Abstract
Cardiotoxicity of doxorubicin (DOX) has gained increasing attention in clinical application. Fuzhengkangfu (FZK) decoction, a traditional Chinese herbal formula of replenishing Qi strengthening spleen, has been used to treat various cardiovascular diseases. However, the chemical composition, the protective effects of FZK, and the underlying mechanisms are yet unclear. In this study, an high-performance liquid chromatography–mass spectrometry (HPLC–MS) analytical method was established for the structural identification of constituents in FZK extracts. Target prediction and enrichment analysis of the identified ingredients were performed. The cell viability was measured via (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) (MTT) assay. The protective effects of FZK on cell survival, mitochondrial membrane potential, intracellular calcium homeostasis, and cell apoptosis were detected. The level of relevant proteins was measured by Western blot. The effect of FZK on the antitumor activity of DOX was evaluated in HeLa cells. A total of 42 major chemical constituents were identified in FZK extracts by HPLC–MS. A comprehensive target prediction of these constituents retrieved 46 pathways, of which several key pathways were related to mitochondrial dysfunction, including metabolic pathways and calcium signaling pathways. Furthermore, FZK ameliorated DOX-induced H9C2 cell apoptosis and increased the Bcl-2/Bax ratio. Also, it moderated the loss of mitochondrial membrane potential and reduced the intracellular calcium overload, which are the major targets of DOX-induced injury. These results confirmed that FZK ameliorates DOX-induced cardiotoxicity via antiapoptotic and mitochondrial protection but does not affect the antitumor activity of DOX.
1. Introduction
Doxorubicin (DOX) is commonly used for the treatment of several types of human malignancies, such as acute leukemia and malignant lymphoma.1 It interferes with DNA and RNA synthesis by inhibiting topoisomerase II, thereby exerting its therapeutic effect.2 However, the clinical application of DOX is limited due to its dose-dependent and cumulative cardiotoxicity.3,4 The exact mechanisms of DOX-induced injury are still not yet understood. It has been hypothesized that several multiple mechanisms may be involved, including DNA damage, oxidative stress, and cell apoptosis.5 Owing to the great significance of DOX in cancer treatment, discovering novel treatment strategies against DOX-induced injury is essential. The therapy using natural and herbal ways to manage human diseases is known for a long time.6 Recently, traditional Chinese medicine (TCM) is gaining worldwide attention due to its holistic concept and systematic viewpoint. Thus, herbal methods are valuable for seeking a novel approach agent against DOX-induced toxicity.
Fuzhengkangfu decoction (FZK), a traditional Chinese herbal formula of reinforcing Qi strengthening spleen, has been extensively applied as a complementary therapy to treat various kinds of cardiovascular diseases. From the perspective of modern physiology, the Qi and spleen system in TCM is a comprehensive concept that integrates anatomy, physiology, and pathology. The system governs ingestion and digestion, blood circulation, nourishing the blood, and engendering the liquid.7 FZK is composed of six medicinal herbs, i.e., Radix codonopsis (Dangshen), Rhizoma atractylodis macrocephalae (Baizhu), Poria (Fulin), Radix saposhnikoviae (Fangfeng), Radix morindae officinalis (Bajitian), and Radix glycyrrhizae (Gancao). These herbs have immunomodulatory, anti-inflammatory, and lipid-regulating effects as confirmed by modern medicine.8−11 Among these, Codonopsis, morindae officinalis exerts myocardial protection, promotes hematopoiesis, and reduces blood deposition.12Poria, atractylodis macrocephalae have the function of diuretic.13 All of these are beneficial for improving cardiac function in patients. FZK has been clinically used to treat various cardiovascular diseases, such as heart failure. However, whether FZK-induced myocardial protection and the protective effect of FZK in the process of DOX-induced cardiotoxicity and its underlying mechanisms are still unclear.
In this study, we integrated the modern analytical technique and bioinformatics to dissect the chemical composition and potential pharmacological effects of FZK. High-performance liquid chromatography–mass spectrometry (HPLC–MS) has been the preferred method for the rapid measurement of various analytes in a complex herbal medicine.14 Network pharmacology is an efficient way to investigate the synergism function mechanism of different constituents from a systematic viewpoint as well as to disclose the molecular mechanisms of TCMs.15,16 Based on the results of chemical composition and target prediction, we further validated whether FZK protects the H9C2 cells against DOX-induced cardiotoxicity and explored the underlying mechanism.
2. Results
2.1. Characterization of Chemical Constituents in FZK
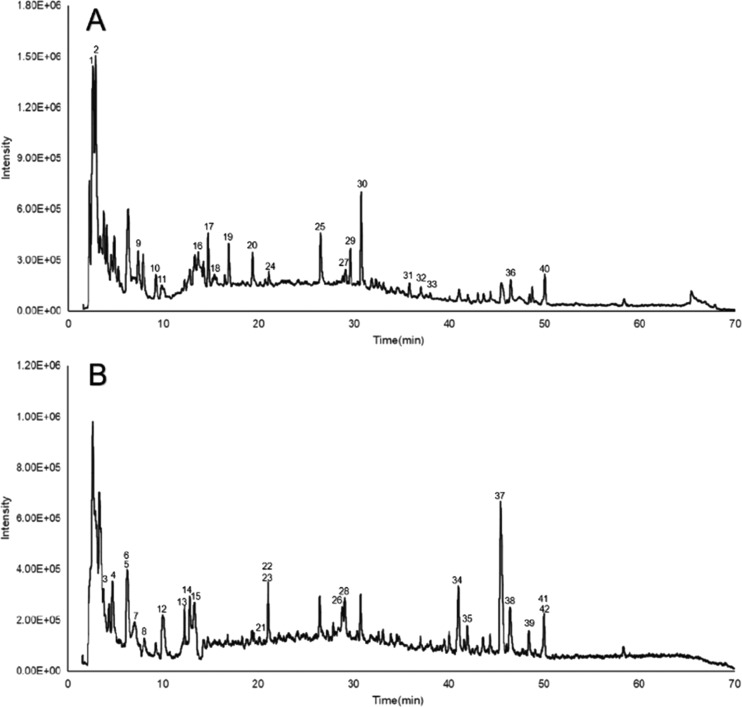
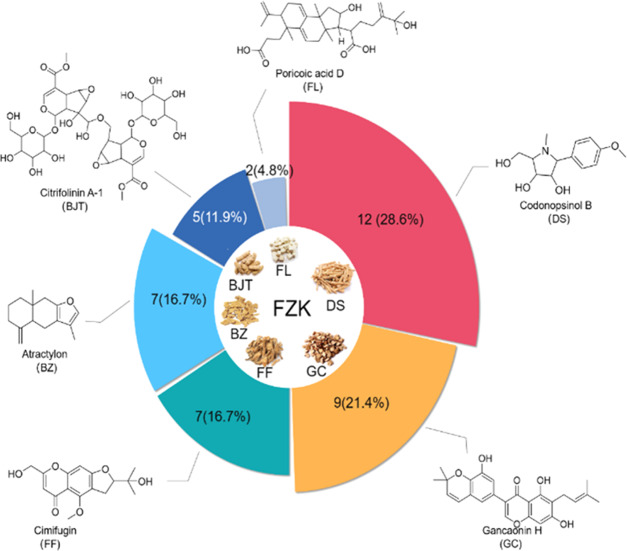
Representative chromatograms obtained by HPLC–MS are shown in Figure 1. A total of 42 compounds (listed in Table 1) were identified according to the MS data combined with literature and database matching,17−23 including 7 flavonoids, 6 amino acids, 5 chromones, 4 alkaloids, 4 volatile oils, 4 terpenoids, 2 glycosides, 2 carbohydrates, 1 nucleoside, 1 coumarin, 1 saponin, and 5 miscellaneous compounds. The chemical constituents of each herb and representative constituents structure identified from FZK are shown in Figure 2, DS, GC, FL, and BZ are the main sources of identified compounds.
