Abstract

Alkaline-earth metal carbonate materials have attracted wide interest because of their high value in many applications. Various sources of carbonate ions (CO32–), such as CO2 gas, alkaline-metal carbonate salts, and urea, have been reported for the synthesis of metal carbonate crystals, yet a slow and sustained CO32– release approach for controlled crystal growth is much desired. In this paper, we demonstrate a new chemical approach toward slow and sustained CO32– release for hydrothermal growth of large alkaline-earth metal carbonate single crystals. Such an approach is enabled by the multiple hydrolysis of a small basic amino acid (arginine, Arg). Namely, the amino groups of Arg hydrolyze to form OH– ions, making the solution basic, and the hydrolysis of the guanidyl group of Arg is hydrothermally triggered to produce urea and ammonia, followed by the hydrolysis of urea to produce CO2 and ammonia and then the release of CO32– because of the reaction between CO2 and the OH– ions hydrolyzed from ammonia. Such a CO32– release behavior enables the slow and controlled growth of various carbonate single crystals over a wide range of pH values. The growth of uniform rhombohedron MgCO3 single crystals with variable morphologies and crystal sizes is studied in detail. The influences of reaction temperature, solution pH, precursor type, and concentration on the morphology and size of the resulting MgCO3 crystals are elucidated. The crystal evolution mechanism is also proposed and discussed with various supportive data.
1. Introduction
Alkaline-earth metal carbonate materials are abundant and have wide applications, such as CO2 sequestration, carriers for drug and functional molecules, biological medicines, refractories, templating synthesis of other materials, and so forth.1−4 As a particular example, magnesite (MgCO3) is a rock-forming mineral associated with carbon sequestration in nature.5 MgCO3 is essential in the manufacture of refractories resistant to high temperature and useful as special powder additives or reinforcing agents for various industrial applications. In addition, the biocompatible and nontoxic nature of MgCO3 has rendered them increasingly attractive as a carrier for drug delivery,6,7 as an adsorbent for wastewater treatment,8 as a carrier for sun-blocking semiconductor nanoparticles for cosmetic applications,9 and so forth. Moreover, MgCO3 is an excellent precursor for the postsynthesis of porous magnesium oxides (MgO), which are promising for CO2 capture,10,11 catalysis,12 and wastewater treatment.13−16 Therefore, controllable growth of MgCO3 crystals with tailored size, morphology, and structure is desirable.
For crystal growth, the carbonate ion (CO32–) source and the release rate of CO32– in solution are critical for the carbonation reaction with alkaline-earth metal cations. For the synthesis of MgCO3 crystals, several typical methods have been reported. The first one is utilization of CO2 gas as the CO32– source. In this way, magnesium carbonate hydrates can be obtained by reacting CO2 gas with Mg(OH)2 suspensions or soluble magnesium salts under alkaline conditions.17,18 In such reactions, the mass transfer of CO2 in water is considered as the rate-determining step for growth of MgCO3 because of the low solubility of CO2 in water. The enhanced CO2 gas flow rate and stirring rate can improve the mass transfer of CO2 but without giving the detailed concentration of carbonate ions in solution to characterize the limiting step for the growth of MgCO3. The use of high-pressure CO2 for the reaction with Mg(OH)2 at a high temperature can significantly improve the reaction kinetics to form MgCO3 crystals.19 The obtained MgCO3 crystals may contain unreacted Mg(OH)2 in their internal cores because of the discrepancy between the fast carbonation kinetics and the slow Mg(OH)2 dissolution rate. In some cases, the addition of precipitation agents, such as ethanol, is necessary in such a tri-phase reaction to facilitate the formation of MgCO3.18 The second method is to use soluble inorganic carbonate salts, such as Na2CO3 or K2CO3, for the growth of MgCO3. Hydromagnesite products can be produced at low temperatures.20,21 These soluble inorganic carbonates dissolve immediately after being added to water; thus, the reactions with Mg2+ cations are very fast, which makes it relatively difficult for slow and controlled crystal growth of MgCO3. The third method is the utilization of soluble organic molecules as the CO32– source. In particular, urea is often used because it can be rapidly hydrolyzed to release a massive amount of CO32– under non-neutral conditions.22,23 The obtained magnesium carbonate hydrates at low temperatures can be transferred to pure MgCO3 crystals under hydrothermal conditions.24 Because the hydrolysis rate of urea is so fast, the release and the concentration of CO32– ions cannot be well controlled, leading to MgCO3 crystals with small and non-uniform sizes. Alternatively, Yang et al. have used hexamethylenetetramine as the CO32– source,25 which produces formaldehyde to react with O2 to produce CO2, thereby reducing the release rate of CO32– and producing micron-sized anhydrous MgCO3 particles under hydrothermal conditions. However, such a reaction is limited by the content of O2 in the closed autoclave. In addition, some other special methods have also been reported. For example, in order to obtain larger MgCO3 crystals, Lou et al. conducted the synthesis in molten sodium at 550 °C. Molten sodium can increase the solubility of MgCO3 such that the number of crystal nuclei decreases and the size of the particles increases.26 Based on the analysis of the prior research, it is still highly desirable to develop a slow and sustained CO32– release pathway for the controllable growth of large MgCO3 crystals.
Amino acids (AAs), which are stable and easy to store, have been widely used for the synthesis of organic and inorganic materials.27,28 They can not only coordinate with metal ions to form complexes for the structure and composition control29 but also can play as structure-directing agents to guide the formation of special structures and crystals.30 In the synthesis of alkaline-earth metal-carbonate crystals, AAs and their polymerized forms (peptides and proteins) have been used to guide the bio-mineralization of CaCO3 with special polymorphs, such as vaterite microspheres,31 smooth mineral films,32 and calcite single crystals.33 In addition, AAs are small amphoteric molecules. They might modify crystal surfaces and change the crystal growth behavior.30,33,34 Moreover, AAs carry one or multiple amino and carboxyl groups on carbon backbones. It is hypothesized that the carboxyl group or the carbon-containing side chains could be used as a CO2 source and the amino group as the OH– source, thus providing a CO32– release approach.35 However, until now, there is little exploration of AAs as a source for CO32– for the controlled growth of metal carbonate crystals.
