CONSPECTUS
Multi-component union tactics in which three or more fragments are rapidly assembled are highly prized in the construction of architecturally complex natural products. Anion Relay Chemistry (ARC), a multi-component union tactic, has just such potential to elaborate structurally diverse scaffolds in a single operation with excellent stereochemical control. Conceptually, the ARC tactic can be divided into two main classes: “Through-Bond”, by the relay of negative charge through the bonding system of a molecule; and “Through-Space”, by the migration of negative charge across space by a transfer agent. “Through-Space” Anion Relay Chemistry, the focus of this Account, can be further subdivided into two types: Type I ARC, originated from Tietze-Schaumann-Smith coupling reaction, which for the first time permits controllable Brook rearrangements to construct unsymmetrical adducts, and as such has been successfully employed in the total syntheses of diverse natural products, including the mycoticins, bryostatin 1, spongistatins, rimocidin, indolizidine alkaloids, and enigmazole A; and Type II ARC, central to which is the design of novel bifunctional linchpins that enable rapid assembly of linear and cyclic fragments with diverse architectural features, ranging from polyols, spiroketals and polyenes to polypropionate scaffolds. Recently, the Type II ARC tactic has been exploited as the key construction tactic in the total syntheses of the spirastrellolides, the cryptocarya acetates, secu’amamine A, mandelalide A and nahuoic acid Ci (Bii). This Account will present the evolution of both the Type I and Type II Anion Relay tactics, in conjunction with some prominent applications.

1. Introduction
Efficient union reactions are fundamental to the construction of complex organic molecules. The challenge of uniting highly functionalized compounds as required in the total synthesis of natural products, however, often requires multiple steps that are required to proceed in high yield with precise stereochemical control.1 Such multistep reaction sequences often involve numerous purifications, thus adding time and material cost at each stage. Anion Relay Chemistry (ARC),2 an effective set of synthetic protocols defined in 2006 for the construction of architecturally complex molecules, stand as a promising method to permit efficient multi-component unions3 in a “single flask” manner.
In the broadest sense, Anion Relay Chemistry (ARC) can be divided into two classes based on how the negative charge migrates: either “Through-Bond” or “Through-Space” (Scheme 1). “Through-Bond” ARC features the transfer of a negative charge through the bonding system of a molecule. A specific example is the propagation of the negative charge via the unsaturated π-system as in 1,4-conjugate addition reactions (Scheme 1). In contrast, “Through-Space” ARC exploits a “carrier” that relays negative charge across space employing δ-bonded intermediates. As generally defined, Brook rearrangements4 (Scheme 2) comprise the transfer of a silyl group from a carbon (1) to an oxygen (2) with concomitant migration of charge resulting in a new carbanion (5) from the initiating alkoxide species (3). This rearrangement, first observed in 1953 by Gilman5 and later defined by Brook and co-workers,4 is now known to proceed by a hypervalent pentacoordinate silicate species (4).6 It is important to note that in the Brook rearrangement “Through-Space” ARC is now controllable (vide infra). Several principal factors are critical in the silyl group transfer between the oxygen and carbon: (1) the strength of the oxygen-metal (i.e., Li, Na, K and etc.) bond; (2) the anion stabilizing ability of the carbon substituents (ASG); and (3) the polarity of the solvent and the temperature of the reaction media.
Scheme 1.
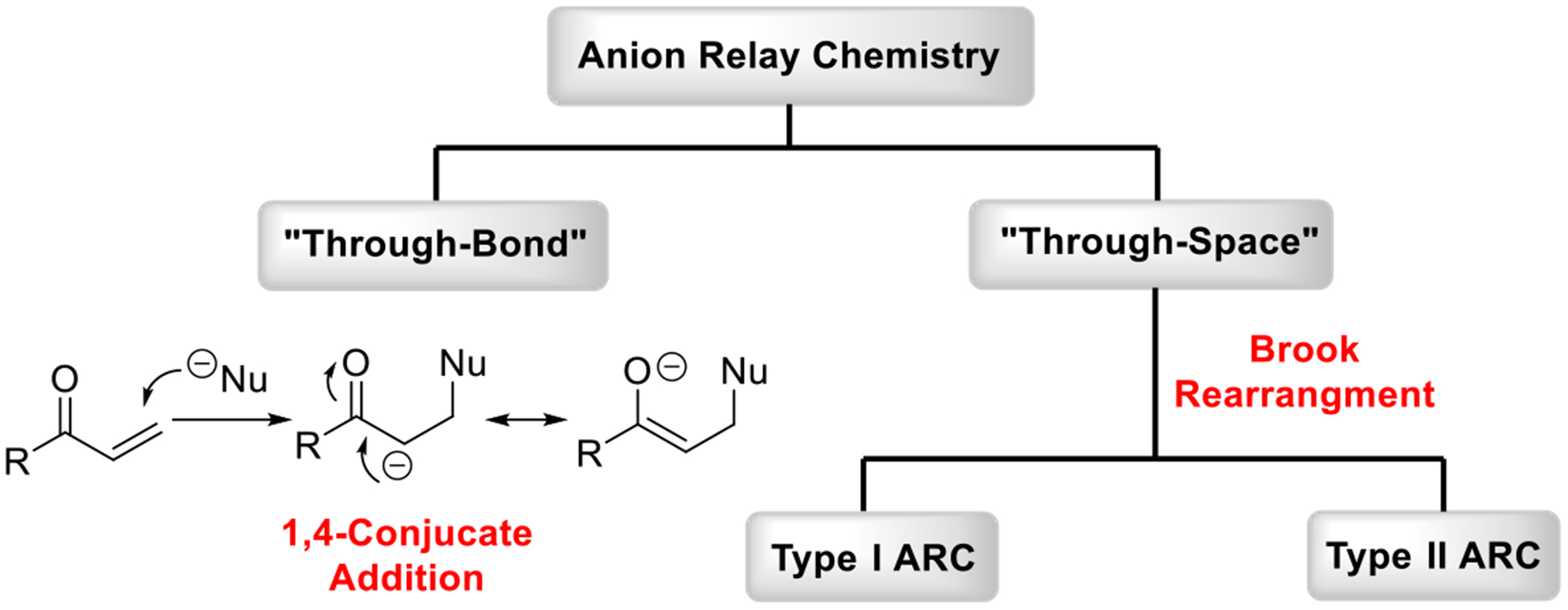
The Hierarchy of Anion Relay Chemistry.
Scheme 2.