Figure 1.
Total ion chromatogram (TIC) in positive mode (A) and negative mode (B) of FZK.
Table 1. Characterization of Chemical Constituents in FZK by HPLC–MSa.
| no. | tR (min) | detected ion (m/z) | formula | identity | source |
|---|---|---|---|---|---|
| 1 | 2.659 | [M + H] + 118.1 | C8H7N | indole | BJT |
| 2 | 2.912 | [M + H] + 116.1 | C5H9NO2 | (2S)-pyrrolidin-1-ium-2-carboxylate | BZ |
| 3 | 3.777 | [M – H] – 173.1 | C6H14N4O2 | protonated arginine | BZ |
| 4 | 4.408 | [M – H] – 549.3 | C32H38O8 | 6,6′-dimethoxygossypol | DS |
| 5 | 6.265 | [M – H] – 191.1 | C10H8O4 | scopoletin | BZ |
| 6 | 6.265 | [M – H] – 191.1 | C11H12O3 | myristicin | DS |
| 7 | 7.149 | [M – H] – 298.1 | C19H38O2 | methyl stearate | DS |
| 8 | 7.401 | [M – H] – 581.3 | C26H30O15 | asperuloside tetraacetate | BJT |
| 9 | 7.437 | [M + H] + 132.1 | C6H13NO2 | (2S,3S)-2-ammonio-3-methylpentanoate | BZ |
| 10 | 9.096 | [M + H] + 182.1 | C13H11N | 3-methyl-9H-carbazole | DS |
| 11 | 9.547 | [M + H] + 182.1 | C9H11NO3 | tyrosine | BZ |
| 12 | 9.961 | [M – H] – 665.3 | C24H42O21 | nystose | BJT |
| 13 | 12.251 | [M – H] – 389.2 | C24H38O4 | diisooctyl phthalate | DS |
| 14 | 12.305 | [M – H] – 389.2 | C25H26O4 | hispaglabridin B | GC |
| 15 | 12.954 | [M – H] – 827.4 | C30H52O26 | 1F-fructofuranosylnystose | BJT |
| 16 | 13.747 | [M + H] + 268.1 | C10H13N5O4 | adenosine | FF /DS |
| 17 | 14.631 | [M + H] + 166.1 | C9H11NO2 | (2S)-2-azaniumyl-3-phenylpropanoate | BZ |
| 18 | 16.437 | [M + H] + 254.2 | C13H19NO4 | codonopsinol B | DS |
| 19 | 16.921 | [M + H] + 268.2 | C14H21NO4 | codonopsine | DS |
| 20 | 19.357 | [M + H] + 205.1 | C11H12N2O2 | tryptophan | DS |
| 21 | 21.049 | [M + H] – 209.1 | C15H30 | 1-pentadecene | DS |
| 22 | 21.103 | [M – H] – 419.2 | C25H24O6 | gancaonin H | GC |
| 23 | 21.103 | [M – H] – 419.2 | C25H24O6 | morusin | GC |
| 24 | 22.365 | [M + H] + 217.2 | C15H20O | atractylon | BZ |
| 25 | 26.476 | [M + H] + 469.2 | C22H28O11 | prim-O-glucosylcimifugin | FF |
| 26 | 28.82 | [M – H] – 549.3 | C26H30O13 | licraside | GC |
| 27 | 28.871 | [M + H] + 257.1 | C15H12O4 | liquiritigenin | GC |
| 28 | 29.126 | [M – H] – 417.2 | C22H26O8 | episyringaresinol | DS |
| 29 | 29.667 | [M – H] + 307.2 | C16H18O6 | cimifugin | FF |
| 30 | 30.749 | [M + H] + 453.2 | C22H28O10 | 5-O-methylvisammioside | FF |
| 31 | 35.851 | [M + H] + 291.2 | C16H18O5 | 5-O-methylvisamminol | FF |
| 32 | 36.987 | [M + H] + 439.2 | C21H26O10 | sec-o-glucosylhamaudol | FF |
| 33 | 37.113 | [M + H] + 439.2 | C27H34O5 | 5-O-methyllicoricidin | GC |
| 34 | 41.044 | [M – H] – 514.4 | C31H46O6 | poricoic acid D | FL |
| 35 | 41.979 | [M – H] – 837.5 | C42H62O17 | licorice saponin G2 | GC |
| 36 | 43.784 | [M + H] + 373.3 | C17H24O9 | tangshenosideII | DS |
| 37 | 45.443 | [M – H] – 498.4 | C31H46O5 | poricoic acid A | FL |
| 38 | 46.344 | [M + H] – 821.5 | C34H46O23 | citrifolinin A-1 | BJT |
| 39 | 49.062 | [M – H] – 407.3 | C20H24O9 | ammijin | FF |
| 40 | 49.986 | [M + H] + 373.3 | C17H24O9 | syringin | DS |
| 41 | 50.041 | [M – H] – 407.3 | C25H28O5 | 3-hydroxyglabrol | GC |
| 42 | 50.221 | [M – H] – (407.3) | C25H28O5 | glyinflanin A | GC |
DS: Radix codonopsis; BZ: Rhizoma atractylodis macrocephalae; FL: Poria; FF: Radix saposhnikoviae; BJT: Radix morindae officinalis; GC: Radix glycyrrhiza.
Figure 2.
Distribution of compound source and chemical structures of representative compounds.
