Abstract
Malabaricol is a unique plant natural product, 3-keto tricarbocyclic triterpenoid, isolated from Ailanthus malabarica. Malabaricol underwent reaction with aromatic aldehydes under alkaline conditions to form 2-arylidene analogs. Indoles and pyrazine ring system fused to the 2,3-position of malabaricol were synthesized. In this ring system of tricarbocyclic triterpenoid, the conformation is such that there is no steric hindrance due to C4 and C10 axial methyl groups and other skeletons. Malabaricol and its synthetic analogues show cytotoxic activity toward lung cancer, which was compared to that of standard doxorubicin.
Introduction
Triterpenoids are prolific in the plant kingdom, while malabaricol, a triterpenoid isolated from the gum exudates of the trunk of Ailanthus malabaricol by Dev,1,2 is the first tricarbocyclic triterpenoid. It is also unusual with a 3-keto group, having a side chain containing tetrahydrofuran group. Its structure was established by many chemical reactions and spectral data.1,2 Further direct correlation of malabaricol with (+)-ambrenolide by circular dichroism provided the structural proof and also absolute stereochemistry at C5, C8, C9, and C10.1,2 The confirmation and stereochemistry of malabaricol structure were disclosed in the work of Van Tamelen,3 who reported nonenzymatic cyclization of squalene-2,3-epoxide (I) using stannic chloride. Thus, Van Tamelen obtained a tricarbocyclic-type triterpenoid skeleton, which suggested that such a structure is feasible.
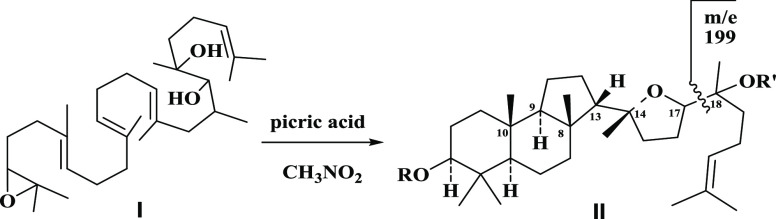
Sharpless also studied the nonenzymatic cyclization of squalene-2,3-epoxide by picric acid4 leading to the synthesis of d,l-malabaricanediol (II) (C3–OH instead of C=O in malabaricol), which revealed the stereochemical positions at C17 and C20, as shown Figure 1. To elaborate further, the Sharpless study confirmed that the chemical tendency of the cyclization of 18,19-dihydroxy squalene-2,3-epoxide I led to the malabaricol skeleton, d,l-malabaricanediol (II),5 while the natural enzyme-catalyzed process in living organisms provided exclusively a vast number of tetra or pentacyclic triterpenoids or lanostanes or steroids.
Figure 1.
Squalene-2,3-epoxide to d,l-malabaracanediol.4
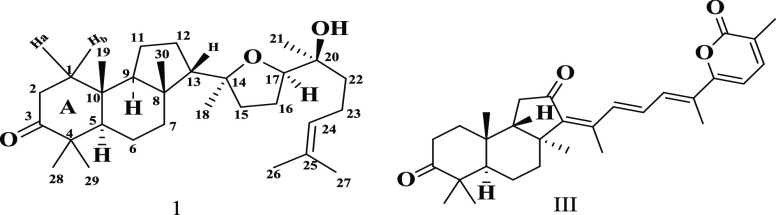
Further, Sharpless, based on biogenetic reasoning, proposed the configuration at C13 and C14.4 Finally, the structure and stereochemistry of malabaricol were confirmed by X-ray analysis on the crystal of malabaricol.6 Subsequent to the isolation of malabaricol 1, a few related tricarbocyclic triterpenoids were isolated from Ailanthus and other species.7−14 Natural tricarbocyclic skeleton isomalabaricanes were also isolated from sponges.15−17 The structure of isomalabaricanes (III) differs from that of malabaricol 1 in the stereochemistry of C9 and C8, as shown in Figure 2.
Figure 2.
Structure of malabaricol and isomalabaricanes.17
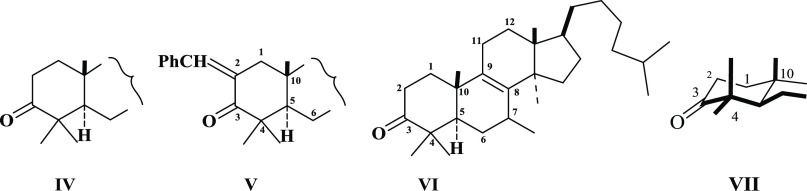
In 1957, Barton et al.18−20 explored sterically and/or electronically the interaction of various functional groups, in tetracyclic triterpenoids, pentacyclic triterpenoids, steroids, and lanost-8-enone (VI), the four types of structures. Barton conducted a conformational study based on the steric effects of the above compounds based on chemical reactions. Thus, Barton studied the condensation of benzaldehyde under alkaline conditions with triterpinoids having 3-keto function. The structures of these starting 3-ketotriterpinoids have a partial structure (IV), which, on base-catalyzed condensation of benzaldehyde with reactive 2-methylene group, led to the formation of benzylidene structure (V). To determine the rate of condensation, the reaction of benzaldehyde with lanost-8-enone (VI) is taken as standard in this case study. Barton investigated the rate of base-catalyzed condensation of the above 3-keto compounds to 2-benzylidene products. Barton also observed that all of the 3-keto compounds formed 2-arylidene compounds, but the rates of formation are different.
Based on kinetic studies, the differences in the rate of formation may be due to the partial steric hindrance at C3-keto due to C4 and C10 axial methyl groups, although these compounds have rigid extended conformations. Thus, the steric effect, although possible, as shown in the partial structure VII due to the C4 and C10 axial methyl groups, does not have a direct role. Another possibility to influence the rate of reaction is by electrostatic effect, space or through bond induction, which is collectively called the inductive effect. This possibility also does not seem to have a role in the rate of formation of 2-benzylidene derivatives. Therefore, the difference in the rate of formation of 3-benzylidene derivatives is due to “conformational transmission”. Barton attributed the differences in the rates of formation of 2-benzylidene formation to the 1:3 interaction between axial methyl groups at C4 and C10 (VII) (Figure 3). Finally, Barton described that methyl groups at C4, C10, and C8 must interact with each other in a 1:3 manner to explain the kinetics of the rate of formation. Therefore, this study provided a method to determine the effects of functional groups found elsewhere in these structures of tetra or pentacyclic triterpenoids, etc. on the reactions of functional groups of ring A. As a result of this study, it is found that the kinetics of this reaction is remarkably influenced by what Barton termed as conformational transmission. This effect is primarily due to distortion in a distant part of the molecule and appears to be transmitted to the reaction site (i.e., 3-CO, 2-CH2– of ring A of triterpenoids or steroids, etc.) through long-range effects like slight angle and bond distortion.
Figure 3.