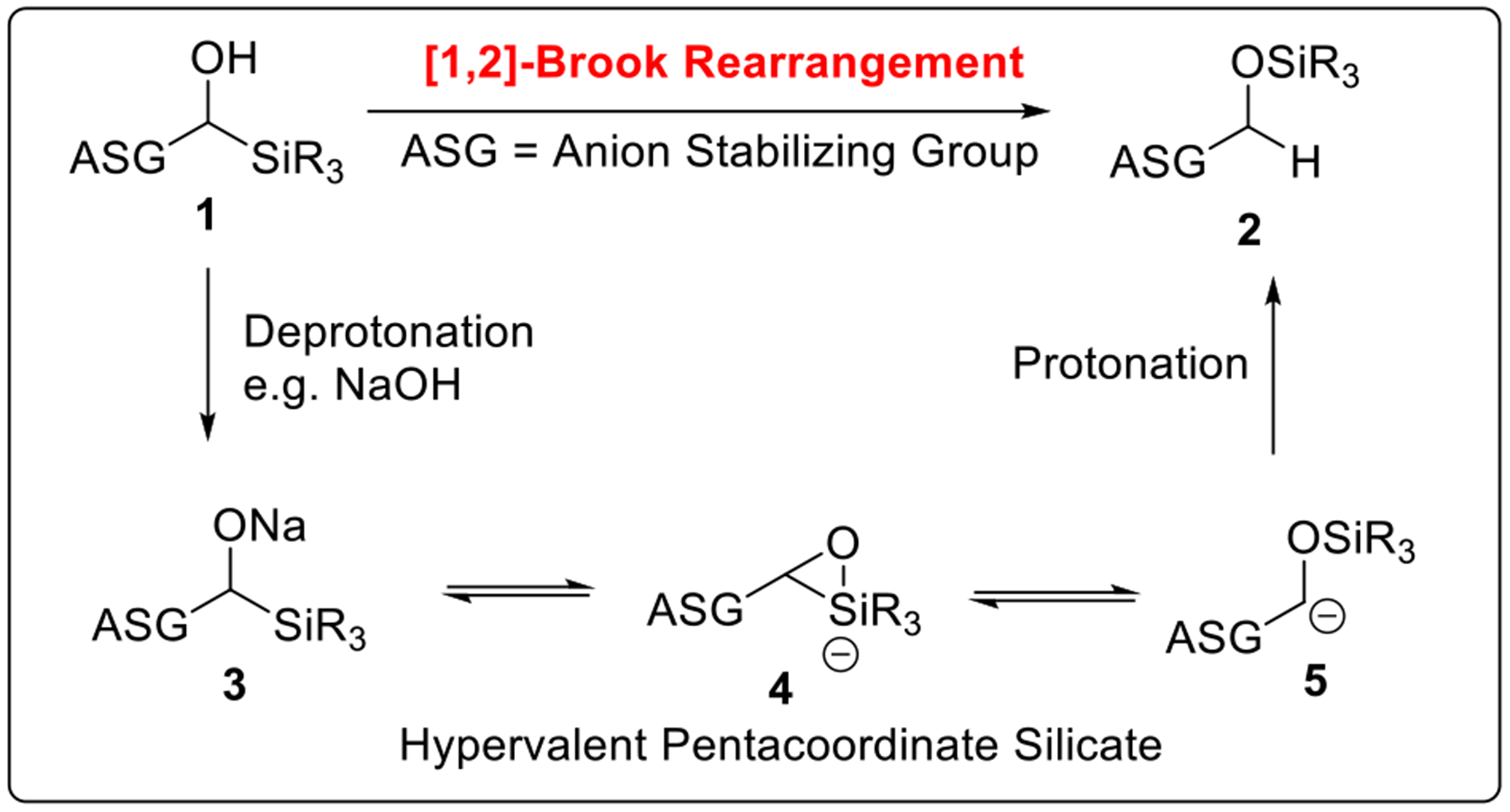
Example of [1,2]-Brook Rearrangement.
In this Account, we will focus on the evolution of the “Through-Space” ARC tactic employing silyl groups as the transfer agent, in conjunction with application to the total synthesis of natural products. This tactic now comprises Type I and Type II ARC (Scheme 3a and 3b), differentiated by the linchpins employed and the final locus of the negative charge after relay. In Type I ARC, a stabilized α-silyl linchpin anion (6) undergoes nucleophilic attack to an electrophile, to form an oxyanion (7), which in turn undergoes a [1,4]-Brook rearrangement with concomitant translocation of the negative charge back to the originating carbon, α to the anion-stabilizing group (ASG). Trapping the latter (8) with diverse electrophiles generates three-component adducts. Alternatively, for Type II ARC, a bifunctional linchpin (i.e., 9) is employed, which contains both an electrophilic and a masked nucleophilic site, the latter possessing a silyl-protected anion-stabilizing group (ASG). Upon nucleophilic addition, the derived oxyanion (10) undergoes a facile [1,4]-Brook rearrangement, unmasking the latent nucleophilicity of the linchpin at a new distal carbon site (11). Subsequent capture with an electrophile yields a three-component adduct.
Scheme 3.