2.2. Predicting the Potential Target of FZK and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis
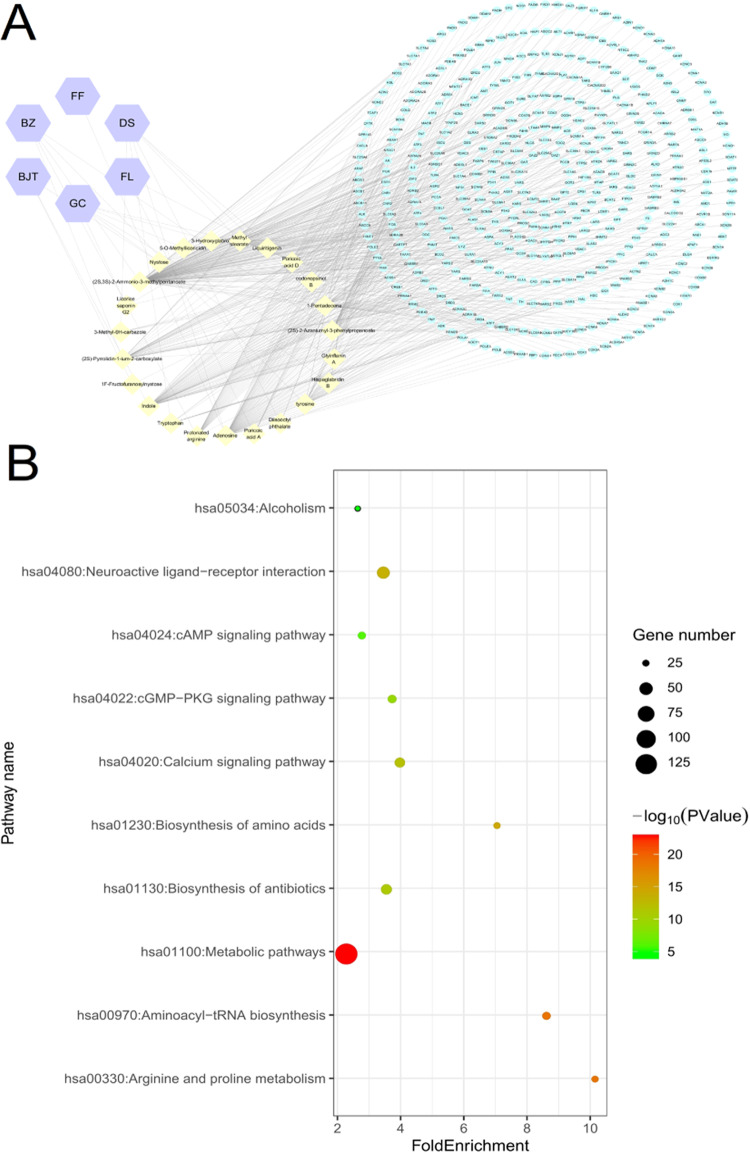
The multifarious chemical constitutes of FZK indicate complex mechanisms of action that warrant a systemic exploration. Therefore, 42 compounds identified by HPLC–MS were uploaded into BATMAN for the prediction of potential targets; target proteins with scores >40 were selected. The analysis by BATMAN retrieved a total of 6 herbal medicine ingredients, and 23 vital components with 446 targets were selected. To establish an intuitive correlation among herbal medicine, compound, and target, a herbal medicine–compound–target network was constructed using Cytoscape 3.2.1 (Figure 3A).
Figure 3.
Potential targets and pathway analysis. (A) Pharmacological network. Purple hexagon: herbal medicine; yellow diamond: components; green oval: targets. (B) Top 10 pathways. The area of the dot indicates the number of enriched genes; the larger the dot, the more the number of genes. The color of the dot represents the significance of P-value; the pathway with intense red color indicates a significant P-value.
Subsequently, a total of 46 pathways with P-value <0.01 were chosen after the completion of analysis by DAVID bioinformatics resource. The top 10 pathways, such as metabolic pathways, neuroactive ligand–receptor interaction, and calcium and cGMP–PKG signaling pathways, consisted of a maximal number of genes (Figure 3B).
2.3. Cell Viability Assay
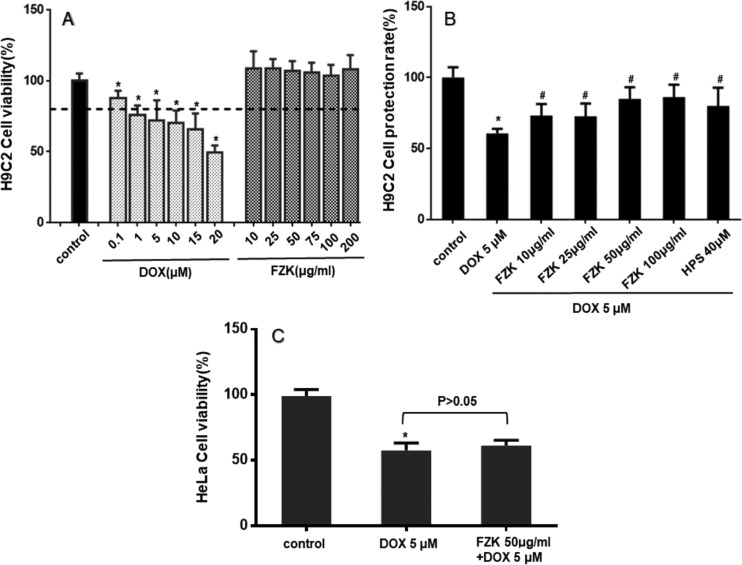
As shown in Figure 4A, DOX treatment-induced H9C2 cell death in a dose-dependent manner. The viability was 87.6 ± 3.1, 75.8 ± 3.9, 72.0 ± 8.2, 70.4 ± 5.0, 65.7 ± 6.4, and 49.2 ± 2.9% for DOX 0.1, 1, 5, 10, 15, and 20 μmol/L, respectively. Moreover, cell death under 10–200 μg/mL FZK treatment was not distinct. In this study, 5 μmol/L DOX was utilized. The effect of pretreatment with FZK on DOX-induced cells is displayed in Figure 4B. The rate of cell protection indicated that pretreatment with FZK attenuates DOX-induced cell death at experimental concentrations (P < 0.05 vs DOX). Thus, the concentration of 50 μg/mL of FZK was selected for subsequent studies.
Figure 4.
Effect of FZK and DOX on cell death. (A) Cytotoxicity: the cell viabilities of H9C2 cells treated with different concentrations of DOX or FZK. (B) Cytoprotective: the cell protection rate of H9C2 cells with or without pretreatment with different concentrations of FZK or HPS (positive control) before DOX. (C) Effect of FZK on the antitumor activity of DOX in HeLa cells. Data are mean ± SD (n = 3). *P < 0.05 vs control; #P < 0.05 vs DOX.
As shown in Figure 4C, after exposure to 5 μM DOX for 24 h, the number of viable HeLa cells significantly reduced to 57.7 ± 3.2% of the control cells (P < 0.05 vs control). The pretreatment with FZK (50 μg/mL) acquired a similar number of viable cells (61.3 ± 2.3% of the control, P > 0.05 vs DOX).