Condensation of benzaldehyde with triterpenoide-3-ketone.18
Results and Discussion
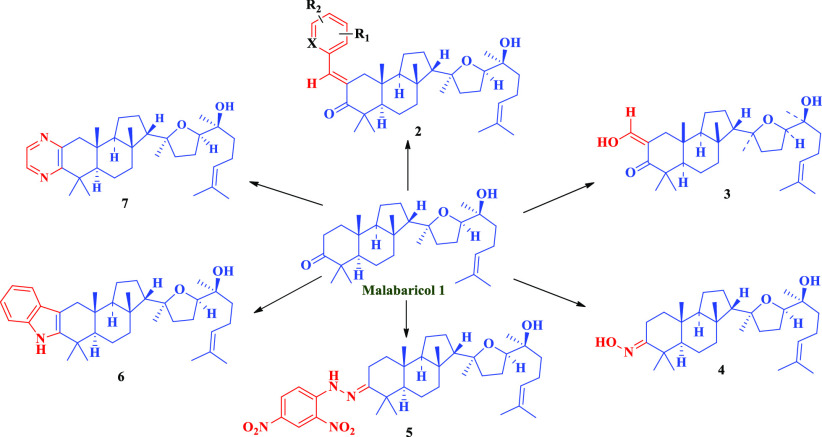
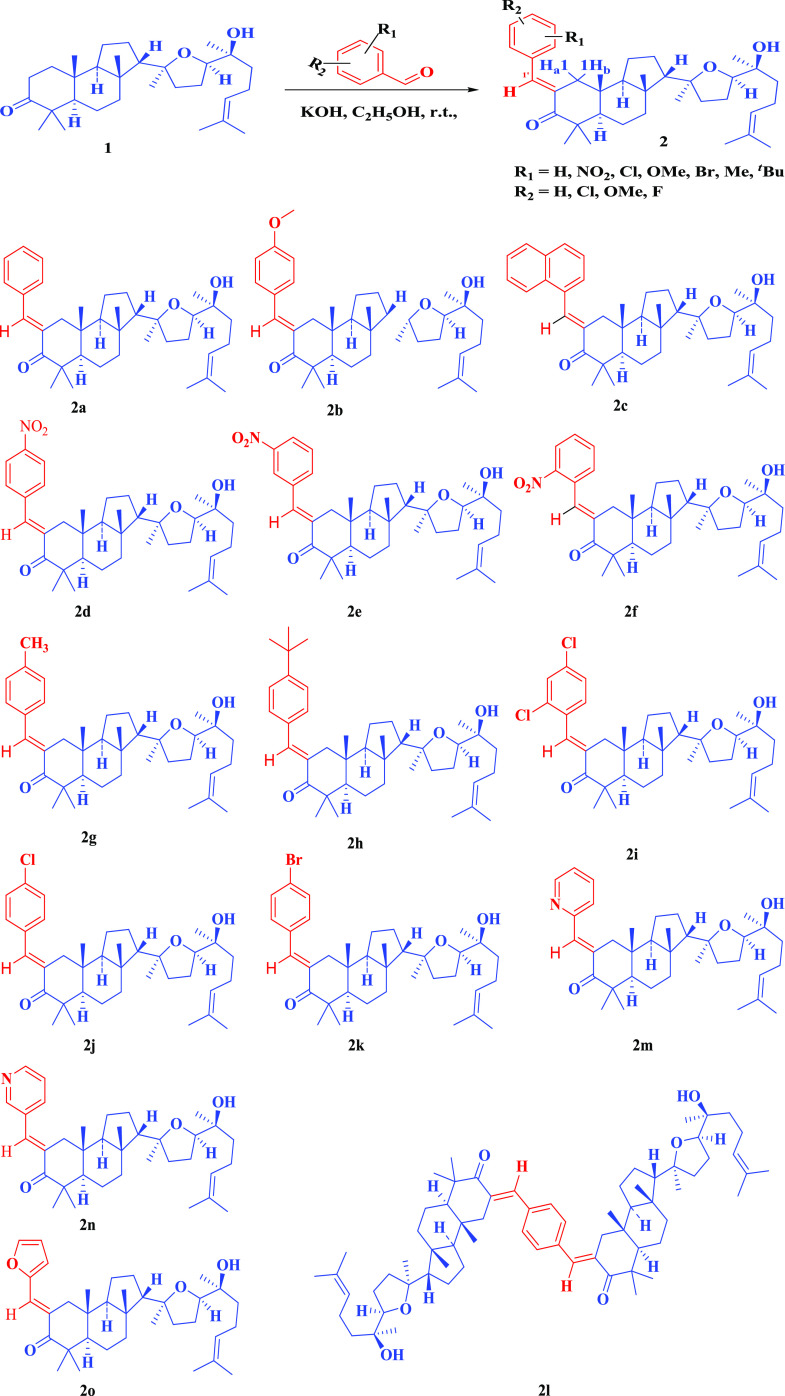
Since malabaricol 1 has a tricarbocyclic ring system, the present study aims at the investigation of the reactivity of the 3-keto group ring and 2-methylene group with various aromatic aldehydes as well as with aromatic hydrazines and 1,2-diamino aliphatic amine. The formation of these compounds depends on the steric disposition of C4 and C10 axial methyl groups and other factors such as the tricarbocyclic nature of malabaricol. The isolation of malabaricol 1, which has 3-keto and reactive C2 methylene group, in our laboratories21 provided us an opportunity to study if 2-arylidene-3-keto compounds will be formed. Such a study is useful to understand the conformation of tricarbocyclic triterpenoids. Thus, malabaricol 1 was condensed with various aryl aldehydes (electron-donating or electron-withdrawing substituents) under alkaline conditions. Malabaricol 1 was also condensed by bulky naphthalene-1-carboxaldehyde and heterocyclic aldehydes. The yields of 2-arylidene malabaricol are between 49 and 71% (Scheme 1). In the 1H NMR spectra of malabaricol C2, two multiplets at δ 2.63–2.47 and δ 2.42–2.29 are observed due to the mutual coupling of two protons at C2 as well as the interaction with two vicinal protons of C1. Further, δ 3.66 (t, J = 6.6 Hz) is due to C17-H and δ 5.08 (m) is due to C24-H. In 2-benzylidene-3-ones, C1-Ha appeared as a doublet at δ 2.31 (d, J1Ha, 1Hb = 15.8 Hz) and C1-Hb appeared as a doublet at δ 2.94 (d, J1Hb, 1Ha = 15.8 Hz). The triplet at δ 3.70 (J = 7.0 Hz) is due to C17-H, the triplet δ 5.12 (J = 7.1 Hz) is due to C24-H, and the singlet at δ 7.54 is due to benzylidene CH. Further, in the IR data of malabaricol, 3-C=O appeared around 1700 cm–1, whereas in benzylidene analogues, 3-C=O appeared at a reduced frequency, around 1680 cm–1 due to extended conjugation. This shows that the arylidene ring is coplanar with ring A, in particular with 3-C=O in all of the arylidene analogues (Scheme 1).
Scheme 1. 2-Arylidene Derivatives of Malabaricol.
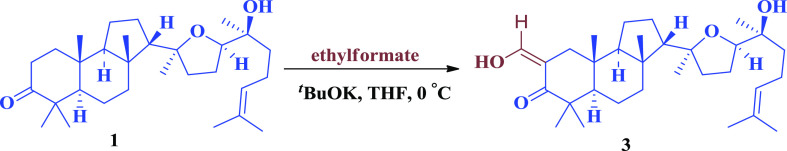
Condensation involving the reaction of C2-methylene with ethylformate under basic conditions yielded an enol (Scheme 2).
Scheme 2. Enol Derivative of Malabaricol.
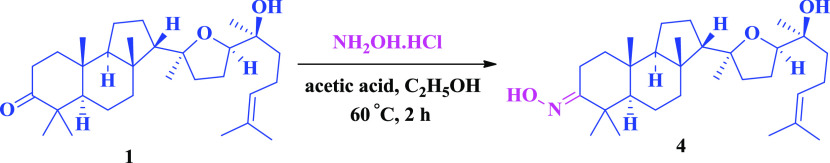
Scheme 3 depicts the formation of 3-oxime with hydroxylamine hydrochloride in acetic medium.
Scheme 3. Oxime Derivative of Malabaricol.
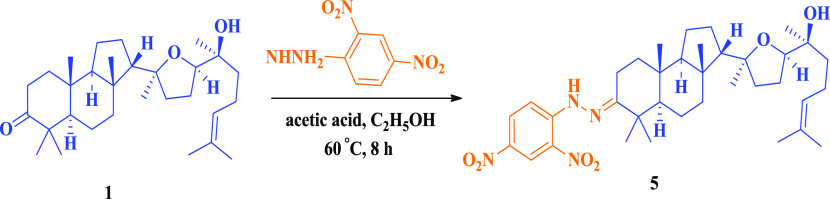
Scheme 4 shows that the condensation of malabaricol 1 with 2,4-dinitrophenylhydrazine in acetic medium at refluxing temperature yielded malabaricol 3-hydrazones. Fused 2′,3′-indole formation is not observed even at elevated temperatures and longer hours of reflux. This is due to the electron-withdrawing effect of the nitro groups.
Scheme 4. Hydrazone Derivative of Malabaricol.