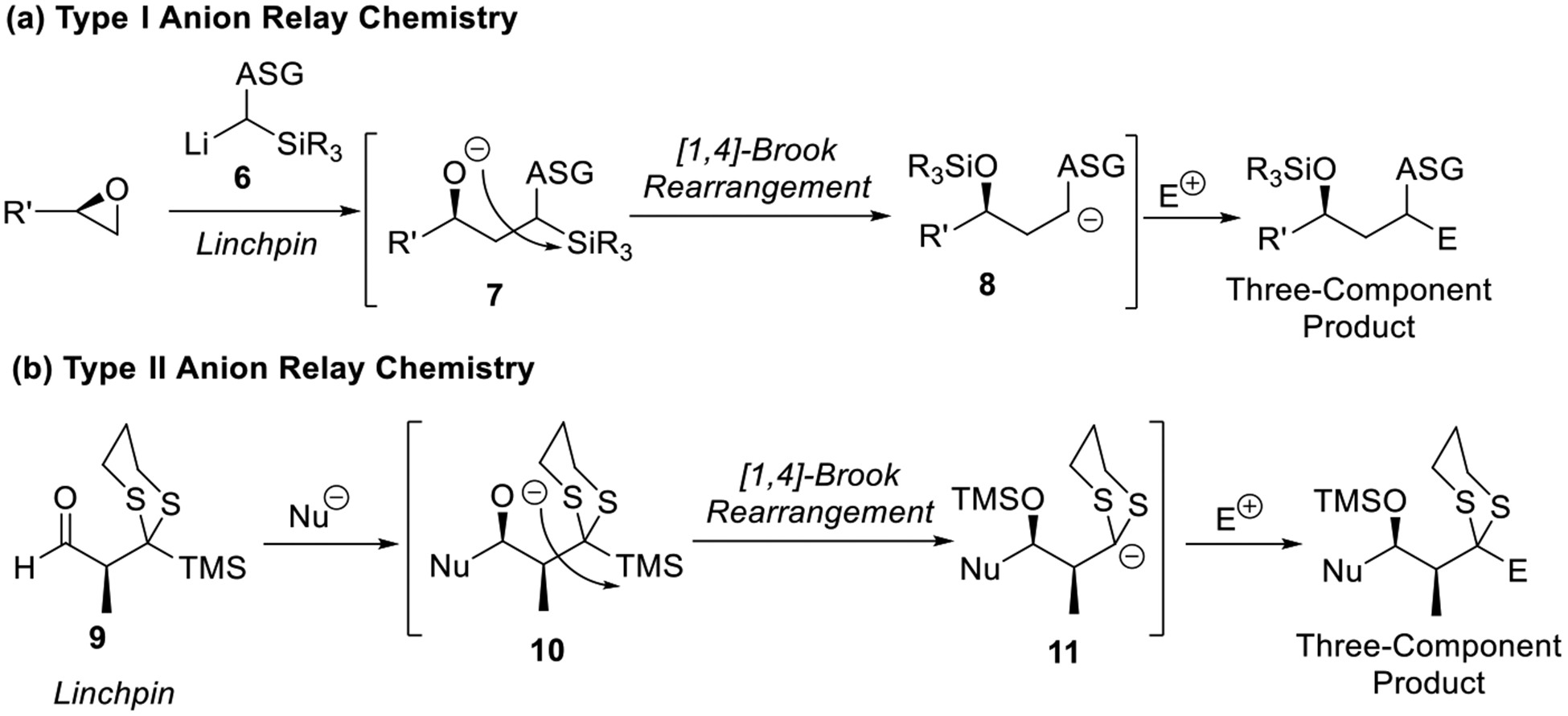
Type I and Type II ARC.
We would emphasize that ARC tactics are not confined to specific linchpins, migration agents (i.e., silyl groups) and/or to specific anion-stabilizing groups (i.e., dithiane). Various bifunctional linchpins have recently been developed (vide infra).7 Anion stabilizing groups (ASGs) such as -CN,7c -Bn7g and -SPh7g have also been validated in our laboratory. Besides silyl groups, the phosphonate group7i has also been employed as the charge transfer agent. We have also demonstrated the feasibility of longer-range through space Anion Relay Chemistry (vide infra).8 The potential of each of the ARC tactics has been demonstrated over the past decade in a number of completed or ongoing synthetic ventures. In this Account, we will illustrate the evolution and application of this multi-component union protocol for the rapid construction of structurally complex scaffolds in a “single-flask” manner.
2. Evolution of Type I ARC and Synthetic Applications
The first example of a multi-component union exploiting Type I ARC was reported by Matsuda in 1979 (Scheme 4a).9 The trimethylsilylacetonitrile (12) served as a viable linchpin. Union of lithio-12 with two epoxides 13 and 14 successfully produced 15 in 73% yield. It is noteworthy that the Brook rearrangement was controlled by raising the reaction temperature after introduction of the initial epoxide (13). Later in 1994, an important breakthrough was made by the Tietze10 and Schaumann groups,11 by introducing 2-trimethylsilyl-1,3-dithiane (16) as a linchpin for Type I ARC (Scheme 4b and 4c). These reactions proved successful in the synthesis of homocoupled (17) or cyclized products (19) without loss of enantiopurity. However, without a controllable Brook rearrangement, the utility of this protocol was restricted in the construction of molecules with C2-symmetry as shown in the synthesis of bryostatin 1 (vide infra)12 (Scheme 7).
Scheme 4.
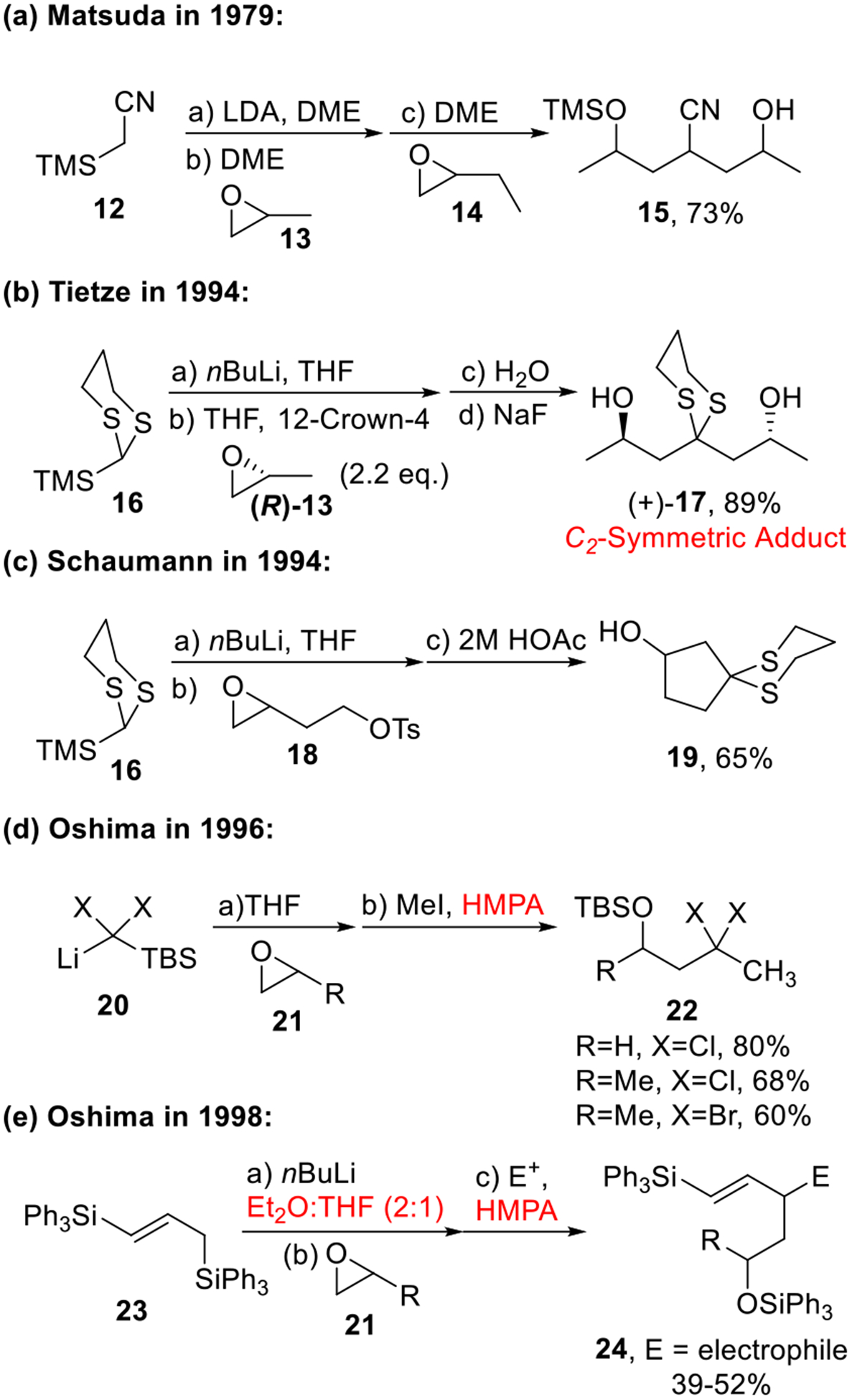
Initial Work on Type I ARC.