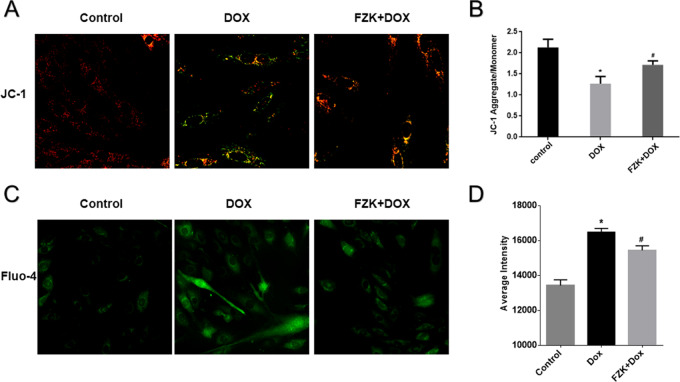
2.4. FZK Protects against DOX-Induced Mitochondrial Membrane Potential (MMP) Loss
JC-1 presents green fluorescence in the cytoplasmic as a monomer and red fluorescence in the mitochondria as aggregates. The transformation of JC-1 fluorescence from red to green indicated MMP loss. Thus, we used the ratio of aggregates and monomers to determine DOX-induced mitochondria injury and the influence of pretreatment with FZK. As shown in Figure 5 A,B, the FZK pretreatment attenuated the DOX-induced mitochondrial injury. In the control group, the ratio of aggregates/monomer was 2.1 ± 0.20. After treatment with DOX, the H9C2 cells showed a lower proportion of JC-1 aggregates in mitochondria due to the dissipation of MMP (1.27 ± 0.99, P < 0.05 vs control). However, this change could be moderated by pretreatment with FZK (1.70 ± 0.57, P < 0.05 vs control and DOX).
Figure 5.
Effect of FZK on MMP and intracellular Ca2+. (A) Representative fluorescent images of JC-1 staining, ×60. (B) Ratio of JC-1 aggregates to JC-1 monomer. (C) Representative fluorescent images of fluo-4, ×40. (D) Fluorescence intensity of calcium. Data are expressed as mean ± SD (n = 3). *P < 0.05 vs control; #P < 0.05 vs DOX.
2.5. FZK-Reduced Intracellular Ca2+ Accumulation Induced by DOX
Ca2+ overload is a critical mechanism responsible for DOX injury. As shown in Figure 5C,D, 5 μM DOX significantly potentiated Fluo-4 fluorescence (P < 0.05 vs control), demonstrating an elevation in intracellular Ca2+ concentration. Consequently, the fluorescence intensity was decreased significantly after FZK pretreatment as compared to that in the DOX group (P < 0.05 vs DOX).
2.6. FZK Protected H9C2 Cells from DOX-Induced Apoptosis
As exhibited in Figure 6, DOX treatment markedly increased the apoptosis in H9C2 cells, which was prevented by pretreatment with FZK. After exposure to DOX, the rate of apoptosis in cells (both early and late) was 56.8 ± 7.4% (P < 0.05 vs control). However, the apoptotic rate of the cells pretreated with FZK decreased significantly to 37.31 ± 7.27% (P < 0.05 vs DOX).
Figure 6.
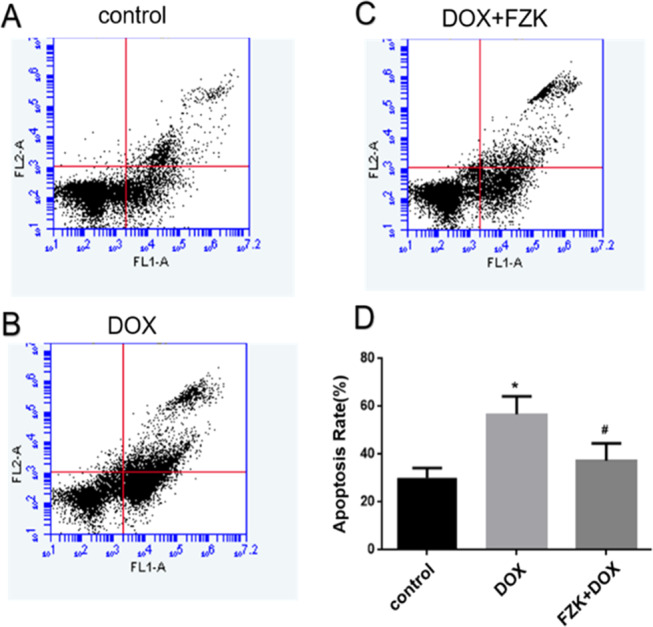
H9C2 cardiomyocyte apoptosis detection. (A) Control group; (B) DOX group; (C) FZK + DOX group; and (D) Ratio of apoptosis. Data are presented as mean ± SD (n = 3). *P < 0.05 vs control; #P < 0.05 vs DOX.
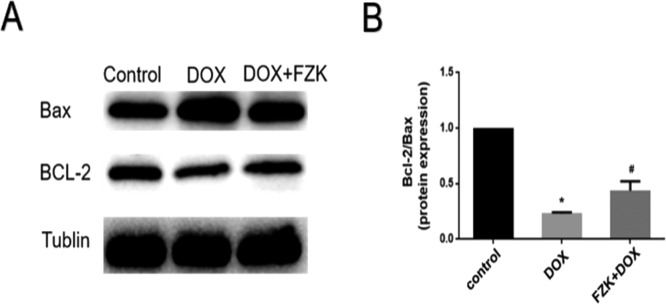
2.7. Expression of Bcl-2/Bax
Bax protein promotes cell apoptosis, and Bcl-2 is an antiapoptotic protein. Bax interacts with Bcl-2 to form a heterodimer and influences the process of cell apoptosis directly. In this study, the Bcl-2/Bax ratio was used to demonstrate the apoptosis degree. DOX-induced apoptosis, accompanied by decreased Bcl-2/Bax ratio (0.23 ± 0.01), and FZK pretreatment attenuated these changes by regulating Bcl-2 and Bax expression (0.43 ± 0.08) (Figure 7).
Figure 7.
Expression of Bax and Bcl-2. (A) Representative images. (B) Quantitative analysis of Bcl-2/Bax. Data are mean ± SD (n = 3). *P ≤ 0.05 vs control; #P ≤ 0.05 vs DOX.
3. Discussion
DOX is an anthraquinone antibiotic for cancer therapy and has been wildly used to treat several types of human malignancies since the late 1960s.24 However, the spectrum of its clinical application is limited due to severe cardiotoxic side effects.25,26 Thus, the discovery of a novel treatment strategy for DOX-induced cardiotoxicity is imperative. H9C2 is a permanent rat-derived cardiomyoblast cell line with morphological characteristics similar to those of undifferentiated embryonic cardiomyocytes. In many studies, the H9C2 cells are commonly used for cell-based heart failure research.27 Thus, H9C2 cells were selected for this study.