Herein, in this paper, by exploring the twenty regular AAs, we report, for the first time, the successful utilization of basic AAs, especially arginine (Arg), to establish a sustained and controlled CO32– release approach for the hydrothermal synthesis of uniform and large alkaline-earth metal carbonate single crystals. A series of crystals, including MgCO3, CaCO3, BaCO3, and SrCO3, can be obtained by this AA-mediated growth method. The synthesis of uniform rhombohedron MgCO3 single crystals with variable sizes and morphologies are studied in detail. The influences of the hydrothermal reaction temperature and time, solution pH values, the AA type, as well as the magnesium salt type and concentration on the growth of MgCO3 crystals are elucidated. The mechanism for the formation of the MgCO3 crystals is explored. The high-temperature-triggered hydrolysis of the guanidyl group of Arg slowly produces CO2 and OH– ions, thus providing a slow and sustained CO32– release pathway for the controllable growth of alkaline-earth metal carbonate single crystals.
2. Results and Discussion
2.1. Amino-Acid-Mediated Growth of Rhombohedron MgCO3 Crystals
Uniform rhombohedron MgCO3 single crystals can be grown from the inorganic salt Mg(NO3)2·6H2O and basic Arg as the CO32– ion source with a molar ratio of 1.0 using the controllable amino-acid-mediated hydrothermal growth method at 200 °C for 48 h. The wide-angle X-ray diffraction (XRD) pattern (Figure 1a) shows a group of sharp peaks that can be assigned to various diffraction planes of a hexagonal magnesite (MgCO3, JCPDS Card no. 08-0479). No other diffraction peaks can be found, indicative of high crystal purity. The crystallite size estimated using the Scherrer Equation on the basis of the most intensive (104) diffraction peak is ∼60.6 nm. The large-area scanning electron microscopy (SEM) image shows that the MgCO3 crystals have a uniform single-crystalline rhombohedron morphology (Figure 2c). The particle-size distribution of the sample is relatively narrow based on analyzing one hundred rhombohedron particles, and the mean particle size is 49.6 ± 0.25 μm (inset in Figure 2c). The magnified SEM image shows that the exposed smooth planes of the rhombohedron can be indexed to the (104) facet (Figure 2d).36 By crushing the crystals to expose the interior, the rhombohedron is single crystalline with the same crystal plane orientation throughout the whole crystal (Figure 2e,f). Inside the broken rhombohedron single crystal, numerous crystal steps of about 1 μm in height for each step can be observed. The clear and ordered lattice fringes in the high-resolution transmission electron microscope (HRTEM) image (Figure 2g) reveals that the particle is single crystalline. The d-spacing is estimated to be ∼2.7 Å, corresponding to the (104) facet of MgCO3. The corresponding selected-area electron diffraction (SAED) pattern (Figure 2h) shows the presence of bright spots, which can be indexed to the (104), (110), and (116) facets of MgCO3 crystals, further revealing that the crystal has a high crystallographic orientation.37
Figure 1.

Wide-angle XRD patterns of the samples obtained by hydrothermally treating the Mg(NO3)2/Arg (molar ratio 1.0) solution at 200 (a), 160 (b), and 120 °C (c) for 48 h.
Figure 2.

SEM (a–f) and HRTEM (g) images and SAED (h) pattern of the samples obtained by hydrothermally treating the Mg(NO3)2/Arg (molar ratio 1.0) solution at 120 °C (a), 160 °C (b), and 200 °C (c–h) for 48 h.
2.2. Influencing Factors on Growth of MgCO3 Crystals
2.2.1. Effect of Hydrothermal Temperature
The hydrothermal temperature plays a critical role in controlling the crystal phase and product yield. With the Mg2+/Arg molar ratio fixed at 1.0 and the heating time fixed at 48 h, TGA results show that the MgCO3 yield significantly increases from ∼3.8% at 120 °C to 67.7% at 160 °C, and then further to 85.1% at 200 °C (Figure S1A,B). The wide-angle XRD pattern of the sample obtained at 120 °C possesses three weakly crystallized phases, namely, a major brucite Mg(OH)2 phase (JCPDS Card no. 44-1482), a moderate MgCO3 phase, and a minor Mg5(CO3)4(OH)2·4H2O phase (JCPDS Card no. 25-0513), respectively (Figure 1c). Accordingly, the SEM image of this sample (Figure 2a) shows the coexistence of two significantly different crystal morphologies, namely, nanoflakes of ∼500 nm in dimension and ∼30 nm in thickness that can be assigned to Mg(OH)2 or Mg5(CO3)4(OH)2·4H2O, and small sized (<5 μm) rhombohedron particles assigned to MgCO3, respectively. With the hydrothermal temperature increased to 160 °C, the wide-angle XRD pattern shows that a predominant MgCO3 phase can be formed in the resultant sample (Figure 1b). This sample is predominantly composed of rhombohedron MgCO3 crystals (Figure 2b). Nevertheless, their surfaces are not smooth but attached with many smaller particles, indicating that there are probably some minor crystal impurities or small-sized MgCO3 crystals. With the temperature further increased to 200 °C, pure and highly crystalline MgCO3 single crystals can be obtained (Figure 2c). The corners of the MgCO3 crystals become acute and smooth with no fragments observed on the crystal surfaces (Figure 2d). On the one hand, the crystallite size of the MgCO3 phase increases from ∼29.3 to 60.6 nm with the temperature increased from 120 to 200 °C (Figure S1C). Meanwhile, the overall mean size of the rhombohedron MgCO3 particles increases from <5 μm at 120 °C to 25.0 ± 0.13 μm at 160 °C and further to 49.6 ± 0.25 μm at 200 °C (Figure S1D). This is because higher temperature results in a high solute oversaturation favoring the growth of large crystals.
2.2.2. Effect of Hydrothermal Time
To investigate the evolution of product species and crystal growth during the hydrothermal process, the hydrothermal time is varied from 1.0 to 48 h with the temperature fixed at 200 °C and the Mg2+/Arg molar ratio fixed at 1.0. The wide-angle XRD pattern shows that a pure Mg(OH)2 phase is present in the sample obtained with a hydrothermal time of 1.0 h (Figure 3a). With the hydrothermal period extended to 3.0 and 6.0 h, a mixed phase composed of a major MgCO3 phase and a minor Mg(OH)2 phase can be observed in the resultant two samples (Figure 3b,c). The content of the Mg(OH)2 phase decreases, while the content of the MgCO3 phase increases (Figure S2A,B). With the hydrothermal time prolonged to 12 h or longer, the pure MgCO3 phase can be obtained (Figure 3d–f). The crystal yield and crystallite size both increase with the extension of the hydrothermal time (Figure S2B,C).
Figure 3.