Scheme 7.
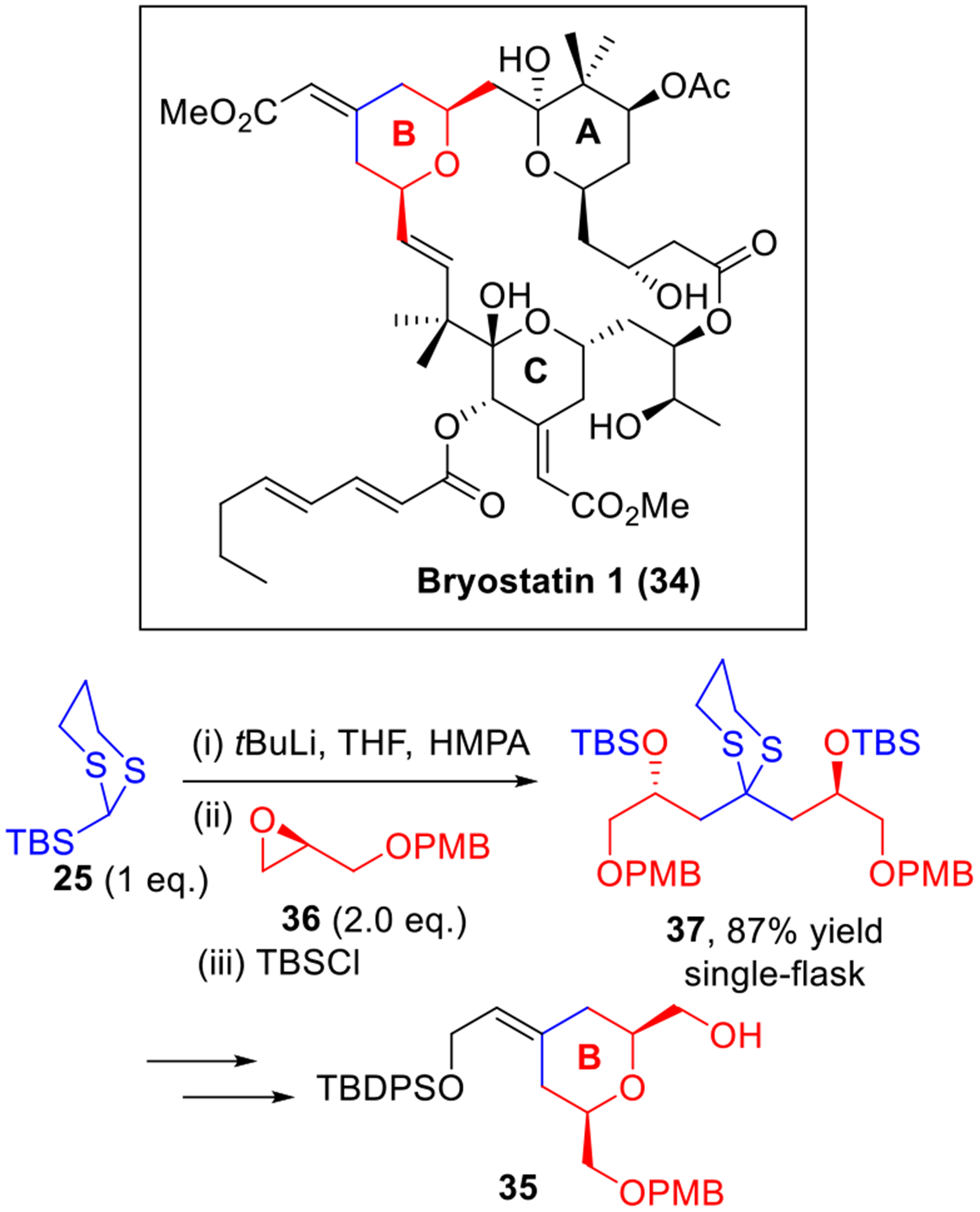
Type I ARC in Hale’s Synthesis of Bryostatin B-Ring.
It was not until 1996 that Oshima and co-workers demonstrated that the Brook rearrangement could be “triggered” by solvents and additives (Scheme 4d–e).13 In their studies, the combination of a mixed solvent (i.e., Et2O/THF)13b or the use of the polar additive HMPA, was required to control the precise timing of the Brook rearrangement, and as such permitted a three-component union with two different electrophiles. Recognizing the potential utility of the multi-component union process in complex molecule synthesis, we developed a multi-component union tactic employing the Tietze silyl dithiane linchpin (i.e., 25) with two different electrophiles.14 Here we utilized a solvent-controlled Brook rearrangement (Type I ARC). Various electrophiles including terminal epoxides, epichlorohydrin, vinyl epoxides, aldehydes, alkyl or allylic halides and aziridines were employed to furnish asymmetric adducts (26a-f) in good yield (Scheme 5). It is noteworthy that the order of electrophile addition could be strategically modified to locate the resultant silyl protecting group. Altering the absolute configuration of the epoxides, followed by removal of the dithiane and stereocontrolled reduction of the derived ketone, provided access to all possible diastereomers of the 1,3-polyol chain.