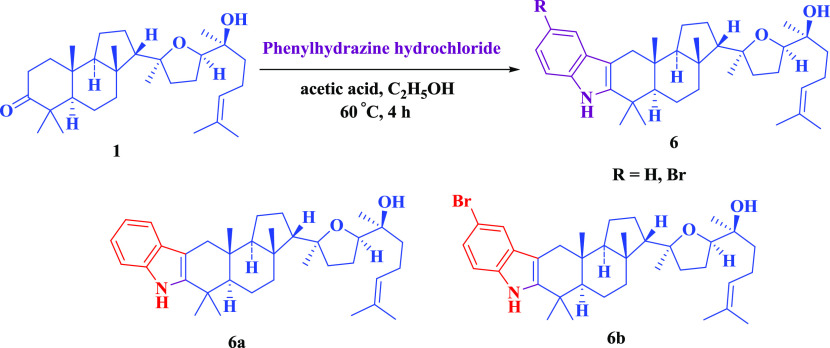
However, at elevated temperatures, the condensation of phenylhydrazine in acetic acid medium at 60 °C yielded 2′,3′-fused indoles. Two examples are given in Scheme 5.
Scheme 5. Indole Derivatives of Malabaricol.
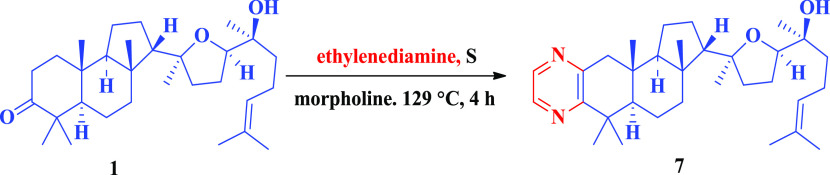
The condensation of malabaricol with 1,2-ethylenediamine (Scheme 6) in the presence of sulfur in morpholine at the refluxing temperature led to the formation 2′,3′-fused pyrazine, involving both 2 and 3 positions of malabaricol and subsequent dehydrogenation.
Scheme 6. Pyrazine Derivatives of Malabaricol.
Thus, the present study revealed that the conformation of malabaricol is favorable for the formation of 3-arylidene analogues and other reactions (Schemes 1–6) enumerated in this paper. This study revealed that the steric hindrance of C4 and C10 axial methyl groups and the tricarbocyclic system do not interfere in the reactivity of ring A, as observed in other triterpinoids.18−20 Malabaricol has been studied for its antifungal activity and metabolic disorders including diabetes without much success.21 These new derivatives of this compound were screened for the cytotoxicity effects on the human cell line.
We have tested all compounds (1–7) for their cytotoxicity against A549 (human lung cancer cell line) cell line with doxorubicin as control. The data is presented in Table 1. Surprisingly, several derivatives including the parent malabaricol showed good activity. Structural activity relationship suggests that among the benzylidine derivatives (2a–l), simple para-substituted compounds did not show any activity except for 2h and 2d. On the other hand ortho, para-dichloro-substituted compound (2i) displayed better activity. In contrast to benzylidene derivatives, the heterocyclic furylidene and pyrolidene analogues showed good activity. While compounds 3 and 5 did not show any cytotoxicity, 4 was active. All 2,3-fused derivatives (6a, 6b, and 7) displayed cytotoxicity. On the basis of activity, further synthetic work on modification of malabaricol 1 will be studied.
Table 1. Cytotoxicity Activity of Malaboricol and Its Synthetic Analogues on Human Lung Cancer Cell Line.
| s. no. | compound number | IC50 at 10 μM |
|---|---|---|
| 1 | 1 | 10.91 ± 0.001 |
| 2 | 2a | 11.41 ± 0.006 |
| 3 | 2b | >50 |
| 4 | 2c | >50 |
| 5 | 2d | 13.24 ± 0.002 |
| 6 | 2e | >50 |
| 7 | 2f | >50 |
| 8 | 2g | >50 |
| 9 | 2h | 12.37 ± 0.004 |
| 10 | 2i | 10.03 ± 0.003 |
| 11 | 2j | >50 |
| 12 | 2k | >50 |
| 13 | 2l | >50 |
| 14 | 2m | 12.81 ± 0.008 |
| 15 | 2n | 12.70 ± 0.006 |
| 16 | 2o | 12.98 ± 0.004 |
| 17 | 3 | >50 |
| 18 | 4 | 12.07 ± 0.001 |
| 19 | 5 | >50 |
| 20 | 6a | 13.99 ± 0.005 |
| 21 | 6b | 13.40 ± 0.007 |
| 22 | 7 | 11.98 ± 0.006 |
| 23 | doxorubicin | 9.10 ± 0.003 |
Conclusions
Malabaricol 1, a multicyclic tricarbocyclic triterpenoid framework, is quite intriguing with multiple stereocenters with druglike structure. A library of compounds were synthesized taking advantage of the reactive functional groups, 3-keto and 2-methylene groups, and screened for cytotoxicity. Malabaricol 1 itself and ortho, para-dichloro-substituted compound (2i) showed promising cytotoxicity comparable to that of doxorubicin. These observations provided further stimuli for the synthesis of a number of novel molecules using malabaricol 1 as a scaffold.
Experimental Section
Material
Malabaricol plant resin was collected from Karnataka, India. Solvents and chemicals were purchased from local vendors and used as received. Reactions were monitored using thin-layer chromatography (TLC) with 0.25 mm E. Merck precoated silica gel plates (60 F254), and visualization was accomplished with UV light, iodine adsorbed on silica gel, or immersion in an ethanolic solution of p-anisaldehyde stain followed by heating. Column chromatography was carried out on silica gel (60–120 mesh). All 1H NMR spectra were recorded on a Bruker 400 or 500 MHz spectrometer, and all C13 NMR spectra were recorded on a Bruker 100 or 125 MHz spectrometer. Chemical shifts (δ) were reported in parts per million (ppm) and calibrated to the residual proton and carbon resonance of CDCl3 (δH = 7.26 and δC = 77.0 ppm). The coupling constants (J) are given in hertz. High-resolution mass spectroscopy (HRMS) was conducted using electrospray ionization (ESI)-time-of-flight techniques. Fourier transform infrared (FTIR) spectra were recorded with a Bruker α spectrophotometer and were reported in cm–1. Cellular viability in the presence of test compounds was determined by 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) microcultured tetrazolium assay. The cells were seeded in flat-bottom (10 000 cells/100 μL) 96-well plates cultured in a medium containing 10% serum and allowed to attach and recover for 24 h in a humid chamber containing 5% CO2. 3-(4,5-Dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) was dissolved in phosphate-buffered saline (PBS) at 5 mg/mL and filtered to sterilize, and a small amount of insoluble residue present in MTT was removed. Different concentrations of compounds were added to the cells. After 48 h, a stock MTT solution (10 μL) was added to the culture plate. The cells were again kept in a CO2 incubator for 2 h. After incubation, 100 μL of dimethyl sulfoxide (DMSO) was added and mixed. The absorbance was read at 562 nm in a plate reader. The results were represented as a percentage of cytotoxicity/viability. All of the experiments were carried out in duplicate. From the percentage of cytotoxicity, the IC50 value was calculated.
Synthesis of 2-Arylidene Derivatives
To a stirred solution of malabaricol 1 (100 mg, 218 μmol) in ethanol (3 mL) was added KOH (73.3 mg, 1.30 mmol) at room temperature. After half an hour, benzaldehyde (67 μL, 654 μmol) was added to the reaction mixture. The resulting mixture was stirred at room temperature for 12 h. The solvent was removed and extracted with diethyl ether (3 × 50 mL), washed with brine, and dried over Na2SO4. The obtained residue was purified by column chromatography (9:91, EtOAc/hexane) to get the desired product 2a (79.8 mg, 67%) as a light green viscous liquid. Yield: IR (Neat) νmax 2962, 2874, 1673, 1449, 1028, 904, 665 cm–1; 1H NMR (500 MHz, CDCl3): δ = 7.54 (s, 1H), 7.46–7.38 (m, 4H), 7.33 (m, 1H), 5.12 (t, J = 7.1 Hz, 1H), 3.70 (t, J = 7.0 Hz, 1H), 2.94 (d, J = 15.8 Hz, 1H), 2.31 (d, J = 15.8 Hz, 1H), 2.17–1.31 (m, 25 H), 1.25 (s, 3H), 1.21 (s, 3H), 1.17 (s, 3H), 1.15 (s, 3H), 0.96 (s, 3H), 0.81 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 207.7, 137.6, 135.9, 133.8, 131.5, 130.3, 128.4, 128.3, 124.5, 85.6, 82.1, 72.7, 59.6, 57.1, 53.1, 45.1, 44.9, 43.9, 38.1, 37.6, 36.3, 36.0, 29.6, 29.5, 26.0, 25.6, 25.1, 24.3, 23.8, 22.3, 22.2, 21.5, 21.4, 17.6, 15.6; HRMS (ESI+) calculated for [C37H54O3 – H]+: 545.3995, found: 545.4008.