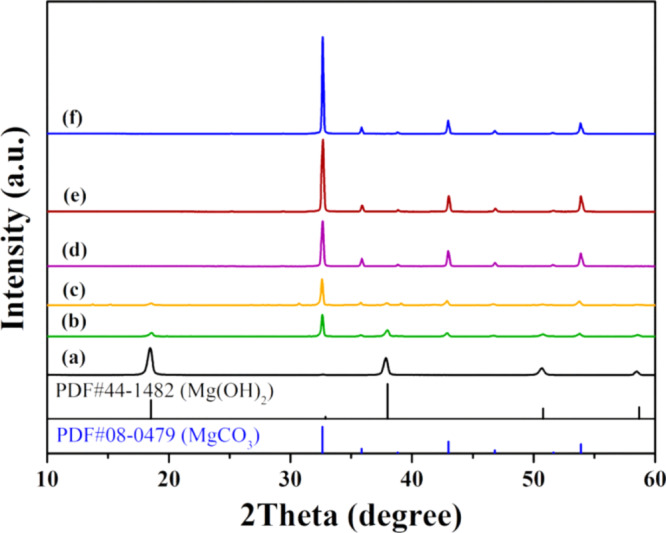
Wide-angle XRD patterns of the samples obtained by hydrothermally treating the Mg(NO3)2/Arg (molar ratio 1.0) solution at 200 °C for 1 (a), 3 (b), 6 (c), 12 (d), 24 (e), and 48 h (f), respectively.
SEM images of the products synthesized at different hydrothermal periods further illustrate the evolution of the two phases of Mg(OH)2 and MgCO3. The Mg(OH)2 phase existing in the samples obtained at a hydrothermal time of 1.0–6.0 h has a hexagonal sheet-like morphology (Figure 4a–d). The sheets are of ∼500 nm in lateral size and ∼30 nm in thickness. The MgCO3 phase with a rhombohedron shape starts to emerge in the sample obtained at a hydrothermal time of 3.0 h (Figure 4b). In addition, there are many sheetlike Mg(OH)2 fragments attached on the surfaces of MgCO3 rhombohedron particles. With the hydrothermal time prolonged to 12 h or more, pure uniform MgCO3 rhombohedron single crystals with smooth surfaces and sharp edges can be observed (Figures 4e,f, and 2d). This is because the Ostwald ripening process occurs with all the small fragments transformed into larger MgCO3 crystals. As a result, increase in the crystallite size and particle size of the MgCO3 rhombohedron crystals can be observed (Figure S2C,D).
Figure 4.

SEM images of the samples obtained by hydrothermally treating the Mg(NO3)2/Arg (molar ratio 1.0) solution at 200 °C for 1 (a), 3 (b,d), 6 (c), 12 (e), and 24 h, respectively.
2.2.3. Effect of the Initial pH Value
Various samples were synthesized at different initial solution pH values (1.0–12.2) with the Mg2+/Arg molar ratio fixed at 1.0 and the hydrothermal conditions fixed at 200 °C for 48 h. Theoretically, with a Mg2+ concentration of 83.3 mmol L–1, the pH at which Mg(OH)2 starts to precipitate is about 9.67, calculated from the solubility product constant of Mg(OH)2 at 25 °C. At high initial pH values (10.5, 11.0, and 12.2), white precipitates can be observed before the hydrothermal treatment, which can be identified as Mg(OH)2 (Figure S3). After the hydrothermal treatment, the pH values decrease to ∼9.5. At the low initial pH values (1.0–8.8), no precipitation can be observed before the hydrothermal treatment. After the hydrothermal treatment, the pH values increase to 7.8–9.2. After the hydrothermal treatment, pure MgCO3 crystals can be obtained at a wide pH range of 1.0–11.1 (Figure 5). Only a higher initial pH value of 12.2 results in a mixed phase of MgCO3 and Mg(OH)2 with a mass ratio of about 3 (Figure S4A). The formation of MgCO3 at all the pH values indicates the occurrence of gradual release of CO32– ions from Arg. The yield of MgCO3 increases from 26.5% at pH 1.0 to 83.6% at pH 11.0 (Figure S4B). This is because the formation of CO32– ions from Arg and precipitation are hindered at low pH values. With a low initial pH value, the nucleation and growth can be slowed down, leading to the growth of MgCO3 crystals with an enhanced crystallinity and a significantly enhanced mean particle size up to 121 ± 0.6 μm (Figures 5, and S4D). In a strongly alkaline environment (initial pH 12.2), part of the Mg2+ ions prefer to form Mg(OH)2 instead of MgCO3 because the high OH– concentration can shift the reaction equilibrium to the Mg(OH)2 direction which is caused by the lower Kspθ value of Mg(OH)2 compared with that of MgCO3.
Figure 5.

Wide-angle XRD patterns of the samples obtained by hydrothermally treating the Mg(NO3)2/Arg (molar ratio 1.0) solution at 200 °C for 48 h at different initial pH values.
SEM image shows that the MgCO3 sample obtained at pH 1.0 presents a spherical rhombohedron morphology with rounded corners (Figure 6a). The crystal rounding may be resulted from the interactions between Mg2+ ions on the crystal surface and organic molecules carrying negative charges under strongly acidic conditions.38 With initial pH values of 3.2–11.0, all the obtained MgCO3 samples show a rhombohedron morphology with sharp edges and exposed (104) facet (Figure 6b–e). The overall particle size increases from 39.5 to 121 μm with the initial pH value decreased from 11.1 to 1.0 (Figures 6a–e, and S4D), indicating that the release of CO32– can be well controlled, and low pH values are favored for the growth of large crystals. The sample grown at pH 12.2 exhibits an irregular crystal morphology with a large number of fractions (Figure 6f). The preferred growth of the (104) facet may be inhibited under strong alkaline conditions, while the other crystal planes are selected to grow, as revealed by the intensified (006) facet (Figure 5).
Figure 6.
SEM images of the samples obtained by hydrothermally treating the Mg(NO3)2/Arg (molar ratio 1.0) solution at 200 °C for 48 h with an initial pH value of 1.0 (a), 3.2 (b) 7.0 (c), 8.8 (d), 11.1 (e), and 12.2 (f), respectively.
2.2.4. Effect of Precursor Concentration
The precursor concentration is an important factor to control the crystallization kinetics and the morphology of resultant crystals.39 Three different precursor concentrations (41.7, 83.3, and 167 mmol L–1) were adopted with the Mg2+/Arg molar ratio fixed at 1.0 and the hydrothermal treatment conditions fixed at 200 °C for 48 h. In all cases, the pure MgCO3 crystal phase can be obtained (Figure S5). The crystallite size decreases slightly from 61.1 to 57.6 nm and the overall particle size also decreases from 49.9 to 35.7 μm with the increase of the precursor concentration (Figure S6B,C). This phenomenon is because the nucleation rate is faster at a higher precursor concentration, leading to the growth of a large number of smaller particles.20,40 All the samples obtained with different precursor concentrations possess the rhombohedron single crystal morphology (Figure S7A,B). It is observed that a higher precursor concentration results in more crystal fragments attached on the surfaces of the rhombohedron crystals.