Scheme 5.
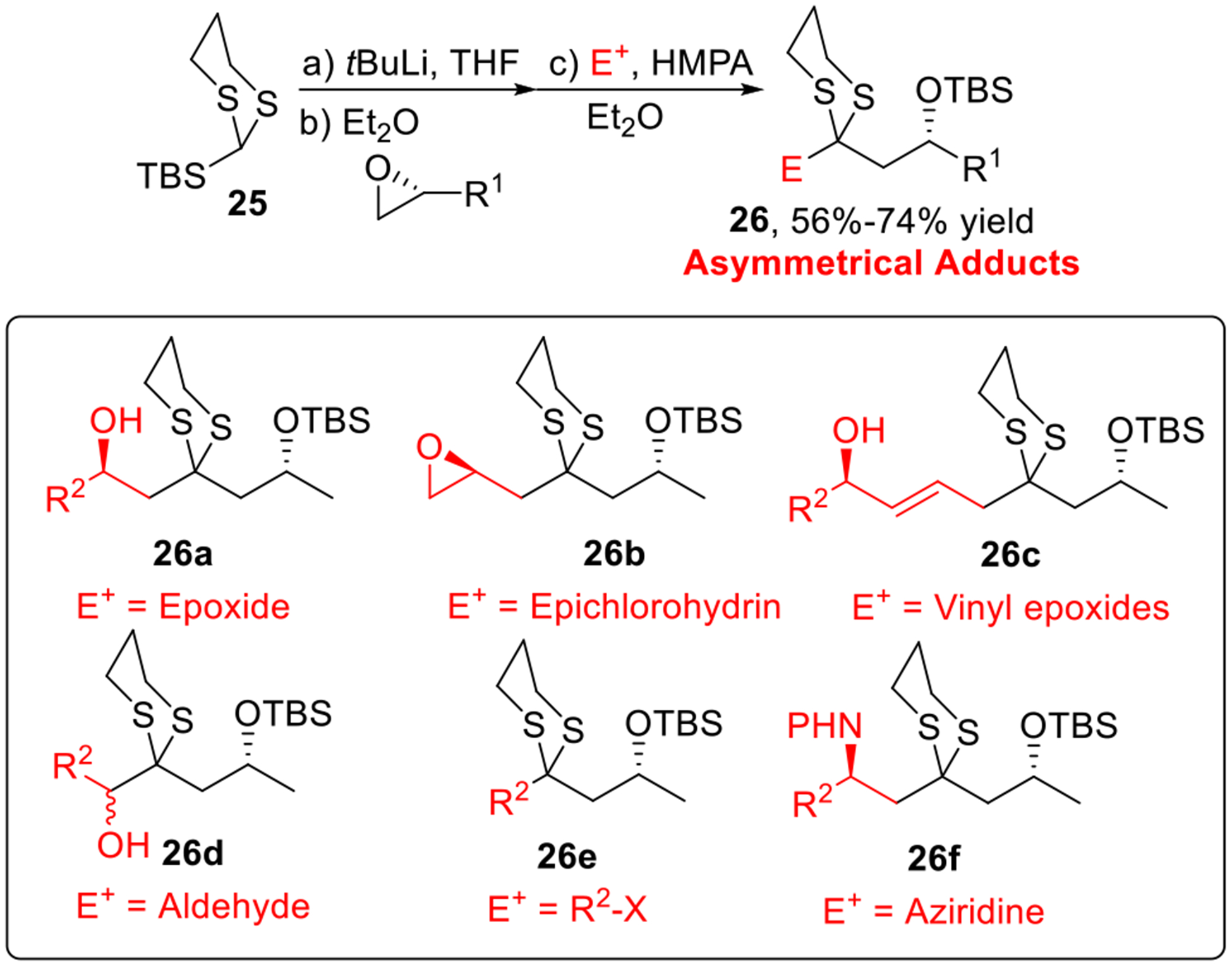
Smith’s Modified Type I ARC.
Type I Anion Relay Chemistry has been harnessed extensively as an effective multi-component union tactic in the concise construction of structurally diverse scaffolds, ranging from polyketides, spiroketals, polyene macrolides to polypropionate scaffolds. Several prominent examples from our and other laboratories will now be presented to illustrate the utility of the Type I multi-component ARC tactic in complex molecule synthesis. Evolution of Type II ARC will follow.
i. The Mycoticins
To showcase the synthetic potential of the multi-component union tactic for the construction of 1,3-polyol moieties, we embarked on an ARC project15 to construct advanced intermediate 27 employed in the Schreiber total synthesis of the mycoticins16 (28-29, Scheme 6). Recognizing the pseudo-C2-symmetry of the polyol backbone, a five-component coupling tactic was designed to rapidly elaborate the carbon skeleton of 27 in a single step. To this end, 2.3 equivalents of epoxide 30 were subjected to nucleophilic attack by 2.5 equivalents of lithio-25 to generated alkoxide 31, which upon the addition of HMPA underwent a solvent-mediated [1,4]-Brook rearrangement. Addition of the second electrophile 32 (1 equiv.) completed the construction of the desired five-component adduct 33 in a 59% yield in a single flask! Pleasingly, with the Type I multi-component ARC tactic, intermediate 27 could then be obtained in eight steps, five steps fewer than the Schreiber’s route.16
Scheme 6.