Compound 2b
Compound 2b was prepared according to the general procedure described above by the reaction between malabaricol 1 and 4-methoxybenzaldehyde and purified by column chromatography (9:91, EtOAc/hexane) as a light green viscous liquid. Yield: 84.7 mg (67.4%); IR (Neat) νmax 2959, 2873, 1669, 1458, 1032, 907, 665 cm–1; 1H NMR (400 MHz, CDCl3): δ = 7.52 (s, 1H), 7.42 (d, J = 8.8 Hz, 2H), 6.94 (d, J = 8.8 Hz, 2H), 5.12 (t, J = 7.0 Hz, 1H), 3.84 (s, 3H), 3.71 (t, J = 6.9 Hz, 1H), 2.92 (d, J = 15.5 Hz, 1H), 2.30 (d, J = 15.5 Hz, 1H), 2.18–1.30 (m, 25H), 1.25 (s, 3H), 1.21 (s, 3H), 1.17 (s, 3H), 1.13 (s, 3H), 0.96 (s, 3H), 0.81 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 207.6, 159.8, 137.5, 132.1, 131.6, 131.5, 128.5, 124.5, 113.9, 85.6, 82.1, 72.7, 59.9, 57.2, 55.2, 52.9, 45.1, 44.9, 43.9, 38.0, 37.5, 36.3, 35.9, 29.7 (2C), 26.0, 25.6, 25.1, 24.3, 23.8, 22.2, 22.1, 21.6, 21.4, 17.6, 15.6; HRMS (ESI+) calculated for [C38H56O4 + H]+: 577.4251, found: 577.4267.
Compound 2c
Compound 2c was prepared according to the general procedure described above by the reaction between malabaricol 1 and 1-naphthaldehyde and purified by column chromatography (9:91, EtOAc/hexane) as a light green viscous liquid. Yield 83.6 mg (64.3%); IR (Neat) νmax 2958, 2853, 1676, 1459, 1078, 907, 666 cm–1; 1H 1 NMR (400 MHz, CDCl3): δ = 7.90 (s, 1H), 7.88–7.80 (m, 3H), 7.71 (s, 1H), 7.54 (dd, J = 8.5, 1.5 Hz, 1H), 7.52–7.47 (m, 1H), 5.12 (t, J = 6.9 Hz, 1H), 3.71 (t, J = 6.8 Hz, 1H), 3.05 (d, J = 16.2 Hz, 1H), 2.42 (d, J = 16.1 Hz, 1H), 2.20–1.31 (m, 26H), 1.26 (s, 3H), 1.22 (s, 3H), 1.19 (s, 6H), 0.96 (s, 3H), 0.83 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 207.6, 137.7, 134.1, 133.4, 133.0, 132.9, 131.5, 130.2, 128.4, 127.8, 127.5 127.5, 126.7, 126.3, 124.5, 85.6, 82.1, 72.7, 59.7, 57.1, 53.1, 45.2, 45.0, 43.9, 38.1, 37.6, 36.4, 36.1, 29.6, 29.5, 26.0, 25.6, 25.1, 24.3, 23.8, 22.3, 22.2, 21.5, 21.3, 17.6, 15.6; HRMS (ESI+) calculated for [C41H56O3 + H]+: 597.4302, found: 597.4301.
Compound 2d
Compound 2d was prepared according to the general procedure described above by the reaction between malabaricol 1 and 4-nitrobenzaldehyde and purified by column chromatography (9:91, EtOAc/hexane) as a light green viscous liquid. Yield 70.4 mg (54.6%); IR (Neat) νmax 2961, 2872, 1678, 1454, 1074, 906, 666 cm–1; 1H NMR (400 MHz, CDCl3): δ = 8.26 (d, J = 8.6 Hz, 2H), 7.55 (d, J = 8.6 Hz, 2H), 7.53 (s, 1H), 5.11 (t, J = 7.0 Hz, 1H), 3.71 (t, J = 7.0 Hz, 1H), 2.87 (d, J = 16.3 Hz, 1H), 2.38–1.33 (m, 26H), 1.25 (s, 3H), 1.21 (s, 3H), 1.18 (s, 3H), 1.17 (s, 3H), 0.97 (s, 3H), 0.82 (s, 3H); 13C NMR (100 MHz): 207.3, 147.8, 142.3, 137.4, 131.5, 130.6, 124.5, 123.6, 85.5, 82.1, 72.8, 59.5, 57.0, 53.1, 45.3, 44.8, 43.9, 38.0, 37.6, 36.2, 36.1, 29.6, 29.3, 25.9, 25.6, 25.1, 24.3, 23.8, 22.3, 22.1, 21.5, 21.3, 17.6, 15.6; HRMS (ESI+) calculated for [C37H53NO5 – H]+: 590.3840, found: 590.3856.
Compound 2e
Compound 2e was prepared according to the general procedure described above by the reaction between malabaricol 1 and 3-nitrobenzaldehyde and purified by column chromatography (9:91, EtOAc/hexane) as a light green viscous liquid. Yield 77.0 mg (59.7%); IR (Neat) νmax 2961, 2871, 1678, 1455, 1074, 905, 665 cm–1; 1H NMR δ 8.28 (s, 1H), 8.18 (dd, J = 1.8, 7.3 Hz, 1H), 7.72 (d, J = 7.7 Hz, 1H), 7.59 (t, J = 7.8 Hz, 1H), 7.52 (s, 1H), 5.11 (t, J = 6.9 Hz, 1H), 3.71 (t, J = 6.7 Hz, 1H), 2.89 (d, J = 15.5 Hz, 1H), 2.35 (d, J = 15.5 Hz, 1H), 2.18–1.29 (m, 19H), 1.68 (s, 3H), 1.62 (s, 3H), 1.25 (s, 3H), 1.21 (s, 3H), 1.18 (s, 3H), 1.17 (s, 3H), 0.97 (s, 3H), 0.84 (s, 3H). 13C NMR (100 MHz): δ 207.1, 148.1, 137.4, 136.5, 135.6, 134.4, 131.3, 129.3, 124.5, 124.3, 122.8, 85.4, 82.0, 72.6, 56.9, 53.0, 45.2, 44.6, 43.8, 37.9, 37.6, 36.0, 29.3, 25.9, 25.5, 25.0, 24.1, 22.1, 22.1, 21.4, 21.2, 17.5, 15.6. HRMS (ESI+) calculated for [C37H53NO5 – OH]+: 575.3975, found: 575.3960.
Compound 2f
Compound 2f was prepared according to the general procedure described above by the reaction between malabaricol 1 and 2-nitrobenzaldehyde and purified by column chromatography (9:91, EtOAc/hexane) as a light green viscous liquid. Yield 68.3 mg (53%); IR (Neat) νmax 2962, 2871, 1679, 1455, 1075, 903, 666 cm–1; 1H NMR (400 MHz, CDCl3): δ = 8.12 (dd, J = 8.1, 1.2 Hz, 1H), 7.64 (ddd, J = 15.0, 7.4, 1.2 Hz, 2H), 7.50 (ddd, J = 15.6, 8.0, 0.8 Hz, 1H), 7.30 (d, J = 7.7 Hz, 1H), 5.10 (t, J = 7.0 Hz, 1H), 3.68 (t, J = 7.3 Hz, 1H), 2.53 (d, J = 15.2 Hz, 1H), 2.09–1.95 (m, 2H), 1.85–1.24 (m, 24H), 1.21 (s, 6H), 1.18 (s, 3H), 1.15 (s, 3H), 0.98 (s, 3H), 0.84 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 207.1, 148.1, 135.8, 134.1, 133.1, 132.2, 131.5, 130.9, 128.7, 124.8, 124.5, 85.5, 82.1, 72.7, 59.4, 56.9, 53.7, 45.9, 44.0, 43.6, 38.0, 37.5, 36.5, 29.6, 29.3, 28.6, 25.9, 25.6, 25.2, 24.3, 22.6, 22.3, 22.1, 21.2, 21.1, 17.5, 15.5. HRMS (ESI+) calculated for [C37H53NO5 + NH4]+: 609.4262, found: 609.4261.