In addition, the Mg2+/Arg molar ratio was also varied. Theoretically, a Mg2+/Arg molar ratio of 1.0 can result in 100% conversion of the Mg2+ cations to metal carbonate, provided that the guanidyl group of Arg can be fully hydrolyzed to form CO32– ions, and these ions can be fully utilized. Practically, a maximum of ∼85.1% carbonation of the Mg2+ cations can be achieved with a Mg2+/Arg molar ratio of 1.0. A lower Mg2+/Arg molar ratio of 0.67 can give a higher conversion (92.2%) of the Mg2+ cations because a higher relative concentration of Arg can produce more CO32– ions. In this case, the obtained sample is composed of the pure MgCO3 phase with a uniform and smooth rhombohedron crystal morphology (Figures S5, S7C). The crystallite size is estimated to be ∼58.5 nm, slightly smaller than that (∼60.6 nm) of the sample obtained at a molar ratio of 1.0 (Figure S6B), which is due to the higher concentration of CO32– ions, resulting in faster nucleation rate and smaller crystallites.
2.2.5. Effect of Various Magnesium Salts
With the Mg2+/Arg molar ratio fixed at 1.0 and the hydrothermal treatment conditions fixed at 200 °C for 48 h, pure MgCO3 crystals with similar rhombohedron single crystal morphology can also be obtained with the use of MgCl2·6H2O and MgSO4 as the precursors (Figures S8–S10). The overall particle size for the sample obtained from the sulfate salt is slightly smaller, and the crystal corners of this sample are more rounded compared with the other two samples obtained from the nitrate and chloride salts. Some recent studies showed that SO42–, a bivalent anion, can reduce the rate of ripening, via adsorption of sulfate ions onto crystal surfaces. The sulfate adsorption can partially poison the nucleation sites on growing crystals and decrease the rate of growth,41 thus causing the formation of smaller-sized crystals with sleek rounded corners.
2.2.6. Effects of the Amino Acid Type
Typical basic AAs (His and Lys) and acidic AAs (Asp and Glu) were also adopted to mediate the crystal growth at 200 °C for 48 h. With His as a precursor, the obtained product shows a crystalline MgCO3 phase and an amorphous carbon phase with a broad diffraction peak at 15–30° (Figure S11). The product is composed of microspheres of ∼6 μm in size (Figure 7a,b). The formation of such a product is because His can be hydrothermally carbonized to microspheres and release CO32– ions. The microspheres act as the growth template for MgCO3 crystallization. With Lys as the precursor, the obtained product consists of two crystal phases, Mg(OH)2 and MgCO3 (Figure S11). The MgCO3 phase only accounts for 27.1 wt % (Figure S12). The product shows a 3D flower-like structure of ∼3 μm in size, which is assembled from nanosheets of ∼30 nm in thickness (Figure 7c,d). The abovementioned result indicates that the release amount of CO32– from His and Lys is much lower. On the other hand, no precipitation can be observed using Asp and Glu after the hydrothermal treatment. Even by adjusting the initial pH value to 10.4 before the hydrothermal reaction, only a small amount of Mg(OH)2 precipitation can be generated (Figure S11). This result indicates that no CO32– ions can be released from decarboxylation of acidic AAs in the adopted experimental conditions.
Figure 7.
SEM images of the products obtained by hydrothermally treating the Mg(NO3)2/AA (molar ratio 1.0) solutions at 200 °C for 48 h using His (a,b) and Lys (c,d) as the carbonate source, respectively.
2.3. Discussion on the Growth Mechanism and the Key Influencing Factors
Based on the abovementioned results, Arg is essential to mediate the growth of MgCO3 single crystals by gradual and sustained release of CO32– ions. The chemical reaction mechanism guiding the formation of MgCO3 is proposed and discussed in detail (Scheme 1, and eqs 1–9). Prior to the hydrothermal treatment, the hydrolysis of the amino groups of Arg (eq 1) causes the mixed solution to be alkaline with an initial pH value of 10.5, which leads to the formation of a small amount of the Mg(OH)2 precipitate (eq 2). During the hydrothermal treatment process, the guanidyl group of Arg starts to hydrolyze slowly to generate ammonia and urea (Scheme 1a and eq 3). Ammonia continues to hydrolyze to produce OH– ions (eq 4) so that more Mg(OH)2 precipitate can be produced (Scheme 1b). This explains the facts that Mg(OH)2 is the major or the only phase of the products obtained with a low temperature (Figure 1c) and/or a short hydrothermal time (Figure 3a). With the enhancement of temperature and/or prolonging of hydrothermal time, more guanidyl groups of the Arg molecules are hydrolyzed. Meanwhile, hydrolysis of the produced urea is continuously proceeded to generate CO2 and more ammonia (eq 5). Then, CO2 reacts with the OH– ions hydrolyzed from ammonia to generate CO32– ions (eq 6). As a result, precipitation of Mg(OH)2 stops, and Mg5(CO3)4(OH)2·4H2O and MgCO3 start to form once the concentration products exceed their Kspθ values (Scheme 1c, and eqs 7 and 8). As Mg5(CO3)4(OH)2·4H2O is thermodynamically unstable, it is only a very minor phase at a low hydrothermal temperature and can be converted to the thermodynamically more stable MgCO3 phase.24,42 With the continuous hydrolysis and production of CO2, Mg2+ and OH– ions are gradually consumed. As a result, the MgCO3 crystals gradually grow larger (Scheme 1d). Simultaneously, the preformed Mg(OH)2 can be gradually dissolved (eq 2 shifting to left), attached to the surface of the formed MgCO3 crystals and converted to MgCO3 (Scheme 1d). Once the hydrolysis of Arg and its product urea is complete, the Mg(OH)2 phase completely disappears, and the CO32– and Mg2+ ions are stoichiometrically converted to MgCO3 crystals (Figure 3d). To support the gradual and sustained hydrolysis of Arg, as shown in eqs 1 and 3–6, by hydrothermally treating an Arg solution alone at 200 °C, the produced amount of CO32– ions from Arg gradually increases with the increase of the hydrothermal time, and the total released amount is identical to the theoretical value (Figure 8). Meanwhile, the final total produced amount (0.23 mol L–1) of NH4+/NH3 is indeed three times of the amount of CO32– ions, indicating gradual and complete hydrolysis of the guanidyl group of Arg. In addition, the pH value of the mixture is lowered to ∼9.0, and the yield of the MgCO3 crystals is up to ∼85.1%. After the chemical conversions are complete, during the final stage of the hydrothermal treatment, an Ostwald ripening process occurs with all the small crystal fragments disappeared and transformed into larger MgCO3 crystals, leading to the preferential growth of smooth and orientated crystal facets,43 which can fill up the edges of the crystals and then sharpen the corners to produce smooth rhombohedrons (Scheme 1e,f).