The present study aimed to confirm the protective effects of FZK against DOX-induced cardiotoxicity. Although the exact mechanisms of DOX-induced injury are yet unclear, several studies provide evidence that excessive generation of ROS is a key contributor to these effects.28 Also, DOX-mediated disturbances of cellular calcium homeostasis interrupt energy metabolism and increase the oxidative stress, further causing DOX-induced cell apoptosis.29 Compelling evidence showed that mitochondria and intracellular calcium play the key role in regulating DOX-induced cardiotoxicity.30 Herein, we proved that FZK pretreatment ameliorated the DOX-induced increase in cytosolic calcium level.
Cardiomyocyte apoptosis is the key event of DOX-induced cardiotoxicity. DOX-induced accumulation of ROS and overload of calcium in mitochondria lead to the dissipation of MMP, activating the mitochondrial permeability transition pore (MPTP). Then, cytochrome c was released, followed by caspase-3 activation and DNA fragmentation, resulting in cell apoptosis.31 The Bcl-2 protein family plays a pivotal role in the process of cardiomyocyte apoptosis via control of mitochondrial cytochrome c release and caspase activation.32 The Bax (proapoptotic) and Bcl-2 (antiapoptotic) proteins belong to this family. Bcl-2 is a crucial cellular component that defends cell apoptosis. Bax triggers mitochondria-mediated apoptosis, which releases apoptogenic factors and leads to mitochondrial dysfunction. These proapoptotic procedures can be antagonized by the antiapoptotic protein Bcl-2.33 In this study, DOX treatment induced apoptosis in H9C2 cells as a result of the loss of MMP, decreased expression of Bcl-2, and increased expression of Bax. Pretreatment with FZK ameliorated DOX-induced apoptosis, attenuated the dissipation of MMP, and increased the Bcl-2/Bax ratio in H9C2 cells. These results demonstrated the protective effect of FZK in DOX-induced cardiotoxicity. Next, we evaluated the effect of FZK on the antitumor activity of DOX in HeLa cells. The (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) (MTT) assay showed that the addition of FZK did not affect the antitumor activity of DOX.
FZK is a TCM containing a variety of active substances with cardiovascular protective properties. The present study aimed to explore the therapeutic role of FZK effectuated by its effective components acting on multiple targets and pathways. We identified 42 compounds by HPLC–MS system. Among these, tanshinone II, liquiritigenin, cimifugin, and scopoletin were reported to be effective in antiapoptosis and antihypertensive effects and inhibition of inflammation.34−38 With the rapid development of bioinformatics, the network pharmacology has gained increasing attention as an effective and innovative approach to uncover the underlying mechanisms of TCM formulas from a holistic perspective. In this study, 46 pathways were identified by KEGG analysis: metabolic pathways, neuroactive ligand–receptor interaction, calcium signaling pathway, and cGMP–PKG signaling pathway. The neuroactive ligand–receptor interaction signaling pathway, which encompasses several genes, can mediate cardioprotection.39 Furthermore, the regulation of neuroactive ligand–receptor interaction might protect the patients’ damaged cardiac function after coronary artery bypass grafting.40 Moreover, the KEGG analysis showed a correlation between calcium and cGMP–PKG signaling pathways and the actions of FZK. The cGMP-dependent protein kinase G (PKG) plays a pivotal role in intracellular environmental homeostasis. Proteins phosphorylated by PKG participate in calcium regulation, muscle contraction, inflammation response, and oxidative stress.41 As stated above, DOX is characterized by disrupted cellular calcium homeostasis, which leads to altered energy metabolism and promotes ROS generation and cell apoptosis. These findings were consistent with our previous experimental results, wherein sustained intracellular calcium homeostasis maintained the mitochondrial function and ameliorated the DOX-induced cell apoptosis. Thus, we inferred that FZK-sustained intracellular calcium homeostasis avoids DOX-induced cardiotoxicity by regulating the calcium pathway.
4. Conclusions
In this study, we confirmed that FZK protects H9C2 cells against DOX-induced cardiotoxicity and increases the antiapoptotic ability but does not affect the antitumor activity of DOX. In addition, we integrated the LC–MS analytical method with bioinformatics to understand the chemical composition and pharmacological effects of FZK. Several pathways, such as neuroactive ligand–receptor interaction, calcium signaling pathway, and cGMP–PKG signaling pathway, were predicted as the potential mechanisms of FZK. Thus, the herbal approach is a vast storehouse for developing new therapeutic agents for the treatment of DOX-induced cardiotoxicity, and FZK might serve as a complementary material.
5. Materials and Methods
5.1. Reagents
Fetal bovine serum (FBS), phosphate-buffered saline (PBS), penicillin-streptomycin, trypsin-EDTA, and Dulbecco’s modified Eagle’s medium (DMEM) were obtained from Gibico BRL (Grand Island, NY). DOX was obtained from Targetmol (Boston, MA). Deionized water was purified using Elga PURELAB flex system (ELGA LabWater, U.K.). HPLC-grade acetonitrile and formic acid were obtained from Merck (Darmstadt, Germany) and Aladdin (Aladdin, China).
The FZK decoction, consisting of 30 g of Dangshen, 15 g of Fuling, 10 g of Fangfeng, 10 g of Baizhu, 10 g of Bajitian, and 8 g of Gancao, was provided by Hangzhou First People’s Hospital (Hangzhou, China). The quality was controlled according to the Standard Operation Procedure of Chinese Pharmacopoeia. The supernatant of the mixture was collected by centrifugation at 12 000 rpm for 15 min.
5.2. Characterization of Major Chemical Constituents in FZK by HPLC–MS
HPLC–MS analysis was performed on an Agilent 1100 system coupled with an Agilent 1946D Quadrupole mass spectrometer, using Waters CORTECS T3 column (100 mm × 4.6 mm i.d., 2.7 μm; Agilent Technologies) maintained at 30 °C. The flow rate was 0.4 mL/min, and the injection volume was 5 μL. The mobile phase consisted of water containing 0.1% v/v formic acid (A) and acetonitrile (B). A gradient program was used for the following profile: 0–5 min, 0–0% B; 5–60 min, 0–60% B; 60–65 min, 60–100 min; and 65–70 min, 100–100%. The detection wavelength was set at 280 nm, and the data were acquired in full scan positive and negative modes under following parameters: mass range, 100–1200 m/z; gas temperature, 350 °C; drying gas, 10.0 L/min; nebulizer pressure, 45 psig; capillary voltage (positive), 4000 V; capillary voltage (negative), 3500 V; fragmentor, 150; gain, 1; and full scan mode.