Compound 2g
Compound 2g was prepared according to the general procedure described above by the reaction between malabaricol 1 and 4-methylbenzaldehyde and purified by column chromatography (9:91, EtOAc/hexane) as a light green viscous liquid. Yield 76.1 mg (62.3%); IR (Neat) νmax 2962, 2874, 1671, 1452, 1073, 906, 666 cm–1; 1H NMR (400 MHz, CDCl3): δ = 7.53 (s, 1H), 7.34 (d, J = 8.1 Hz, 2H), 7.21 (d, J = 8.0 Hz, 2H), 5.12 (t, J = 6.9 Hz, 1H), 3.71 (t, J = 6.9 Hz, 1H), 2.93 (d, J = 16.2 Hz, 1H), 2.38 (s, 3H), 2.34–2.27 (m, 1H), 2.14–1.30 (m, 25H), 1.25 (s, 3H), 1.21 (s, 3H), 1.16 (s, 3H), 1.14 (s, 3H), 0.96 (s, 3H), 0.81 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 207.7, 138.6, 137.7, 133.0, 132.9, 131.5, 130.4, 129.1, 124.5, 85.6, 82.1, 72.7, 59.8, 57.1, 53.0, 45.0, 45.0, 43.9, 38.0, 37.6, 36.3, 35.9, 29.6, 26.0, 25.6, 25.1, 24.3, 23.8, 22.2, 22.1, 21.5, 21.3, 21.3, 17.6, 15.6; HRMS (ESI+) calculated for [C38H56O3 + H]+: 561.4302, found: 561.4308.
Compound 2h
Compound 2h was prepared according to the general procedure described above by the reaction between malabaricol 1 and 4-tert-butylbenzaldehyde and purified by column chromatography (9:91, EtOAc/hexane) as a light green viscous liquid. Yield 83.0 mg (63.2%); IR (Neat) νmax 2957, 2852, 1672, 1461, 1078, 908, 665 cm–1; 1H NMR (500 MHz, CDCl3): δ = 7.52 (s, 1H), 7.46–7.38 (m, 4 H), 5.12 (t, J = 7.1 Hz, 1H), 3.71 (t, J = 6.8 Hz, 1H), 2.96 (d, J = 16.0 Hz, 1H), 2.33 (d, J = 16.0 Hz, 1H), 2.17–1.39 (m, 25H), 1.34 (s, 9H), 1.25 (s, 3H), 1.21 (s, 3H), 1.17 (s, 3H), 1.13 (s, 3H), 0.96 (s, 3H), 0.81 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 207.8, 151.8, 137.5, 133.0, 133.0, 131.5, 130.3, 125.4, 124.5, 85.6, 82.1, 72.7, 57.1, 53.0, 45.1, 45.0, 43.9, 38.1, 37.6, 35.9, 34.7, 31.1, 29.8, 25.6, 25.1, 24.3, 22.2, 22.2, 21.6, 21.4, 17.6, 15.6; HRMS (ESI+) calculated for [C41H62O3 + H]+: 603.4772, found: 603.4776.
Compound 2i
Compound 2i was prepared according to the general procedure described above by the reaction between malabaricol 1 and 2,4-dichlorobenzaldehyde and purified by column chromatography (9:91, EtOAc/hexane) as a light green viscous liquid. Yield 86.2 mg (64.3%); IR (Neat) νmax 2960, 2872, 1678, 1466, 1054, 904, 665 cm–1; 1H NMR (500 MHz, CDCl3): δ = 7.56 (d, J = 1.9 Hz, 1H), 7.44 (d, J = 2.0 Hz, 1H), 7.28–7.19 (m, 2H), 5.11 (t, J = 7.2 Hz, 1H), 3.69 (t, J = 7.2 Hz, 1H), 2.68 (d, J = 15.8 Hz, 1H), 2.18–1.29 (m, 26H), 1.22 (s, 3H), 1.19 (s, 3H), 1.18 (s, 3H), 1.15 (s, 3H), 0.95 (s, 3H), 0.82 (s, 3H); 13C NMR (100 MHz, CDCl3): 207.1, 136.2, 135.5, 134.4, 133.3, 132.8, 131.5, 130.8, 129.5, 126.6, 124.5, 85.5, 82.1, 72.7, 59.7, 57.1, 53.5, 45.6, 44.0, 38.0, 37.5, 36.3, 29.6, 29.3, 28.9, 25.9, 25.6, 25.2, 24.3, 23.8, 22.6, 22.3, 22.1, 21.3, 21.2, 17.6, 15.5; HRMS (ESI+) calculated for [C37H52Cl2O3 – H]+: 613.3216, found: 613.3210.
Compound 2j
Compound 2j was prepared according to the general procedure described above by the reaction between malabaricol 1 and 4-chlorobenzaldehyde and purified by column chromatography (9:91, EtOAc/hexane) as a light green viscous liquid. Yield 72.1 mg (57%); IR (Neat) νmax 2958, 2853, 1678, 1462, 1078, 908, 666 cm–1; 1H NMR (400 MHz, CDCl3): δ = 7.48 (d, J = 1.4 Hz, 1H), 7.39–7.32 (m, 4H), 5.12 (t, J = 7.0 Hz, 1H), 3.71 (t, J = 7.0 Hz, 1H), 2.87 (d, J = 16.2 Hz, 1H), 2.31–2.20 (m, 2H), 2.18–1.31 (m, 22H), 1.26 (s, 3H), 1.25 (s, 3H), 1.21 (s, 3H), 1.16 (s, 3H), 1.15 (s, 3H), 0.96 (s, 3H), 0.81 (s, 3H); 13C NMR (125 MHz, CDCl3): δ = 207.5, 136.2, 134.3, 131.5, 131.4, 128.6, 124.5, 85.6, 82.1, 72.7, 59.7, 57.2, 53.0, 45.1, 44.8, 43.9, 38.0, 37.6, 36.0, 29.6, 29.6, 29.5, 29.3, 25.9, 25.6, 25.1, 24.3, 23.8, 22.6, 22.2, 22.1, 21.5, 21.3, 17.6, 15.6; HRMS (ESI+) calculated for [C37H53O3Cl – OH]+: 563.3656, found: 563.3642.
Compound 2k
Compound 2k was prepared according to the general procedure described above by the reaction between malabaricol 1 and 4-bromobenzaldehyde and purified by column chromatography (9:91, EtOAc/hexane) as a light green viscous liquid. Yield 75.4 mg (55.4%); IR (Neat) νmax 2963, 2874, 1674, 1453, 1073, 906, 666 cm–1; 1H NMR (400 MHz, CDCl3): δ = 7.53 (d, J = 8.4 Hz, 2H), 7.45 (s, 1H), 7.31–7.25 (m, 2H), 5.12 (t, J = 7.0 Hz, 1H), 3.71 (t, J = 6.9 Hz, 1H), 2.86 (d, J = 16.5 Hz, 1H), 2.38 (s, 1H), 2.26 (d, J = 16.5 Hz,1H), 2.18–1.29 (m, 24H), 1.25 (s, 3H), 1.21 (s, 3H), 1.16 (s, 3H), 1.14 (s, 3H), 0.96 (s, 3H), 0.80 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 207.5, 136.2, 134.7, 134.5, 131.6, 131.5, 131.5, 124.5, 122.6, 85.5, 82.1, 72.7, 59.6, 57.1, 53.0, 45.1, 44.8, 43.9, 38.0, 37.6, 36.0, 29.4, 25.9, 25.6, 25.1, 24.3, 23.8, 22.2, 22.1, 21.5, 21.3, 17.6, 15.6; HRMS (ESI+) calculated for [C37H53BrO3 – H]+: 623.3100, found: 623.3139.