 |
1 |
| 2 |
 |
3 |
| 4 |
| 5 |
| 6 |
| 7 |
| 8 |
| 9 |
Scheme 1. Schematic Illustration of the AA-Mediated Crystal Growth Process of MgCO3 Single Crystals under Hydrothermal Conditions: (a) Reaction Pathway, (b) Formation of Mg(OH)2 Nanosheets, (c) Nucleation and Formation of Small MgCO3 Crystals, (d) MgCO3 Crystal Growth and Transformation of Mg(OH)2 to MgCO3, and (e,f) Formation of Large MgCO3 Single Crystals.

Figure 8.

Time-dependent CO32– release profiles by hydrothermally treating different AAs and urea solutions at various pH values at 200 °C. All the solute concentrations are fixed at 0.083 mol L–1. The dashed line is the theoretical maximum CO32– concentration that can be produced from the Arg solutions.
An intriguing aspect of the growth method is that the slow and sustained generation of CO32– ions leads to the controllable growth of large MgCO3 single crystals. Because the CO32– ions are generated slowly in situ and a large amount of the Mg2+ ions are first precipitated to Mg(OH)2, the concentrations of the two ions are relatively low over the reaction process. Therefore, the solute oversaturation is low, and thus, the nucleation rate is slow. Once MgCO3 nucleus and small crystals are formed, the Mg2+ and CO32– ions are gradually adsorbed to the (104) facet of the MgCO3 crystals and grow continuously, resulting in the formation of large rhombohedron MgCO3 single crystals. In a control experiment, the hydrothermal products of a mixed solution of Na2CO3 and Mg(NO3)2·6H2O with an initial pH value of 10.9 are composed of polydispersed mixed MgCO3 and Mg(OH)2 crystals with small sizes (<5 μm) and irregular shapes (Figures S13, S14), which is because of the fast and uncontrollable nucleation and growth process. The Arg molecules are crucial for the slow and sustained release of CO32– ions for controlled crystallization. By the hydrothermal treatment of an Arg solution at 200 °C for various time periods with various initial pH values of 3.0–11.1, the slow and sustained CO32– release behavior can be validated (Figure 8). The released amount increases with the prolonged hydrothermal time and with the increase of the pH value. For the other basic AAs His and Lys, the generated amounts of CO32– ions are rather low, and even no CO32– ions can be generated from the acidic AAs Asp and Glu (Figure 8), leading to low or no product yields after the hydrothermal treatment. The results are in agreement with the previous reports that Arg has a higher rate of the decomposition constant compared with His and Lys, and acidic AAs like Asp is more prone to de-amination under high temperatures.44,45 These results also confirm that the decarboxylation of AAs is limited under the adopted conditions (≤200 °C), and the generation of CO32– ions from Arg is mainly because of the hydrolysis of the guanidyl group and then the hydrolysis of its product urea (Scheme 1a, and eqs 3 and 5). However, pure urea hydrolyzes to produce CO32– ions too fast under non-neutral conditions (Figure 8), inducing uncontrolled crystal growth.
Another intriguing aspect of the growth method is that MgCO3 single crystals with controllable crystal sizes can be obtained over a wide range of initial pH values, even at very low pH values (1.0 or lower). At low pH values, no Mg(OH)2 precipitate can be observed before the hydrothermal treatment. During the hydrothermal treatment, the hydrolysis of Arg can produce ammonia and urea. Subsequently, the hydrolysis of urea can produce more ammonia and CO2. A part of the produced ammonia can naturalize the acid (eq 9). The excessive amount of ammonia can hydrolyze to produce OH– ions, which can react with CO2, leading to the formation of CO32– ions and nucleation of MgCO3 crystals. At low initial values, the formation of CO32– is even slower (Figure 8), and thus, the nucleation rate of MgCO3 is retarded. With less MgCO3 crystal nucleus formed, larger-sized MgCO3 single crystals can be grown (Figure 6a).
2.4. Versatility in Growth of Alkaline-Earth Metal Carbonate Crystals
The facile AA-mediated hydrothermal crystal growth method is versatile for the synthesis of a series of metal carbonate crystals. With a fixed metal ion/Arg molar ratio of 1.0 and fixed hydrothermal conditions of 200 °C for 48 h, CaCO3, BaCO3, and SrCO3 crystals can be obtained. The CaCO3 sample possesses a pure calcite phase (Figure 9a). The sample has a rhombohedral morphology with the particle size ranging from about 30 to 100 μm (Figure 10a,b). The BaCO3 sample possesses a pure witherite phase (Figure 9b). The crystal grows along the (130) crystal plane. The BaCO3 crystal shows a hexagonal prism morphology ended with two hexagonal pyramids, and the size of the prism is mainly 50 and 8 μm (Figure 10c,d). The SrCO3 sample possesses a pure strontianite phase (Figure 9c). The SrCO3 crystals show a hexagonal prism morphology with a particle size of 4–65 μm (Figure 10e,f). This method may also be applicable for the synthesis of other metal carbonate crystals.
Figure 9.

Wide-angle XRD patterns of the CaCO3 (a), BaCO3 (b), and SrCO3 (c) crystals obtained hydrothermally treating the metal nitrate/Arg solutions (metal ion/Arg molar ratio 1.0) at 200 °C for 48 h.
Figure 10.
SEM images of the CaCO3 (a,b), BaCO3 (c,d), and SrCO3 (e,f) crystals obtained by hydrothermally treating the metal nitrate/Arg solutions (metal ion/Arg molar ratio 1.0) at 200 °C for 48 h.