5.3. Potential Targets and Enrichment Analysis
Herein, BATMAN-TCM, a bioinformatics analysis tool of TCM, was selected to predict the potential targets for the ingredients mentioned above.42 The targets, obtained from the DrugBank, KEGG, and TTD databases, were ranked according to the interactions between potential targets and resemblance to known targets, based on scores from high to low; those with scores >40 were selected. The network visualization software Cytoscape (ver. 3.2.1) was used to build an herbal medicine–constituents–target protein pharmacological network based on the data acquired by previous steps.
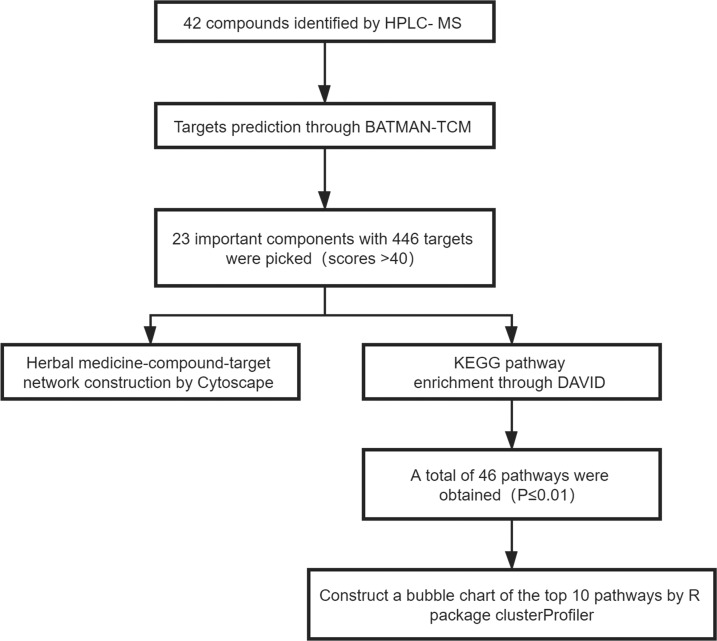
DAVID (ver. 6.7) was applied for (KEGG) pathway enrichment analysis.43 The data were analyzed using the R package clusterProfiler (ver. 3.6.0), with a threshold value of P ≤ 0.01. Also, the general flowchart of target network construction and pathway enrichment is shown in Figure 8.
Figure 8.
Research process of target network construction and pathway enrichment.
5.4. Cell Culture and Treatment
H9C2 and HeLa cells, obtained from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China), were cultured in DMEM with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C under an atmosphere of 5% CO2. The H9C2 cells were treated with 0.1, 1, 5, 15, or 20 μM DOX and 10, 25, 50, 75, 100, 150, or 200 μg/mL FZK for 24 h to acquire the appropriate concentration of DOX and FZK for the current study. The three groups were established according to different treatments: cells without additional treatment in the control group; 5 μM DOX was applied for 24 h to cells in the DOX group; and 50 μg/mL FZK was applied for 4 h, followed by 5 μM DOX for 24 h to cells in FZK + DOX group.
5.5. Viability Assay by MTT Assay
Cell viability was assessed by the MTT assay. The cells were seeded at a density of 5 × 104/well in 96-well plates and received different treatments. Quercetin (Hupisu, HPS), a type of flavonoid, has been reported to exert protective effects against DOX-induced cell death44 and hence served as the positive control. Then, 100 μL of MTT (5 mg/mL) was added to each well for 4 h, followed by dimethyl sulfoxide. The optical density (OD) was measured at 580 nm using a microtiter plate reader.
5.6. Measurement of MMP
A fluorescence microscope was used to detect the loss of mitochondrial membrane potential by JC-1 staining. The H9C2 cells were treated with JC-1 for 30 min at 37 °C after intervention as described above and scanned using ImageXpress Micro Confocal High-Content Imaging System (Molecular Devices), with a 60× Plan Fluor objective. The fluorescent values were analyzed by the analysis module of MetaXpress High-Content Image and Analysis Software (Molecular Devices) system. The monomeric of JC-1 was detected at 475 and 536 nm as the green excitation and emission wavelengths, respectively, while the aggregation of JC-1 was detected at 530 and 593 nm as red excitation and emission wavelengths, respectively.
5.7. Measurement of Intracellular Ca2+ Levels by Fluo-4
H9C2 cells were seeded in 96-well plates, incubated with or without 50 μg/mL FZK, and loaded with 5 μM Fluo-4 (Beyotime, China) at 37 °C for 60 min, according to the manufacturer’s protocol. The intracellular Ca2+ concentration was evaluated by measuring the fluorescence intensity after administrating DOX, excitation at 488 nm, and emission at 510 nm using ImageXpress Micro Confocal High-Content Imaging System. The fluorescent values were analyzed by the analysis module of MetaXpress High-Content Image and Analysis Software system.
5.8. Apoptosis Measurement
The rate of apoptosis was measured by flow cytometry assay using Annexin V fluorescein isothiocyanate/propidium iodide (Annexin V-FITC/PI) double staining. The cells in the logarithmic phase were seeded in six-well plates. After the treatment, as mentioned above, the cells were collected by 0.05% trypsin, washed twice using PBS, and incubated in 500 μg/mL of binding buffer. Then, 5 μL of Annexin V-FITC and 10 μL of PI were added to each well and cultured in the dark for 15 min at room temperature. The apoptotic rate of each sample was measured by flow cytometry (Accuri C6, BD).
5.9. Western Blotting
The concentration of the total protein of each group was quantified by BCA assay kit (ThermoFisher). An equivalent of protein was separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to poly(vinylidene fluoride) (PVDF) membrane (Millipore, Bedford, MA). After blocking with 10% skim milk, the membranes were probed with primary antibodies (Bcl-2, 1:1000; Bax 1:1000; Tublin 1:1000, Beyotime) overnight at 4 °C, followed by incubation with secondary antibodies. The immunoreactive bands were analyzed using ChemiDoc Touch Imaging System and Image Lab software (Bio-Rad).
5.10. Statistical analysis
The data are expressed as mean ± SD. Statistical significance was determined using one-way analysis of variance (ANOVA, GraphPad Prism 6 Software). P-value <0.05 was considered statistically significant.
Acknowledgments
This study was supported by the Traditional Chinese Medicine Science and Technology Plan Project of Zhejiang Province (2019ZQ038) and the Traditional Chinese Medicine Science and Technology Plan Project of Zhejiang Province (2017ZQ021).
Author Contributions
⊥ Y.Z. and M.L. contributed equally to this study.
The authors declare no competing financial interest.