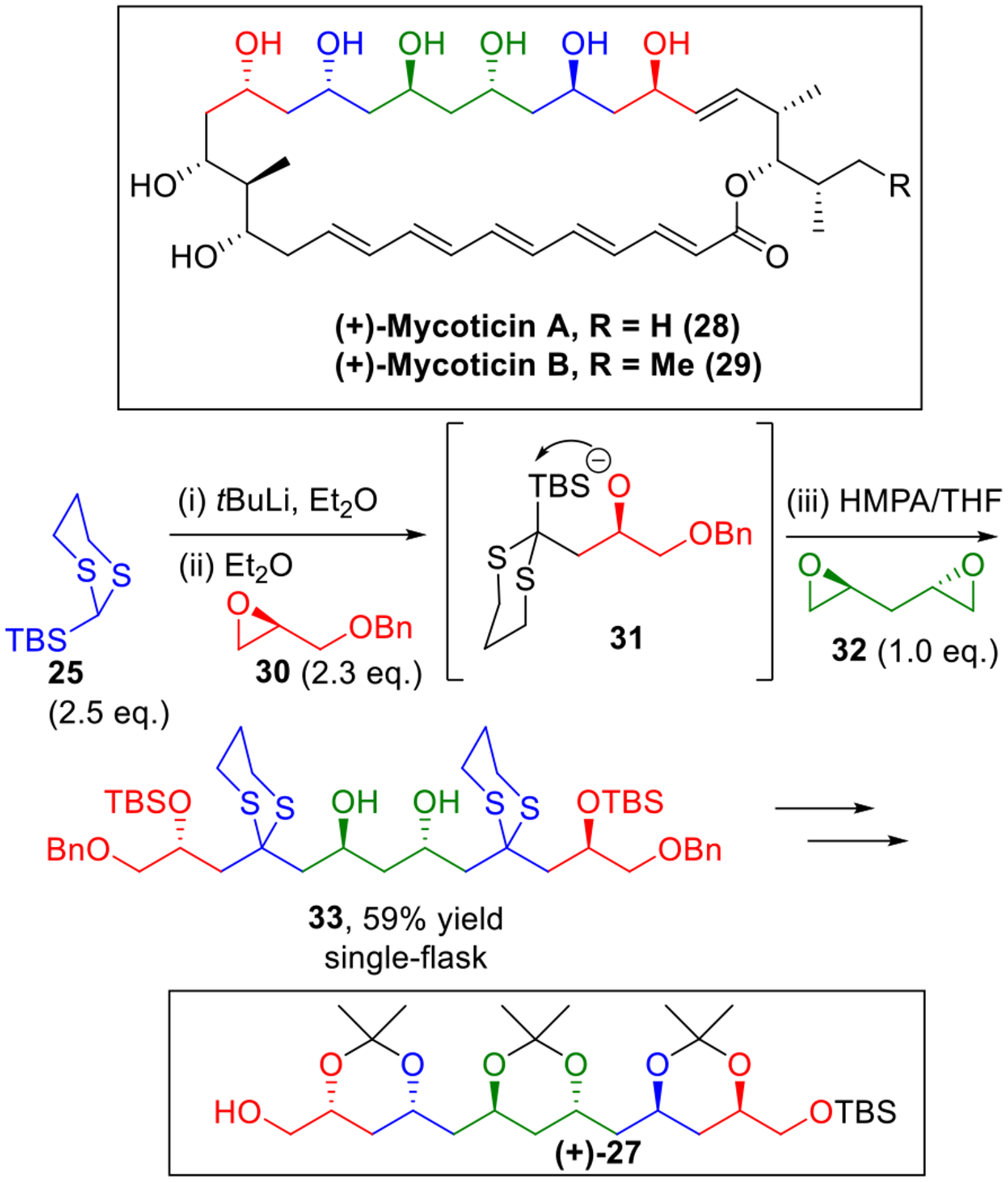
Five-Component Type I ARC in the Synthesis of Mycoticins.
ii. Bryostatin 1
Bryostatin 1 (34, Scheme 7), a member of an architecturally intriguing family of antitumor macrolides, displays considerable clinical promise in the treatment of cancer.17 In 2000, Hale and co-workers reported their synthetic studies for the construction of the bryostatin B-ring intermediate (35).12 Recognizing the inherent C2-symmetry of this subunit, Hale developed a strategy exploiting Tietze’s Type I ARC tactic to readily access the B-ring linear precursor (37). This reaction sequence began with lithiation of linchpin 25, followed by treatment with two equivalents of epoxide 36. Capture of the resulting alkoxide with TBSCl furnished the bisalkylated adduct 37 in 87% yield in a single flask! The adduct 37 was then further elaborated to the bryostatin B-ring subunit 35.
iii. The Spongistatins
The spongistatins (38-39, Scheme 8) comprise marine sponge metabolites with extraordinarily potent antitumor bioactivities.18 The scarcity of spongistatins as well as their complex structures, has attracted considerable attention in synthetic community.19 Since 2001, we have developed several generations of scalable total syntheses of both spongistatins 1 and 2,20 and later in 2008 a gram-scale synthesis of spongistatin 1(38) was completed.20c From the retrosynthetic perspective, both the AB- and CD-spiroketal fragments (40 and 41) in spongistatin 1 share a high level of symmetry in the carbon backbones, thus making them ideal synthetic targets for the Type I ARC tactic. Thus, on a two-gram scale, Type I ARC multi-component union protocols were developed to permit rapid construction of the AB- and CD-spiroketal fragments. To this end, deprotonation of 42 with t-BuLi, followed by addition of epoxide 43 produced intermediate alkoxide 44. A solvent-mediated [1,4]-Brook rearrangement triggered by HMPA, followed by addition of epoxide 45 furnished the linear AB-spiroketal precursor 46 in 58% yield in a single flask. Similarly, the three-component union of linchpin 25 with epoxides 47 and 49 provided, by the one-pot Type I ARC tactic, the CD-spiroketal linear precursor 50 of the spongistatins in a 69% yield. Notably, both Type I ARC reactions have been carried out on a 10 g scale, which proved to be the cornerstone for success in our gram-scale synthesis of spongistatin 1 (38).
Scheme 8.