3. Conclusions
In summary, this work offers a novel approach for controllable hydrothermal growth of large alkaline-earth metal carbonate single crystals with variable crystal sizes. The key to the synthesis is the slow and sustained release of CO32– ions from AAs mediating the crystal growth rate. Most importantly, the amino group of Arg is hydrolyzed to make the solution alkaline, and the guanidyl group hydrolyzes slowly to produce ammonia and urea, followed by the hydrolysis of urea producing CO2 and more ammonia and then the release of CO32– via the reaction between CO2 and OH– ions hydrolyzed from ammonia. Such a CO32– release behavior allows the slow and controlled growth of various single crystals including MgCO3, CaCO3, BaCO3, and SrCO3. The typical MgCO3 products show a single crystalline rhombohedron morphology with uniform and controllable micron sizes (30.0–121 μm). A temperature of ≥160 °C is essential to trigger the CO32– release reaction for carbonation and MgCO3 crystal formation. Over the crystal growth process, the Mg2+ ions are first partially converted to Mg(OH)2, and then, Mg5(CO3)4(OH)2·4H2O forms, followed by the growth of anhydrous MgCO3 crystals. With the prolonging of the hydrothermal treatment, all the Mg2+ ion sources can be converted into MgCO3, and the Ostwald ripening process can make the crystals larger with smooth and sharpened corners. MgCO3 single crystals with various sizes and morphologies have been obtained by varying the magnesium salt type, AAs type, precursor concentration, and solution pH values. The solution pH value shows the most significant influence with a lower pH value favored for the slow growth of larger crystals. The findings in this paper may provide new insights into the hydrothermal decomposition of AAs and establish a general protocol for the synthesis of other metal carbonates and their derivative metal oxides.
4. Experimental Section
A series of alkaline-earth metal carbonate crystals can be obtained using the AA-mediated growth method. The synthesis of MgCO3 was studied in detail. The details about the chemicals used for the synthesis can be found in the Supporting Information. In a typical synthesis process of the rhombohedron MgCO3 crystals, 1.28 g of Mg(NO3)2·6H2O (5.0 mmol) and 0.87 g of Arg (5.0 mmol) with a molar ratio of 1.0 were sequentially dissolved into 60 mL of ultrapure water in a 100 mL beaker. The aqueous mixture with a pH value of 10.5 was transferred into a 100 mL Teflon-lined stainless-steel autoclave, placed in an oven at ambient temperature, and then heated to a specific temperature for the hydrothermal treatment for a certain period. The temperature was varied between 120 and 200 °C, and the heating time was varied between 1 and 48 h to study the crystallization process with the formation of different crystals. After cooling to room temperature, the precipitate was collected by filtration and washed three times with ultrapure water and ethanol. The precipitate was then dried at 60 °C under vacuum overnight.
A series of experimental parameters were considered for the synthesis. First, because the mixed solutions of Mg(NO3)2·6H2O and Arg were basic, to avoid the formation of Mg(OH)2 precipitation at the initial mixing stage and to study the influence of the pH value on crystallization, a range of pH values from 1.0 to 12 were adopted for the synthesis. Second, different Mg2+ and Arg concentrations with the fixed Mg2+/Arg molar ratio of 1.0 were adopted for the synthesis. Third, various magnesium salts were also adopted for the synthesis with the Mg2+/Arg molar ratio fixed at 1.0. In addition, several other basic AAs including lysine (demoted as Lys) and histidine (denoted as His) and acidic AAs including glutamic acid (denoted as Glu) and aspartic acid (denoted as Asp) (their molecular structures are shown in Scheme S1) were also adopted to try to synthesize magnesium carbonates. A Mg2+/AA molar ratio of 1.0 for Lys and His, and 2.0 for Asp and Glu were adopted. In all the above experiments, the temperature was set at 200 °C, and the heating time was fixed at 48 h.
In addition, in order address the versatility of the synthesis method, a series of other alkaline-earth metal (Ca, Ba and Sr) carbonate crystals were also synthesized using their nitrate salts and Arg with a molar ratio of 1.0. The synthesis process was the same as that for the synthesis of MgCO3 as described above. The conditions were fixed at a heating temperature of 200 °C and a heating time of 48 h.
A series of techniques were adopted to characterize the physicochemical properties of the obtained samples. The details about the characterization and measurement can be found in the Supporting Information.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (nos. 21875153), the Natural Science Foundation of Jiangsu Province (BK20150312). We acknowledge the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions and the Project of Scientific and Technologic Infrastructure of Suzhou (SZS201708) for support. We thank Muzi Chen (Testing and Analysis Centre of Soochow University) for characterization assistance in HRTEM.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01719.
Analyses on the composition, crystallites and particle sizes, and additional XRD, SEM characterization of the products obtained at different conditions (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Sanna A.; Uibu M.; Caramanna G.; Kuusik R.; Maroto-Valer M. M. A Review of Mineral Carbonation Technologies to Sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. 10.1039/c4cs00035h. [DOI] [PubMed] [Google Scholar]