References
- Carvalho C.; Santos R. X.; Cardoso S.; Correia S.; Oliveira P. J.; Santos M. S.; Moreira P. I. Doxorubicin: the good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285. 10.2174/092986709788803312. [DOI] [PubMed] [Google Scholar]
- Pommier Y.; Leo E.; Zhang H.; Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Štěrba M.; Popelova O.; Vavrova A.; Jirkovsky E.; Kovarikova P.; Gersl V.; Simunek T. Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection. Antioxid. Redox Signaling 2013, 18, 899–929. 10.1089/ars.2012.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. L.; Wang Z. V.; Ding G.; Tan W.; Luo X.; Criollo A.; Xie M.; Jiang N.; May H.; Kyrychenko V.; Schneider J. W.; Gillette T. G.; Hill J. A. Doxorubicin Blocks Cardiomyocyte Autophagic Flux by Inhibiting Lysosome Acidification. Circulation 2016, 133, 1668–1687. 10.1161/CIRCULATIONAHA.115.017443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Daim M. M.; Kilany O. E.; Khalifa H. A.; Ahmed A. A. M. Allicin ameliorates doxorubicin-induced cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Cancer Chemother. Pharmacol. 2017, 80, 745–753. 10.1007/s00280-017-3413-7. [DOI] [PubMed] [Google Scholar]
- Wu B. N.; Huang Y. C.; Wu H. M.; Hong S. J.; Chiang L. C.; Chen I. J. A highly selective beta1-adrenergic blocker with partial beta2-agonist activity derived from ferulic acid, an active component of Ligusticum wallichii Franch. J. Cardiovasc. Pharmacol. 1998, 31, 750–757. 10.1097/00005344-199805000-00014. [DOI] [PubMed] [Google Scholar]
- Yang X.; Jia C. Understanding association of spleen system with earth on traditional Chinese medicine theory. J. Tradit. Chin. Med. 2013, 33, 134–136. 10.1016/S0254-6272(13)60115-6. [DOI] [PubMed] [Google Scholar]
- Pastorino G.; Cornara L.; Soares S.; Rodrigues F.; Oliveira M. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. 10.1002/ptr.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. H.; Xin H. L.; Xu Y. M.; Shen Y.; He Y. Q.; Hsien Y.; Lin B.; Song H. T.; Juan L.; Yang H. Y.; Qin L. P.; Zhang Q. Y.; Du J. Morinda officinalis How. - A comprehensive review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2018, 213, 230–255. 10.1016/j.jep.2017.10.028. [DOI] [PubMed] [Google Scholar]
- Kreiner J.; Pang E.; Lenon G. B.; Yang A. W. H. Saposhnikoviae divaricata: a phytochemical, pharmacological, and pharmacokinetic review. Chin. J. Nat. Med. 2017, 15, 255–264. 10.1016/S1875-5364(17)30042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B.; Zhang Q. L.; Hua J. W.; Cheng W. L.; Qin L. P. The traditional uses, phytochemistry, and pharmacology of Atractylodes macrocephala Koidz.: A review. J. Ethnopharmacol. 2018, 226, 143–167. 10.1016/j.jep.2018.08.023. [DOI] [PubMed] [Google Scholar]
- Gao S. M.; Liu J. S.; Wang M.; Cao T. T.; Qi Y. D.; Zhang B. G.; Sun X. B.; Liu H. T.; Xiao P. G. Traditional uses, phytochemistry, pharmacology and toxicology of Codonopsis: A review. J. Ethnopharmacol. 2018, 219, 50–70. 10.1016/j.jep.2018.02.039. [DOI] [PubMed] [Google Scholar]
- Rios J.-L. Chemical constituents and pharmacological properties of Poria cocos. Planta Med. 2011, 77, 681–691. 10.1055/s-0030-1270823. [DOI] [PubMed] [Google Scholar]
- Liu P.; Li Z.; Qian D.; Li W.; Shang E. X.; Duan J. A. Simultaneous determination of bioactive components in essential oil of Xiang-Fu-Si-Wu Formula in Beagle dog plasma by UPLC-MS/MS and its application to pharmacokinetics. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2013, 929, 63–69. 10.1016/j.jchromb.2013.04.022. [DOI] [PubMed] [Google Scholar]
- Hopkins A. L. Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- Tang H.; He S.; Zhang X.; Luo S.; Zhang B.; Duan X.; Zhang Z.; Wang W.; Wang Y.; Sun Y. A Network Pharmacology Approach to Uncover the Pharmacological Mechanism of XuanHuSuo Powder on Osteoarthritis. J. Evidence-Based Complementary Altern. Med. 2016, 2016, 3246946 10.1155/2016/3246946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa T.; Watari T.; Ashizawa K.; Hanada D.; Yanagiya T.; Watanabe N.; Terada T.; Tomoda Y.; Fujii S. Development of an improved rapid BACpro(R) protocol and a method for direct identification from blood-culture-positive bottles using matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J. Microbiol. Methods 2018, 148, 138–144. 10.1016/j.mimet.2018.04.005. [DOI] [PubMed] [Google Scholar]
- Wadman M. “Rapid onset” of transgender identity ignites storm. Science 2018, 361, 958–959. 10.1126/science.361.6406.958. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Qiao X.; Liu C. F.; Ji S.; Feng L. M.; Qian Y.; Guo D. A.; Ye M. Metabolites identification of glycycoumarin, a major bioactive coumarin from licorice in rats. J. Pharm. Biomed. Anal. 2014, 98, 287–295. 10.1016/j.jpba.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Wei J.; Yang M. Simultaneous Analysis of Iridoid Glycosides and Anthraquinones in Morinda officinalis Using UPLC-QqQ-MS/MS and UPLC-Q/TOF-MS(E). Molecules 2018, 23, 1070. 10.3390/molecules23051070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Yang J.; Cai Z. Chemical investigation on Sijunzi decoction and its two major herbs Panax ginseng and Glycyrrhiza uralensis by LC/MS/MS. J. Pharm. Biomed. Anal. 2006, 41, 1642–1647. 10.1016/j.jpba.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Ichikawa M.; Ohta S.; Komoto N.; Ushijima M.; Kodera Y.; Hayama M.; Shirota O.; Sekita S.; Kuroyanagi M. Rapid identification of triterpenoid saponins in the roots of Codonopsis lanceolata by liquid chromatography-mass spectrometry. J. Nat. Med. 2008, 62, 423–429. 10.1007/s11418-008-0270-z. [DOI] [PubMed] [Google Scholar]
