlorpyrifos and bioremediation of contaminated soil. Int. Biodeterior. Biodegrad. 2008, 62, 204–209. 10.1016/j.ibiod.2007.12.005. [DOI] [Google Scholar]
- Farghaly M. F. M.; Zayed S. M.; Soliman S. M. Deltamethrin degradation and effects on soil microbial activity. J. Environ. Sci. Health, Part B 2013, 48, 575–581. 10.1080/03601234.2013.774900. [DOI] [PubMed] [Google Scholar]
- Singh B. K.; Walker A. Microbial degradation of organophosphorus compounds. FEMS Microbiol. Rev. 2006, 30, 428–471. 10.1111/j.1574-6976.2006.00018.x. [DOI] [PubMed] [Google Scholar]
- Cycoń M.; Wojcik M.; Piotrowska-Seget Z. Biodegradation of the organophosphorus insecticide diazinon by Serratia sp. and Pseudomonas sp. and their use in bioremediation of contaminated soil. Chemosphere 2009, 76, 494–501. 10.1016/j.chemosphere.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Angioni A.; Garau A.; Caboni P.; Russo M. T.; Farris G. A.; Zara S.; Cabras P. Gas chromatographic ion trap mass spectrometry determination of zoxamide residues in grape, grape processing, and in the fermentation process. J. Chromatogr. A 2005, 1097, 165–170. 10.1016/j.chroma.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Randazzo C. L.; Fava G.; Tomaselli F.; Romeo F. V.; Pennino G.; Vitello E.; Caggia C. Effect of kaolin and copper based products and of starter cultures on green table olive fermentation. Food Microbiol. 2011, 28, 910–919. 10.1016/j.fm.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Ohshiro K.; Kakuta T.; Sakai T.; Hirota H.; Hoshino T.; Uchiyama T. Biodegradation of organophosphorous insecticides by bacteria isolated from turf green oil. J. Ferment. Bioeng. 1996, 82, 299–305. 10.1016/0922-338X(96)88823-4. [DOI] [Google Scholar]
- Kumral A.; Korukluoglu M.; Romero C.; De Castro A.; Ruiz-Barba J. L.; Brenes M. Phenolic inhibitors involved in the natural fermentation of Gemlik cultivar black olives. Eur. Food Res. Technol. 2013, 236, 101–107. 10.1007/s00217-012-1859-8. [DOI] [Google Scholar]
- Torriani S.; Felis G. E.; Dellaglio F. Differentiation of Lactobacillus plantarum, L. pentosus, and L. paraplantarum by recA gene sequence analysis and multiplex PCR assay with recA gene-derived primers. Appl. Environ. Microbiol. 2001, 67, 3450–3454. 10.1128/AEM.67.8.3450-3454.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özay G.; Borcaklı M. Effect of brine replacement and salt concentration on the fermentation of naturally black olives. Food Res. Int. 1995, 28, 553–559. 10.1016/0963-9969(95)00054-2. [DOI] [Google Scholar]
- Tassou C. C.; Panagou E. Z.; Katsaboxakis K. Z. Microbiological and physicochemical changes of naturally black olives fermented at different temperatures and NaCl levels in the brines. Food Microbiol. 2002, 19, 605–615. 10.1006/fmic.2002.0480. [DOI] [Google Scholar]
- Agilent Quechers Selection Guide. https://www.agilent.com/cs/library/selectionguide/public/5990-8590EN.pdf (accessed Dec, 2019).
- SANTE/11813/2017. Guidance Document on Analytical Quality Control and Method validation Procedures for Pesticides Residues Analysis in Food and Feed. European Commission Directorate-General for Health and Food Safety. (rev.0). https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_wrkdoc_2017-11813.pdf (accessed Dec, 2019).
- JMP version 7.0.2. SAS Institute: Cary, NC, 2007.