Compound 2l
Compound 2l was prepared according to the general procedure described above by the reaction between malabaricol 1 and phthalaldehyde and purified by column chromatography (9:91, EtOAc/hexane) as a light green viscous liquid. Yield 109.3 mg (49.4%); IR (Neat) νmax 2968, 2879, 1678, 1452, 1025, 908, 668 cm–1; 1H NMR (400 MHz, CDCl3): δ = 7.54 (d, J = 1.0 Hz, 1H), 7.48 (s, 2H), 5.12 (t, J = 7.0 Hz, 1H), 3.71 (t, J = 6.7 Hz, 1H), 2.95 (d, J = 16.4 Hz, 1H), 2.34 (d, J = 16.4 Hz, 1H), 2.16–1.31 (m, 20H), 1.69 (s, 3H), 1.62 (s, 3H), 1.26 (s, 3H), 1.21 (s, 3H), 1.18 (s, 3H), 1.15 (s, 3H), 0.97 (s, 3H), 0.82 (s, 3H). 13C NMR (100 MHz, CDCl3): δ = 207.4, 136.7, 135.9, 134.4, 131.3, 130.3, 124.5, 85.5, 82.0, 72.6, 57.0, 52.9, 45.0, 44.9, 43.8, 38.0, 37.6, 36.2, 35.9, 29.5, 25.9, 25.6, 25.1, 24.2, 22.2, 22.1, 21.5, 21.3, 17.5, 15.6. HRMS (ESI+) calculated for [C68H102O6 + Na]+: 1037.7569, found: 1037.7578.
Compound 2m
Compound 2m was prepared according to the general procedure described above by the reaction between malabaricol 1 and 3-pyridinebenzaldehyde and purified by column chromatography (9:91, EtOAc/hexane) as a light green viscous liquid. Yield 79.8 mg (67%); IR (Neat) νmax 2965, 2875, 1678, 1454, 1021, 906, 665 cm–1; 1H NMR (500 MHz, CDCl3): δ = 8.69 (d, J = 1.6 Hz, 1H), 8.55 (dd, J = 4.8, 1.5 Hz, 1H), 7.73 (m,1H), 7.47 (s, 1H), 7.38–7.33 (m, 1H), 5.11 (t, J = 7.0 Hz, 1H), 3.71 (t, J = 6.8 Hz, 1H), 2.87 (d, J = 16.3 Hz, 1H), 2.40–2.27 (m, 1H), 2.15–1.30 (m, 25H), 1.24 (s, 3H), 1.21 (s, 3H), 1.18 (s, 3H), 1.16 (s, 3H), 0.96 (s, 3H), 0.82 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 207.7, 137.6, 135.9, 133.8, 131.5, 130.3, 128.4, 128.3, 124.5, 85.6, 82.1, 72.7, 59.9, 57.2, 53.1, 45.1, 44.9, 43.9, 38.1, 37.6, 36.4, 36.0, 29.6, 29.5, 26.0, 25.6, 25.1, 24.3, 23.8, 22.3, 22.2, 21.5, 21.4, 17.6, 15.6; HRMS (ESI+) calculated for [C36H53NO3 + H]+: 548.4098, found: 548.4110.
Compound 2n
Compound 2n was prepared according to the general procedure described above by the reaction between malabaricol 1 and 2-pyridinecarboxaldehyde and purified by column chromatography (9:91, EtOAc/hexane) as a light green viscous liquid. Yield 82.7 mg (69.3%); IR (Neat) νmax 2958, 2852, 1679, 1457, 1076, 906, 665 cm–1; 1H NMR (500 MHz, CDCl3): δ = 8.69 (dd, J = 4.7, 0.9 Hz, 1H), 7.69 (td, J = 7.7, 1.8 Hz, 1H), 7.42 (dd, J = 2.7, 1.5 Hz, 1H), 7.38 (d, J = 7.9, Hz 1H), 7.18 (dddd, J = 12.3, 7.4, 4.8, 0.7 Hz, 1H), 5.11 (t, J = 7.0 Hz, 1H), 3.70 (t, J = 6.8 Hz, 1H), 3.41 (d, J = 17.3 Hz, 1H), 2.48 (d, J = 17.3, Hz, 1H), 2.14–1.97 (m, 3H), 1.79–1.31 (m, 22H), 1.24 (s, 3H), 1.20 (s, 3H), 1.18 (s, 3H), 1.14 (s, 3H), 0.96 (s, 3H), 0.83 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 208.5, 155.5, 149.4, 138.2, 136.0, 134.5, 131.4, 126.6, 124.5, 122.2, 85.6, 82.0, 72.7, 59.9, 56.8, 53.0, 45.2, 43.8, 38.0, 37.5, 36.2, 35.8, 29.6, 29.4, 26.0, 25.6, 25.1, 24.3, 23.8, 22.1, 21.5, 21.3, 17.6, 15.8; HRMS (ESI+) calculated for [C36H53NO3 + H]+: 548.4098, found: 548.4111.
Compound 2o
Compound 2o was prepared according to the general procedure described above by the reaction between malabaricol 1 and furan-2-carbaldehyde and purified by column chromatography (9:91, EtOAc/hexane) as a light green viscous liquid. Yield 78.0 mg (66.7%); IR (Neat) νmax 2959, 2872, 1671, 1455, 1074, 907, 666 cm–1; 1H NMR (400 MHz, CDCl3): δ = 7.55 (d, J = 1.5 Hz, 1H), 7.34–7.31 (m, 1H), 6.59 (d, J = 3.4 Hz, 1H), 6.50 (dd, J = 3.3, 1.7 Hz, 1H), 5.12 (t, J = 7.0 Hz, 1H), 3.70 (t, J = 7.2 Hz, 1H), 3.02 (d, J = 17.3 Hz, 1H), 2.27 (d, J = 17.4 Hz, 1H), 2.18–1.89 (m, 2H), 1.89–1.32 (m, 17H), 1.68 (d, J = 0.6 Hz, 1H), 1.62 (s, 3H), 1.26 (s, 3H), 1.25 (s, 3H), 1.21 (s, 3H), 1.17 (s, 3H), 1.10 (s, 3H), 0.98 (s, 3H), 0.85 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 207.2, 152.5, 144.3, 131.5, 130.9, 124.5, 124.3, 115.3, 112.1, 85.6, 82.1, 72.7, 57.1, 52.8, 45.1, 44.8, 43.9, 38.1, 37.6, 35.5, 29.7, 29.6, 25.6, 25.0, 24.3, 22.2, 22.1, 21.6, 21.4, 17.6, 16.0; HRMS (ESI+) calculated for [C35H52O4 + H]+: 537.3938, found: 537.3930.
Compound 3
To a stirred solution of malabaricol 1 (100 mg, 218 μmol) and ethylformate (88 μL, 1.09 mmol) in tetrahydrofuran (THF) at 0 °C was added potassium tert-butoxide (98 mg, 872 mmol), and stirring was continued for 4 h. The reaction mixture was quenched with saturated NH4Cl (5 mL) at 0 °C, followed by concentration of solvent. The reaction mixture was extracted with ethyl acetate (3 × 30 mL), washed with brine, dried over Na2SO4, and concentrated. The obtained residue was purified by column chromatography (12:88, EtOAc/hexane) to give 3 (75.5 mg, 71.2%) as a light greenish viscous liquid. IR (Neat) νmax 2965, 2873, 1711, 1453, 1029, 905, 666 cm–1; 1H NMR (500 MHz, CDCl3): δ = 14.9 (d, J = 3.2 Hz, 1H), 8.56 (d, J = 2.8, Hz, 1H), 5.11 (t, J = 7.0 Hz, 1H), 3.69 (t, J = 7.0 Hz, 1H), 2.21 (d, J = 14.3, Hz, 1H), 2.15–1.24 (m, 29H), 1.20 (s, 6H), 1.11 (s, 3H), 0.96 (s, 3H), 0.85 (s, 3H); 13C NMR (100 MHz, CDCl3): 191.0, 188.1, 131.5, 124.5, 105.8, 85.6, 82.2, 72.7, 59.4, 57.1, 52.4, 44.0, 40.0, 38.1, 37.6, 36.5, 36.0, 28.4, 25.9, 25.6, 25.3, 24.3, 23.9, 22.1, 21.1, 20.5, 17.6, 15.0; HRMS (ESI+) calculated for [C31H50O4 + H]+: 487.3782, found: 487.3770.