- Lee S.; Choi E.; Yang S. M.; Ryoo R.; Moon E.; Kim S. H.; Kim K. H. Bioactive compounds from sclerotia extract of Poria cocos that control adipocyte and osteoblast differentiation. Bioorg. Chem. 2018, 81, 27–34. 10.1016/j.bioorg.2018.07.031. [DOI] [PubMed] [Google Scholar]
- Jain D. Cardiotoxicity of doxorubicin and other anthracycline derivatives. J Nucl Cardiol 2000, 7, 53–62. 10.1067/mnc.2000.103324. [DOI] [PubMed] [Google Scholar]
- Fu L. X.; Waagstein F.; Hjalmarson A. A new insight into adriamycin-induced cardiotoxicity. Int. J. Cardiol. 1990, 29, 15–20. 10.1016/0167-5273(90)90267-9. [DOI] [PubMed] [Google Scholar]
- Singal P. K.; Iliskovic N.; Li T.; Kumar D. Adriamycin cardiomyopathy: pathophysiology and prevention. FASEB J. 1997, 11, 931–936. 10.1096/fasebj.11.12.9337145. [DOI] [PubMed] [Google Scholar]
- Yu H.; Chen B.; Ren Q. Baicalin relieves hypoxia-aroused H9c2 cell apoptosis by activating Nrf2/HO-1-mediated HIF1alpha/BNIP3 pathway. Artif. Cells, Nanomed., Biotechnol. 2019, 47, 3657–3663. 10.1080/21691401.2019.1657879. [DOI] [PubMed] [Google Scholar]
- Tocchetti C. G.; Carpi A.; Coppola C.; Quintavalle C.; Rea D.; Campesan M.; Arcari A.; Piscopo G.; Cipresso C.; Monti M. G.; De Lorenzo C.; Arra C.; Condorelli G.; Di Lisa F.; Maurea N. Ranolazine protects from doxorubicin-induced oxidative stress and cardiac dysfunction. Eur. J. Heart Failure 2014, 16, 358–366. 10.1002/ejhf.50. [DOI] [PubMed] [Google Scholar]
- Moore L.; Landon E. J.; Cooney D. A. Inhibition of the cardiac mitochondrial calcium pump by adriamycin in vitro. Biochem. Med. 1977, 18, 131–138. 10.1016/0006-2944(77)90084-9. [DOI] [PubMed] [Google Scholar]
- Solem L. E.; Heller L. J.; Wallace K. B. Dose-dependent increase in sensitivity to calcium-induced mitochondrial dysfunction and cardiomyocyte cell injury by doxorubicin. J. Mol. Cell. Cardiol. 1996, 28, 1023–1032. 10.1006/jmcc.1996.0095. [DOI] [PubMed] [Google Scholar]
- Renu K.; Valsala Gopalakrishnan A. Deciphering the molecular mechanism during doxorubicin-mediated oxidative stress, apoptosis through Nrf2 and PGC-1alpha in a rat testicular milieu. Reprod. Biol. 2019, 19, 22–37. 10.1016/j.repbio.2019.02.004. [DOI] [PubMed] [Google Scholar]
- Sugioka R.; Shimizu S.; Funatsu T.; Tamagawa H.; Sawa Y.; Kawakami T.; Tsujimoto Y. BH4-domain peptide from Bcl-xL exerts anti-apoptotic activity in vivo. Oncogene 2003, 22, 8432–8440. 10.1038/sj.onc.1207180. [DOI] [PubMed] [Google Scholar]
- Cheng E. H.; Wei M. C.; Weiler S.; Flavell R. A.; Mak T. W.; Lindsten T.; Korsmeyer S. J. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell 2001, 8, 705–711. 10.1016/S1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Xie X. W. Liquiritigenin attenuates cardiac injury induced by high fructose-feeding through fibrosis and inflammation suppression. Biomed. Pharmacother. 2017, 86, 694–704. 10.1016/j.biopha.2016.12.066. [DOI] [PubMed] [Google Scholar]
- Hong H. J.; Liu J. C.; Chen P. Y.; Chen J. J.; Chan P.; Cheng T. H. Tanshinone IIA prevents doxorubicin-induced cardiomyocyte apoptosis through Akt-dependent pathway. Int. J. Cardiol. 2012, 157, 174–179. 10.1016/j.ijcard.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Han B.; Dai Y.; Wu H.; Zhang Y.; Wan L.; Zhao J.; Liu Y.; Xu S.; Zhou L. Cimifugin Inhibits Inflammatory Responses of RAW264.7 Cells Induced by Lipopolysaccharide. Med. Sci. Monit. 2019, 25, 409–417. 10.12659/MSM.912042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan K. K. S.; Jayakumar D.; Velusamy P.; Srinivasan A.; Mohan T.; Ravi D. B.; Uthamaraman S.; Sathyamoorthy Y. K.; Rajasekaran N. S.; Periandavan K. Morinda citrifolia and Its Active Principle Scopoletin Mitigate Protein Aggregation and Neuronal Apoptosis through Augmenting the DJ-1/Nrf2/ARE Signaling Pathway. Oxid. Med. Cell. Longevity 2019, 2019, 2761041 10.1155/2019/2761041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunas-Herrera H.; Tortoriello J.; Herrera-Ruiz M.; Martinez-Henandez G. B.; Zamilpa A.; Santamaria L. A.; Lorenzana M. G.; Lombardo-Earl G.; Jimenez-Ferrer E. Acute and Chronic Antihypertensive Effect of Fractions, Tiliroside and Scopoletin from Malva parviflora. Biol. Pharm. Bull. 2019, 42, 18–25. 10.1248/bpb.b18-00355. [DOI] [PubMed] [Google Scholar]
- Lauss M.; Kriegner A.; Vierlinger K.; Noehammer C. Characterization of the drugged human genome. Pharmacogenomics 2007, 8, 1063–1073. 10.2217/14622416.8.8.1063. [DOI] [PubMed] [Google Scholar]
- Wang J.; Cheng J.; Zhang C.; Li X. Cardioprotection Effects of Sevoflurane by Regulating the Pathway of Neuroactive Ligand-Receptor Interaction in Patients Undergoing Coronary Artery Bypass Graft Surgery. Comput. Math. Methods Med. 2017, 2017, 3618213 10.1155/2017/3618213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaiber M.; Kruse M.; Volker K.; Schroter J.; Feil R.; Freichel M.; Gerling A.; Feil S.; Dietrich A.; Londono J. E.; Baba H. A.; Abramowitz J.; Birnbaumer L.; Penninger J. M.; Pongs O.; Kuhn M. Novel insights into the mechanisms mediating the local antihypertrophic effects of cardiac atrial natriuretic peptide: role of cGMP-dependent protein kinase and RGS2. Basic Res. Cardiol. 2010, 105, 583–595. 10.1007/s00395-010-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Guo F.; Wang Y.; Li C.; Zhang X.; Li H.; Diao L.; Gu J.; Wang W.; Li D.; He F. BATMAN-TCM: a Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine. Sci. Rep. 2016, 6, 21146 10.1038/srep21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D. W.; Sherman B. T.; Lempicki R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Kaiserová H.; Simunek T.; van der Vijgh W. J.; Bast A.; Kvasnickova E. Flavonoids as protectors against doxorubicin cardiotoxicity: role of iron chelation, antioxidant activity and inhibition of carbonyl reductase. Biochim. Biophys. Acta 2007, 1772, 1065–1074. 10.1016/j.bbadis.2007.05.002. [DOI] [PubMed] [Google Scholar]