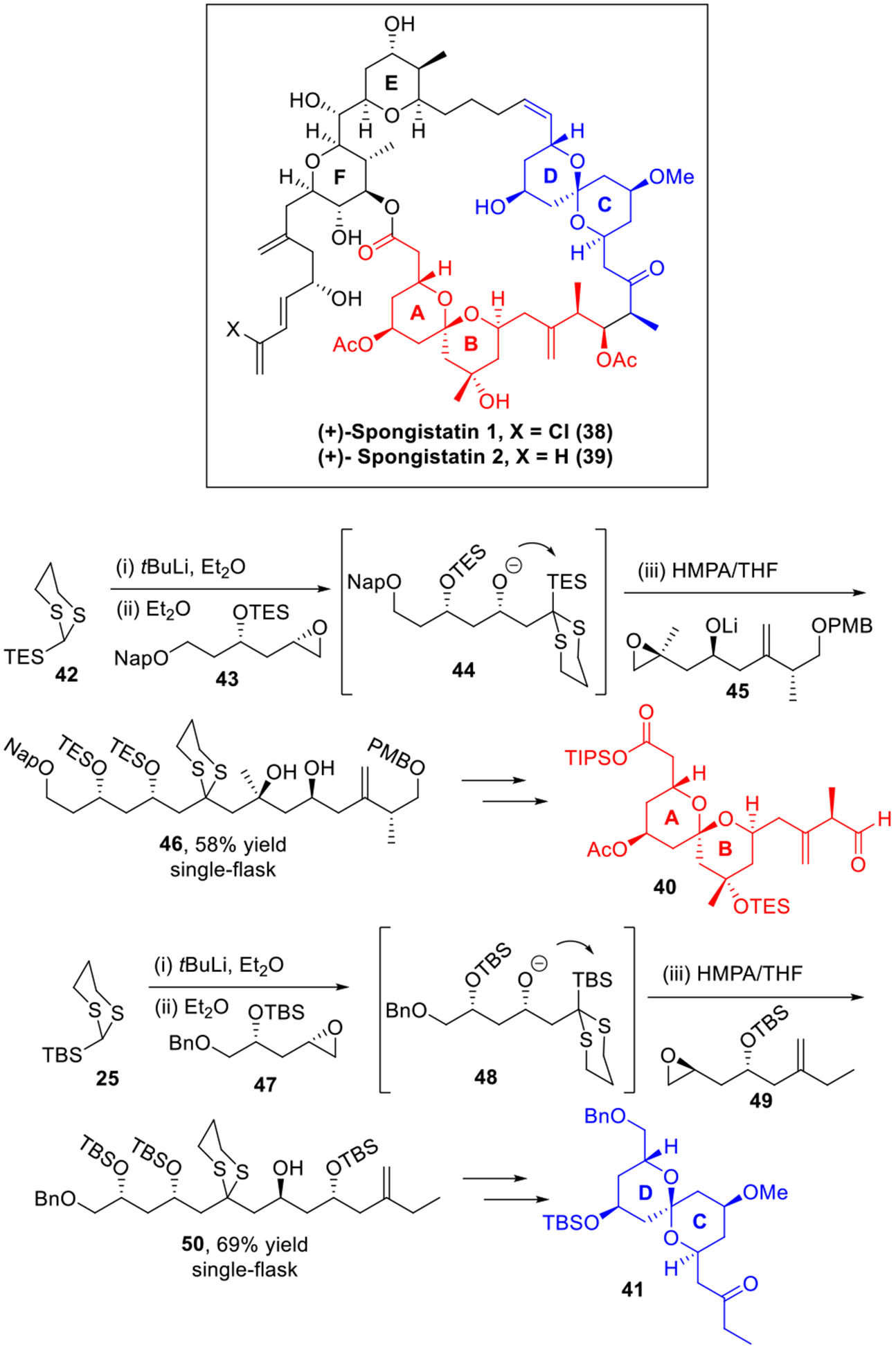
Construction of the AB- and CD-Spiroketal Fragment
iv. (+)-Rimocidin
(+)-Rimocidin (51, Scheme 9), an architecturally antifungal polyene macrolide, comprises a 28-membered aglycone, possessing an all-trans tetraene, an internal hemiketal and nine stereogenic centers.21 We reported the construction of the advanced C(1–19) polyol fragment of rimocidin in 2003,22 and later in 2009, we completed the synthesis of the macrocyclic core of the (+)-rimocidin aglycone.23 The central feature of our synthetic strategy highlights the utility of the multi-component Type I ARC tactic to construct the linear carbon skeleton of the C(1–19) polyol fragment in one step (Scheme 9).
Scheme 9.
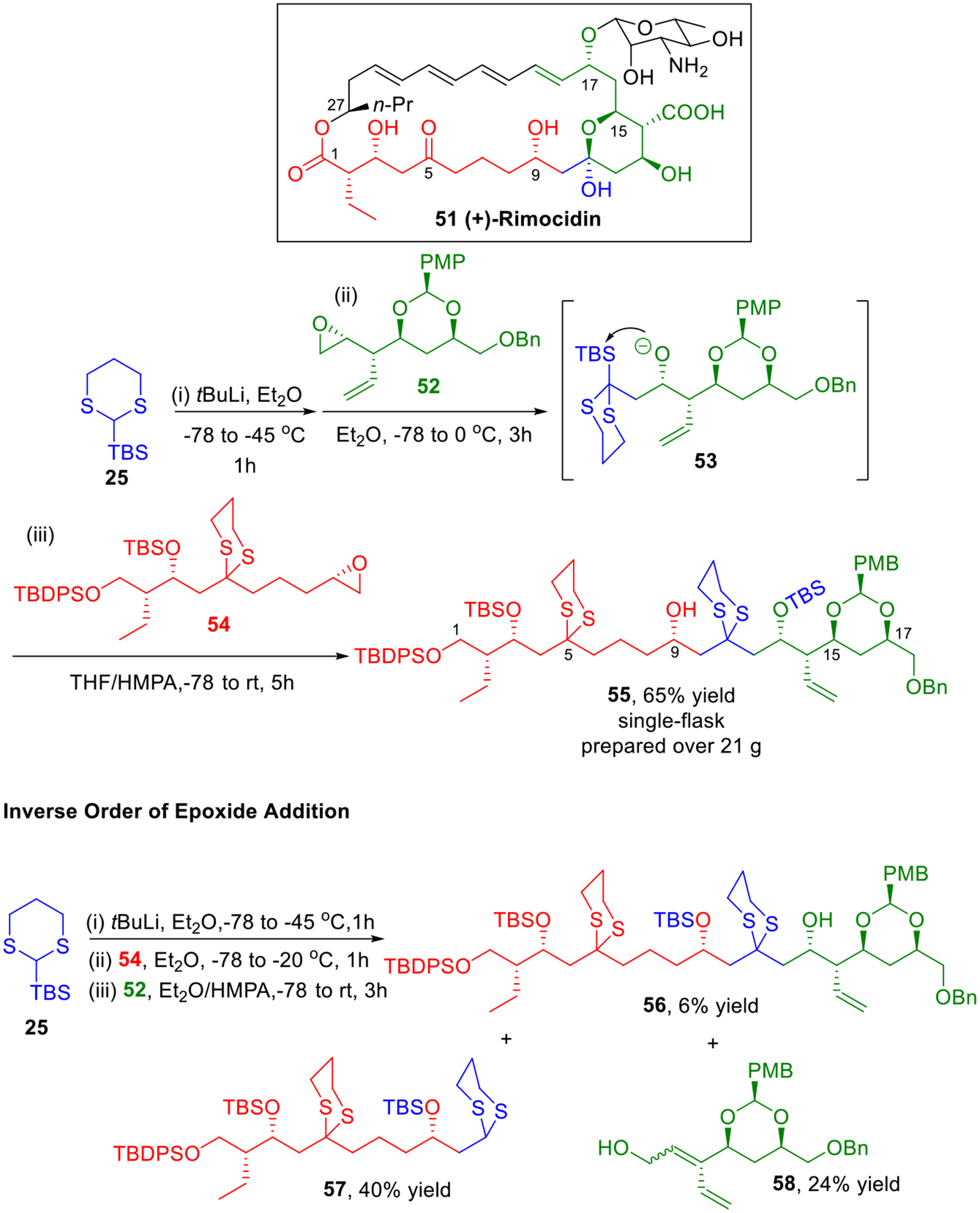
Construction of the C(1–19) Fragment of Rimocidin.