Compound 4
To a stirred solution of malabaricol 1 (100 mg, 218 μmol) and hydroxylamine hydrochloride (75.8 mg, 1.09 mmol) in ethanol at room temperature was added acetic acid (100 μL), and the reaction mixture was refluxed for 4 h. The reaction mixture was concentrated, extracted with ethyl acetate (3 × 30 mL), washed with brine, dried over Na2SO4, and concentrated. The obtained residue was purified by column chromatography (1:9, EtOAc/hexane) to give 4 (47.9 mg, 46.4%) as a light greenish viscous liquid. IR (Neat) νmax 2964, 2873, 1452, 1072, 930, 665 cm–1; 1H NMR (400 MHz, CDCl3): δ = 9.19–8.81 (brs, 1H), 5.11 (t, J = 7.0 Hz, 1H), 3.67 (t, J = 6.9 Hz, 1H), 3.12–3.02 (m, 1H), 2.27–2.16 (m, 1H), 2.15–1.24 (m, 21H), 1.68 (d, J = 0.7 Hz, 3H), 1.61 (s, 3H), 1.18 (s, 3H), 1.18 (s, 3H), 1.16 (s, 3H), 1.06 (s, 3H), 0.95 (s, 6H); 13C NMR (100 MHz, CDCl3): δ = 166.7, 131.4, 124.6, 85.6, 82.1, 72.7, 58.4, 56.1, 44.0, 40.2, 39.0, 38.1, 37.5, 36.9, 36.8, 27.3, 26.0, 25.9, 25.6, 24.3, 23.8, 22.9, 22.1, 21.0, 20.0, 17.5, 17.0, 15.4; HRMS (ESI+) calculated for [C30H51NO3 + H]+: 474.3942, found: 474.3952.
Compound 5
To a stirred solution of malabaricol 1 (100 mg, 218 μmol) and 2,4-dinitrophenylhydrazine (130 mg, 654 μmol) in ethanol at room temperature was added acetic acid (100 μL), and the reaction mixture was refluxed for 4 h. The reaction mixture was concentrated, extracted with ethyl acetate (3 × 30 mL), washed with brine, dried over Na2SO4, and concentrated. The obtained residue was purified by column chromatography (1:9, EtOAc/hexane) to give 5 (93.6 mg, 67.2%) as an orange-red solid. Mp 109–111 °C; IR (Neat) νmax 2966, 2872, 1451, 1071, 921, 666 cm–1; 1H NMR (500 MHz, CDCl3): δ = 11.7 (s, 1H), 9.12 (d, J = 2.5 Hz, 1H), 8.29 (dd, J = 2.4, 9.4 Hz, 1H), 7.95 (d, J = 9.6 Hz, 1H), 5.10 (t, J = 7.0 Hz, 1H), 3.68 (t, J = 6.8 Hz, 1H), 2.64 (d, J = 15.5 Hz, 1H), 2.55–2.44 (m, 1H), 2.15–1.96 (m, 3H), 1.96–1.24 (m, 18H), 1.68 (s, 3H), 1.61 (s, 3H), 1.31 (s, 3H), 1.20 (s, 3H), 1.19 (s, 3H), 1.17 (s, 3H), 0.98 (s, 3H), 0.96 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 166.8, 145.5, 137.4, 131.5, 128.9, 124.5, 123.5, 116.4, 85.5, 82.2, 72.7, 58.2, 55.7, 44.1, 42.1, 38.6, 38.1, 37.5, 36.5, 28.8, 25.7, 25.6, 24.3, 23.8, 22.1, 21.0, 20.6, 20.5, 17.5, 15.2; HRMS (ESI+) calculated for [C36H54N4O6 + H]+: 639.4116, found: 639.4103.
Compound 6a
To a stirred solution of malabaricol 1 (100 mg, 218 μmol) and phenylhydrazine hydrochloride (95 mg, 654 μmol) in ethanol at room temperature was added acetic acid (100 μL), and the reaction mixture was refluxed for 4 h. The reaction mixture was concentrated, extracted with ethyl acetate (3 × 30 mL), washed with brine, dried over Na2SO4, and concentrated. The obtained residue was purified by column chromatography (1:9, EtOAc/hexane) to give indole 6a (70.9 mg, 61.2%) as a light greenish viscous liquid. IR (Neat) νmax 2963, 2876, 1459, 1074, 901, 666 cm–1; 1H NMR (400 MHz, CDCl3): δ = 7.75 (s, 1H), 7.43 (d, J = 7.6 Hz, 1H), 7.29 (d, J = 7.6 Hz, 1H), 7.11 (td, J = 1.2, 7.1 Hz, 1H), 7.05 (td, J = 1.2, 7.7 Hz, 1H), 5.11 (t, J = 7.0 Hz, 1H), 3.70 (t, J = 6.8 Hz, 1H), 2.69 (d, J = 15.1 Hz, 1H), 2.25 (d, J = 15.1 Hz, 1H), 2.15–1.22 (m, 20H), 1.68 (s, 3H), 1.61 (s, 3H), 1.31 (s, 3H), 1.24 (s, 3H), 1.22 (s, 3H), 1.20 (s, 3H), 1.00 (s, 3H), 0.88 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 141.0, 136.0, 131.4, 128.2, 124.6, 120.9, 118.8, 118.0, 110.2, 107.0, 85.8, 82.1, 72.8, 57.8, 53.6, 44.2, 38.1, 37.7, 37.5, 33.9, 31.0, 26.9, 25.6, 25.4, 24.3, 22.9, 22.6, 22.2, 21.3, 20.3, 17.6, 16.2, 14.1; HRMS (ESI+) calculated for [C36H53NO2 + H]+: 532.4149, found: 532.4156.
Compound 6b
Compound 6b was prepared according to the procedure for 6a described above by the reaction between malabaricol 1 and 4-bromophenylhydrazine hydrochloride and purified by column chromatography (9:91, EtOAc/hexane) as a light green viscous liquid. Yield 84.7 mg (63.7%); IR (Neat) νmax 2962, 2876, 1458, 1076, 901, 665 cm–1; 1H NMR (400 MHz, CDCl3): δ = 7.79 (s, 1H), 7.55 (d, J = 1.5 Hz, 1H), 7.20–7.13 (m, 2H), 5.11 (t, J = 6.9 Hz, 1H), 3.70 (t, J = 6.8 Hz, 1H), 2.63 (d, J = 14.9 Hz, 1H), 2.21 (d, J =14.9 Hz, 1H), 2.25–1.16 (m, 19H), 1.68 (s, 3H), 1.61 (s, 3H), 1.30 (s, 3H), 1.25 (s, 3H), 1.21 (s, 3H), 1.20 (s, 3H), 0.99 (s, 3H), 0.86 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 142.9, 134.5, 131.5, 130.0, 124.5, 123.5, 120.7, 112.0, 111.6, 106.9, 85.7, 82.0, 72.7, 57.6, 53.5, 44.1, 38.1, 37.7, 37.5, 37.3, 34.0, 31.9, 30.9, 29.6, 25.6, 25.4, 24.3, 22.9, 22.6, 22.1, 21.2, 20.2, 17.6, 16.1; HRMS (ESI+) calculated for [C36H52BrNO2 + H]+: 610.3254, found: 610.3258.
Compound 7
To a stirred solution of malabaricol 1 (100 mg, 218 μmol) ethylenediamine (78 μL, 1.09 mmol) in morpholine at room temperature, sulfur (41.8 mg, 1.3 mmol) was added and then the reaction mixture was refluxed for 4 h. The reaction mixture was concentrated, and the obtained residue was directly purified by column chromatography (1:9, EtOAc/hexane) to give 7 (73.4 mg, 68.1%) as a light greenish viscous liquid. IR (Neat) νmax 2962, 2874, 1452, 1075, 903, 665 cm–1; 1H NMR (400 MHz, CDCl3): δ = 8.41 (dd, J = 0.7, 2.3 Hz, 1H), 8.27 (d, J = 2.5 Hz, 1H), 5.11 (t, J = 7.1 Hz, 1H), 3.71 (t, J = 7.1 Hz, 1H), 2.91 (d, J = 16.5 Hz, 1H), 2.57 (d, J = 16.5 Hz, 1H), 2.16–1.40 (m, 18H), 1.68 (s, 3H), 1.61 (s, 3H), 1.41–1.28 (m, 1H), 1.32 (s, 3H), 1.30 (s, 3H), 1.24 (s, 3H), 1.20 (s, 3H), 1.00 (s, 3H), 0.84 (s, 3H); 13C NMR (125 MHz, CDCl3): δ = 159.8, 150.7, 142.2, 141.4, 131.4, 124.5, 85.6, 82.1, 72.7, 57.2, 53.4, 49.1, 44.0, 39.3, 38.1, 37.6, 36.5, 36.2, 31.6, 26.0, 25.6, 25.2, 24.2, 23.8, 22.1, 21.1, 17.5, 16.0; HRMS (ESI+) calculated for [C32H50N2O2 + H]+: 495.3945, found: 495.3932.