- Cai W.-Y.; Feng L.-D.; Liu S.-H.; Zhu J.-J. Hemoglobin-CdTe-CaCO3@Polyelectrolytes 3D Architecture: Fabrication, Characterization, and Application in Biosensing. Adv. Funct. Mater. 2008, 18, 3127–3136. 10.1002/adfm.200800531. [DOI] [Google Scholar]
- Lenders J. J. M.; Dey A.; Bomans P. H. H.; Spielmann J.; Hendrix M. M. R. M.; de With G.; Meldrum F. C.; Harder S.; Sommerdijk N. A. J. M. High-Magnesian Calcite Mesocrystals: A Coordination Chemistry Approach. J. Am. Chem. Soc. 2012, 134, 1367–1373. 10.1021/ja210791p. [DOI] [PubMed] [Google Scholar]
- Lauth V.; Loretz B.; Lehr C.-M.; Maas M.; Rezwan K. Self-Assembly and Shape Control of Hybrid Nanocarriers Based on Calcium Carbonate and Carbon Nanodots. Chem. Mater. 2016, 28, 3796–3803. 10.1021/acs.chemmater.6b00769. [DOI] [Google Scholar]
- García del Real P.; Maher K.; Kluge T.; Bird D. K.; Brown G. E.; John C. M. Clumped-Isotope Thermometry of Magnesium Carbonates in Ultramafic Rocks. Geochim. Cosmochim. Acta 2016, 193, 222–250. 10.1016/j.gca.2016.08.003. [DOI] [Google Scholar]
- Vall M.; Zhang P.; Gao A.; Frykstrand S.; Cheung O.; Strømme M. Effects of Amine Modification of Mesoporous Magnesium Carbonate on Controlled Drug Release. Int. J. Pharm. 2017, 524, 141–147. 10.1016/j.ijpharm.2017.03.063. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Zardán Gómez de la Torre T.; Forsgren J.; Bergström C. A. S.; Strømme M. Diffusion-Controlled Drug Release From the Mesoporous Magnesium Carbonate Upsalite. J. Pharm. Sci. 2016, 105, 657–663. 10.1002/jps.24553. [DOI] [PubMed] [Google Scholar]
- Vall M.; Strømme M.; Cheung O. Amine-Modified Mesoporous Magnesium Carbonate as an Effective Adsorbent for Azo Dyes. ACS Omega 2019, 4, 2973–2979. 10.1021/acsomega.8b03493. [DOI] [Google Scholar]
- Åhlén M.; Cheung O.; Strømme M. Amorphous Mesoporous Magnesium Carbonate as a Functional Support for UV-Blocking Semiconductor Nanoparticles for Cosmetic Applications. ACS Omega 2019, 4, 4429–4436. 10.1021/acsomega.8b03498. [DOI] [Google Scholar]
- Zhao X.; Ji G.; Liu W.; He X.; Anthony E. J.; Zhao M. Mesoporous MgO Promoted with NaNO3/NaNO2 for Rapid and High-Capacity CO2 Capture at Moderate Temperatures. Chem. Eng. J. 2018, 332, 216–226. 10.1016/j.cej.2017.09.068. [DOI] [Google Scholar]
- Gao W.; Zhou T.; Wang Q. Controlled Synthesis of MgO with Diverse Basic Sites and its CO2 Capture Mechanism under Different Adsorption Conditions. Chem. Eng. J. 2018, 336, 710–720. 10.1016/j.cej.2017.12.025. [DOI] [Google Scholar]
- Zhu K.; Hu J.; Kübel C.; Richards R. Efficient Preparation and Catalytic Activity of MgO(111) Nanosheets. Angew. Chem., Int. Ed. 2006, 45, 7277–7281. 10.1002/anie.200602393. [DOI] [PubMed] [Google Scholar]
- Gao P.; Tian X.; Yang C.; Zhou Z.; Li Y.; Wang Y.; Komarneni S. Fabrication, Performance and Mechanism of MgO Meso-/Macroporous Nanostructures for Simultaneous Removal of As(iii) and F in a Groundwater System. Environ. Sci.: Nano 2016, 3, 1416–1424. 10.1039/c6en00400h. [DOI] [Google Scholar]
- Purwajanti S.; Zhou L.; Ahmad Nor Y.; Zhang J.; Zhang H.; Huang X.; Yu C. Synthesis of Magnesium Oxide Hierarchical Microspheres: A Dual-Functional Material for Water Remediation. ACS Appl. Mater. Interfaces 2015, 7, 21278–21286. 10.1021/acsami.5b05553. [DOI] [PubMed] [Google Scholar]
- Oladoja N. A.; Chen S.; Drewes J. E.; Helmreich B. Characterization of Granular Matrix Supported Nano Magnesium Oxide as an Adsorbent for Defluoridation of Groundwater. Chem. Eng. J. 2015, 281, 632–643. 10.1016/j.cej.2015.07.007. [DOI] [Google Scholar]
- Zheng X.; Huang M.; You Y.; Fu X.; Liu Y.; Wen J. One-pot Synthesis of Sandwich-like MgO@Carbon with Enhanced Sorption Capacity of Organic Dye. Chem. Eng. J. 2018, 334, 1399–1409. 10.1016/j.cej.2017.10.156. [DOI] [Google Scholar]
- Han B.; Qu H.; Niemi H.; Sha Z.; Louhi-Kultanen M. Mechanistic Study of Magnesium Carbonate Semibatch Reactive Crystallization with Magnesium Hydroxide and CO2. Ind. Eng. Chem. Res. 2014, 53, 12077–12082. 10.1021/ie501706j. [DOI] [Google Scholar]
- Chen G.; Song X.; Dong C.; Sun S.; Sun Z.; Yu J. Mineralizing CO2 as MgCO3·3H2O Using Abandoned MgCl2 Based on a Coupled Reaction–Extraction–Alcohol Precipitation Process. Energy Fuels 2016, 30, 7551–7559. 10.1021/acs.energyfuels.6b01297. [DOI] [Google Scholar]
- Fricker K. J.; Park A.-H. A. Investigation of the Different Carbonate Phases and Their Formation Kinetics during Mg(OH)2 Slurry Carbonation. Ind. Eng. Chem. Res. 2014, 53, 18170–18179. 10.1021/ie503131s. [DOI] [Google Scholar]
- Wang J.; Li Z. Crystallization and Agglomeration Kinetics of Hydromagnesite in the Reactive System MgCl2-Na2CO3-NaOH-H2O. Ind. Eng. Chem. Res. 2012, 51, 7874–7883. 10.1021/ie300213c. [DOI] [Google Scholar]
- Zhang Z.; Zheng Y.; Zhang J.; Zhang Q.; Chen J.; Liu Z.; Liang X. Synthesis and Shape Evolution of Monodisperse Basic Magnesium Carbonate Microspheres. Cryst. Growth Des. 2007, 7, 337–342. 10.1021/cg060544y. [DOI] [Google Scholar]
- Ghouri Z. K.; Barakat N. A. M.; Alam A.-M.; Alsoufi M. S.; Bawazeer T. M.; Mohamed A. F.; Kim H. Y. Synthesis and Characterization of Nitrogen-Doped & CaCO3-Decorated Rreduced Graphene Oxide Nanocomposite for Electrochemical Supercapacitors. Electrochim. Acta 2015, 184, 193–202. 10.1016/j.electacta.2015.10.069. [DOI] [Google Scholar]