Acknowledgments
The authors thank Dr. Srivari Chandrasekhar, Director, CSIR-IICT, for the generous support. All authors thank Ministry of AYUSH, New Delhi, India, for financial support as part of natural product programme under the title GAP-0623.IICT/pubs./2019/426.
Glossary
Abbreviations
- tBuOK
potassium tert-butoxide
- S
sulfur
- NMR
nuclear magnetic resonance
- A549
adenocarcinomic human alveolar basal epithelial cells
- AYUSH
Ayurveda Yoga Unani Siddah Homoeopathy
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01525.
Copies of 1H and 13C NMR spectra; general information; general experimental methods; synthesis of 2-arylidene derivatives, compound 2b, compound 2c, and and compound 2d; 1H NMR spectrum of compound 1 in CDCl3; and 13C NMR spectrum of compound 1 in CDCl3 (PDF)
Author Contributions
S.S.B. and L.S.R.K. performed the experiments and contributed equally to this work. V.S. studied the cytotoxicity. N.K. designed the study and supervised the necessary work. All authors have given approval to the final version of the manuscript.
Pharmacopoeia Commission for Indian Medicine and Homoeopathy, Ministry of AYUSH, Govt. of India.
The authors declare no competing financial interest.
Supplementary Material
References
- Chawla A.; Dev S. A. New class of triterpenoids from ailanthus-malabarica DC derivatives of malabaricane. Tetrahedron Lett. 1967, 8, 4837–4843. 10.1016/S0040-4039(01)89615-5. [DOI] [Google Scholar]
- Sobti R. R.; Dev S. A direct correlation of (+)-malabaricol with (+)-ambreinolide. Tetrahedron Lett. 1968, 9, 2215–2217. 10.1016/S0040-4039(00)89723-3. [DOI] [Google Scholar]
- Van Tamelen E. E.; Willet J.; Schwartz M.; Nadeau R. Nonenzymic laboratory cyclization of squalene 2,3-oxide. J. Am. Chem. Soc. 1966, 88, 5937–5938. 10.1021/ja00976a048. [DOI] [PubMed] [Google Scholar]
- Sharpless K. B. d,l-Malabaricanediol. The first cyclic natural product derived from squalene in a nonenzymic process. J. Am. Chem. Soc. 1970, 92, 6999–7001. 10.1021/ja00726a064. [DOI] [Google Scholar]
- Van Tamelen E. E. Bioorganic chemistry: sterols and acrylic terpene terminal expoxides. Acc. Chem. Res. 1968, 1, 111–120. 10.1021/ar50004a003. [DOI] [Google Scholar]
- Paton W. F.; Paul I. C.; Bajaj A. G.; Dev S. The structure of malabaricol. Tetrahedron Lett. 1979, 20, 4153–4145. 10.1016/S0040-4039(01)86530-8. [DOI] [Google Scholar]
- Thongnest S.; Boonsombat J.; Prawat H.; Mahidol C. Ailanthusins A-G and nor-lupane triterpenoids from Ailanthus triphysa. Phytochemistry 2017, 134, 98–105. 10.1016/j.phytochem.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Achanta P. S.; Gattu R. K.; Belvotagi A. R. V.; Akkinepally R. R.; Bobbala R. K.; Achanta A. R. V. N. New malabaricane triterpenes from the oleoresin of Ailanthus malabarica. Fitoterapia 2015, 100, 166–173. 10.1016/j.fitote.2014.11.022. [DOI] [PubMed] [Google Scholar]
- Srinivas P. V.; Rao R. R.; Rao J. M. Two new tetracyclic triterpenes from the heartwood of Ailanthus excelsa Roxb. Chem. Biodiversity 2006, 3, 930–934. 10.1002/cbdv.200690095. [DOI] [PubMed] [Google Scholar]
- Marner F. J.; Freyer A.; Lex J. Triterpenoids from gum mastic, the resin of Pistacia lentiscus. Phytochemistry 1991, 30, 3709–3712. 10.1016/0031-9422(91)80095-I. [DOI] [Google Scholar]
- Faini F.; Castillo M.; Torres R.; Monache G. D.; Gacs-Baitz E. Malabaricane triterpene glucosides from Adesmia aconcaguensis. Phytochemistry 1995, 40, 885–890. 10.1016/0031-9422(95)00056-D. [DOI] [Google Scholar]
- Jakupovic J.; Eid F.; Bohlmann F.; El-Dahmy S. Malabaricane derivatives from Pyrethrum santolinoides. Phytochemistry 1987, 26, 1536–1538. 10.1016/S0031-9422(00)81855-5. [DOI] [Google Scholar]
- Arai Y.; Hirohara M.; Ageta H. Fern constituents: Three new skeletal triterpenoid hydrocarbons isolated from Polypodiodesniponica. Tetrahedron Lett. 1989, 30, 7209–7212. 10.1016/S0040-4039(01)93936-X. [DOI] [Google Scholar]
- Liang C.-Q.; Shi Y.-M.; Li X. Y.; Luo R.-H.; Li Y.; Zheng Y.-T.; Zhang H.-B.; Xiao W. L.; Sun H.-D. Kadcotriones a–c: Tricyclic triterpenoids from Kadsura coccinea. J. Nat. Prod. 2013, 76, 2350–2354. 10.1021/np400546z. [DOI] [PubMed] [Google Scholar]
- Aoki S.; Sanagawa M.; Watanabe Y.; Setiawan A.; Arai M.; Kobayashi M. Novel isomarabarican triterpenes, exhibiting selective anti-proliferative activity against vascular endothelial cells, from marine sponge Rhabdastrella globostellata. Bioorg. Med. Chem. 2007, 15, 4818–4828. 10.1016/j.bmc.2007.04.070. [DOI] [PubMed] [Google Scholar]
- Ravi B. N.; Wells R. J.; Croft K. D. Malabaricane triterpenes from a fijian collection of the sponge Jaspisstellifera. J. Org. Chem. 1981, 46, 1998–2001. 10.1021/jo00323a006. [DOI] [Google Scholar]
- McCabe T.; Clardy J.; Minale L.; Pizza C.; Zollo F.; Riccio R. A triterpenoid pigment with the isomalabaricane skeleton from the marine sponge stelletta sp. Tetrahedron Lett. 1982, 23, 3307–3310. 10.1016/S0040-4039(00)87601-7. [DOI] [Google Scholar]
- Barton D. H. R.; Head A. J.; May P. J. Long-range effects in alicyclic systems. Part II. The rates of condensation of some triterpenoid ketones with benzaldehyde. J. Chem. Soc. 1957, 935–944. 10.1039/jr9570000935. [DOI] [Google Scholar]
- Barton D. H. R.; McCapra F.; May P. J.; Thudium F. Long-range effects in alicyclic systems. Part III. The relative rates of condensation of some steroid and triterpenoid ketones with benzaldehyde. J. Chem. Soc. 1960, 1297–1311. 10.1039/jr9600001297. [DOI] [Google Scholar]
- Ourisson G.; Crabbe P.; Rodig O. R.. Tetracyclic Triterpenes; Holden-Day Inc.: San Francisco, USA, 1961; pp 24–106. [Google Scholar]
- a Miller L.; Adam K. P.; Milburn M. V.; Cobb J. E.; Evans A. M.; Zhang Q. WO Patent WO2016/269921A12016.; b Kallepu S.; Sanjeev K.; Chegondi R.; Mainkar P. S.; Chandrasekhar S. Benzyne insertion onto β-Keto esters of polycyclic natural products: Synthesis of benzo octacyclo scaffolds. Org. Lett. 2018, 20, 7121–7124. 10.1021/acs.orglett.8b03070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.