- Fujita Y.; Taylor J. L.; Gresham T. L. T.; Delwiche M. E.; Colwell F. S.; Mcling T. L.; Petzke L. M.; Smith R. W. Stimulation of Microbial Urea Hydrolysis in Groundwater to Enhance Calcite Precipitation. Environ. Sci. Technol. 2008, 42, 3025–3032. 10.1021/es702643g. [DOI] [PubMed] [Google Scholar]
- Xing Z.; Hao Q.; Ju Z.; Xu L.; Qian Y. Synthesis of MgCO3 Microcrystals at 160 °C Starting from Various Magnesium Sources. Mater. Lett. 2010, 64, 1401–1403. 10.1016/j.matlet.2010.03.042. [DOI] [Google Scholar]
- Ni S.; Li T.; Yang X. Hydrothermal Synthesis of MgCO3 and its Optical Properties. J. Alloys Compd. 2011, 509, 7874–7876. 10.1016/j.jallcom.2011.04.064. [DOI] [Google Scholar]
- Lou Z.; Chen Q.; Zhu Y.; Zhang Y.; Gao J. Growth of Magnesium Carbonate Single Crystal in Supercritical Carbon Dioxide–Molten Sodium System. Cryst. Growth Des. 2004, 4, 415–417. 10.1021/cg034224f. [DOI] [Google Scholar]
- Gao X.; Chen Z.; Yao Y.; Zhou M.; Liu Y.; Wang J.; Wu W. D.; Chen X. D.; Wu Z.; Zhao D. Direct Heating Amino Acids with Silica: A Universal Solvent-Free Assembly Approach to Highly Nitrogen-Doped Mesoporous Carbon Materials. Adv. Funct. Mater. 2016, 26, 6649–6661. 10.1002/adfm.201601640. [DOI] [Google Scholar]
- Zhang J.; Liu X.; Guo X.; Wu S.; Wang S. A General Approach to Fabricate Diverse Noble-Metal (Au, Pt, Ag, Pt/Au)/Fe2O3 Hybrid Nanomaterials. Chem.—Eur. J. 2010, 16, 8108–8116. 10.1002/chem.201000096. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Gao X.; Wei X.; Wang X.; Li Y.; Wu T.; Guo J.; Gu Q.; Wu W. D.; Chen X. D.; Wu Z.; Zhao D. Directly Anchoring Fe3C Nanoclusters and FeNx Sites in Ordered Mesoporous Nitrogen-Doped Graphitic Carbons to Boost Electrocatalytic Oxygen Reduction. Carbon 2017, 121, 143–153. 10.1016/j.carbon.2017.05.078. [DOI] [Google Scholar]
- Marzec B.; Green D. C.; Holden M. A.; Coté A. S.; Ihli J.; Khalid S.; Kulak A.; Walker D.; Tang C.; Duffy D. M.; Kim Y.-Y.; Meldrum F. C. Amino Acid-Assisted Incorporation of Dye Molecules within Calcite Crystals. Angew. Chem., Int. Ed. 2018, 57, 8623–8628. 10.1002/anie.201804365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum G.; Reddy T. N.; Kumar K. P.; Dhevendar K.; Singh S.; Amarnath M.; Misra S.; Rangari V. K.; Rana R. K. In Situ Strategy to Encapsulate Antibiotics in a Bioinspired CaCO3 Structure Enabling pH-Sensitive Drug Release Apt for Therapeutic and Imaging Applications. ACS Appl. Mater. Interfaces 2016, 8, 22056–22063. 10.1021/acsami.6b07177. [DOI] [PubMed] [Google Scholar]
- Dai L.; Cheng X.; Gower L. B. Transition Bars during Transformation of an Amorphous Calcium Carbonate Precursor. Chem. Mater. 2008, 20, 6917–6928. 10.1021/cm800760p. [DOI] [Google Scholar]
- Green D. C.; Ihli J.; Kim Y.-Y.; Chong S. Y.; Lee P. A.; Empson C. J.; Meldrum F. C. Rapid Screening of Calcium Carbonate Precipitation in the Presence of Amino Acids: Kinetics, Structure, and Composition. Cryst. Growth Des. 2016, 16, 5174–5183. 10.1021/acs.cgd.6b00741. [DOI] [Google Scholar]
- Innocenti Malini R.; Finney A. R.; Hall S. A.; Freeman C. L.; Harding J. H. The Water–Amorphous Calcium Carbonate Interface and Its Interactions with Amino Acids. Cryst. Growth Des. 2017, 17, 5811–5822. 10.1021/acs.cgd.7b00874. [DOI] [Google Scholar]
- Ramachandran M. S.; Easwaramoorthy D.; Rajasingh V.; Vivekanandam T. S. N-Chlorosuccinimide-Promoted Oxidative Decarboxylation of α-Amino Acids in Aqueous Alkaline Medium. Bull. Chem. Soc. Jpn. 1990, 63, 2397–2403. 10.1246/bcsj.63.2397. [DOI] [Google Scholar]
- Song R.-Q.; Xu A.-W.; Antonietti M.; Cölfen H. Calcite Crystals with Platonic Shapes and Minimal Surfaces. Angew. Chem., Int. Ed. 2009, 48, 395–399. 10.1002/anie.200803383. [DOI] [PubMed] [Google Scholar]
- Egerton R. F.; Li P.; Malac M. Radiation Damage in the TEM and SEM. Micron 2004, 35, 399–409. 10.1016/j.micron.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Du H.; Amstad E. Water: How does it Influence the CaCO3 Formation?. Angew. Chem., Int. Ed. 2020, 59, 1798–1816. 10.1002/anie.201903662. [DOI] [PubMed] [Google Scholar]
- Raz S.; Weiner S.; Addadi L. Formation of High-Magnesian Calcites via an Amorphous Precursor: Phase Possible Biological Implications. Adv. Mater. 2000, 12, 38–42. . [DOI] [Google Scholar]
- Kulak A. N.; Iddon P.; Li Y.; Armes S. P.; Colfen H.; Paris O.; Wilson R. M.; Meldrum F. C. Continuous Structural Evolution of Calcium Carbonate Particles: A Unifying Model of Copolymer-Mediated Crystallization. J. Am. Chem. Soc. 2007, 129, 3729–3736. 10.1021/ja067422e. [DOI] [PubMed] [Google Scholar]
- Bots P.; Benning L. G.; Rodriguez-Blanco J. D.; Roncal-Herrero T.; Shaw S. Mechanistic Insights into the Crystallization of Amorphous Calcium Carbonate (ACC). Cryst. Growth Des. 2012, 12, 3806–3814. 10.1021/cg300676b. [DOI] [Google Scholar]
- Sandengen K.; Jøsang L. O.; Kaasa B. Simple Method for Synthesis of Magnesite (MgCO3). Ind. Eng. Chem. Res. 2008, 47, 1002–1004. 10.1021/ie0706360. [DOI] [Google Scholar]
- Xu S.; Ye Z.; Wu P. Biomimetic Controlling of CaCO3 and BaCO3 Superstructures by Zwitterionic Polymer. ACS Sustainable Chem. Eng. 2015, 3, 1810–1818. 10.1021/acssuschemeng.5b00387. [DOI] [Google Scholar]
- Abdelmoez W.; Nakahasi T.; Yoshida H. Amino Acid Transformation and Decomposition in Saturated Subcritical Water Conditions. Ind. Eng. Chem. Res. 2007, 46, 5286–5294. 10.1021/ie070151b. [DOI] [Google Scholar]
- Sato N.; Quitain A. T.; Kang K.; Daimon H.; Fujie K. Reaction Kinetics of Amino Acid Decomposition in High-Temperature and High-Pressure Water. Ind. Eng. Chem. Res. 2004, 43, 3217–3222. 10.1021/ie020733n. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