Beginning with the Type I ARC tactic, deprotonation of linchpin 25 followed by addition of epoxide 52 results in an alkoxide 53 that undergoes a [1,4]-Brook rearrangement upon solvent polarity change (addition of HMPA); addition of the second epoxide 54 then leads to the formation of 55 in 65% yield in a single flask. It is noteworthy that this large-fragment union reaction could be scaled up with over 21 grams of the required adduct 55 prepared! During our study of this Type I ARC reaction, we noticed that the order of epoxide addition has a dramatic influence on the result. Simply reversing the order addition (first epoxide 54 then epoxide 52) produced adduct 56 in only 6% yield. Instead the major product 57 was derived from initial dithiane addition followed by proton capture. Diene 58 was also obtained in 25%, which indicates that the dithiane anion of 57 rather than acting as a nucleophile, undergoes deprotonation of the allylic position of epoxide 52, thus leading to diene 58 by ring-opening.
v. Indolizidine Alkaloids
(−)-Indolizidine 223AB (59, Scheme 10) and frog alkaloid (−)-205B (60) belong to a class of Neotropic frog toxins isolated by Daly et al.24 The indolizidine backbone we envisioned could be constructed by a Type I multi-component ARC union of linchpin 25 with an epoxide and an aziridine as the two electrophiles (Scheme 10).25 For example, in our total synthesis of (−)-indolizidine 223AB, reaction of 25 with epoxide 61, followed by addition of the electrophilic aziridine 62 provides 63 in 56% yield, which was then elaborated to the final natural product 59 in four steps. For the architecturally more challenging alkaloid 60, a similar strategy for construction of the tricyclic core was achieved. The Type I ARC protocol utilizing linchpin 25, epoxide 64 and aziridine 65 furnished 66 in 53% yield in a single flask, which in turn was converted to (−)-205B (60).
Scheme 10.
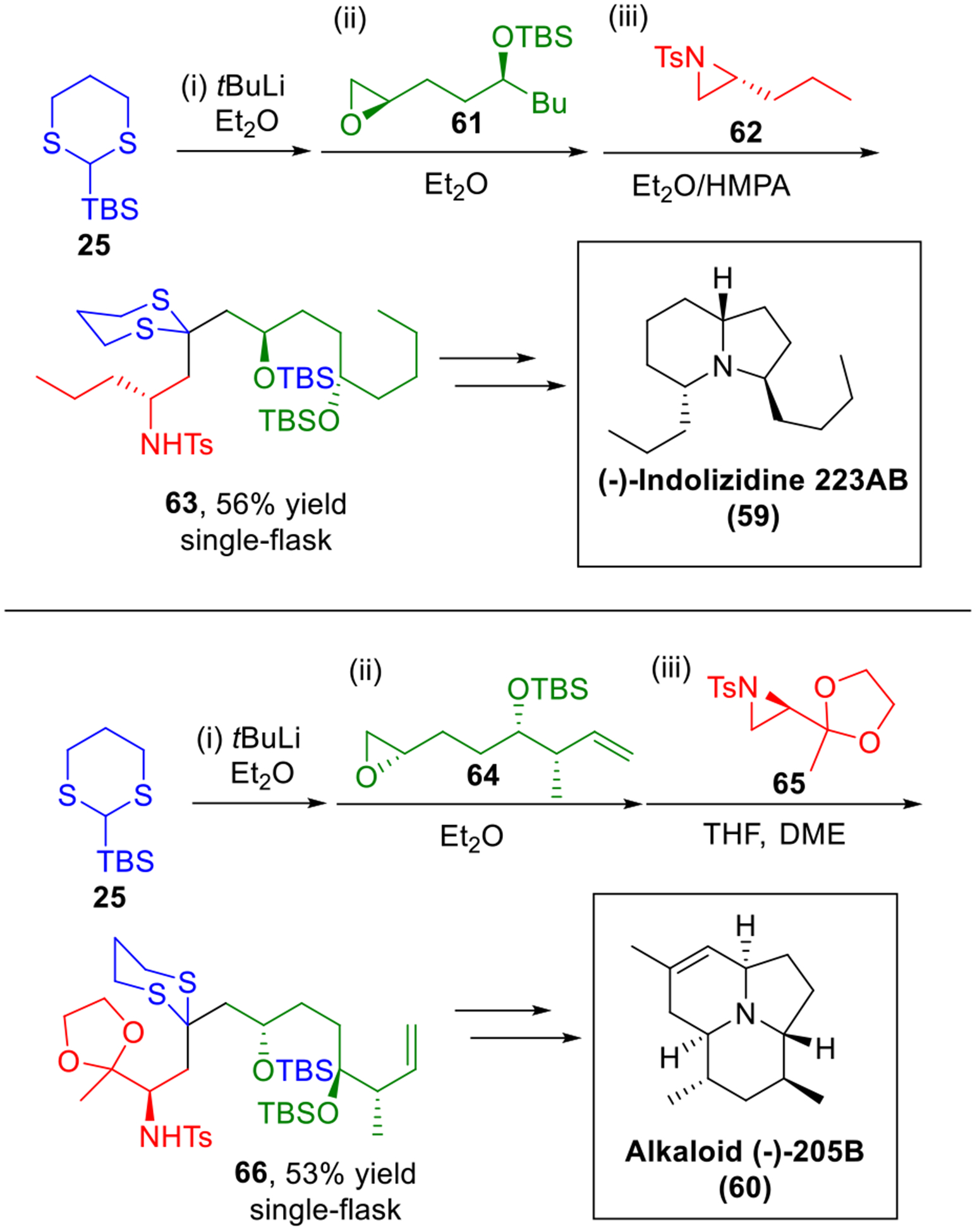
Synthesis of the Indolizidine Natural Products.
vi. (−)-Enigmazole A
In 2015, we completed the total synthesis of (−)-enigmazole A (67, Scheme 11),26 a potent cytotoxic natural product against the NCI 60 human tumor cell lines.27 The late-stage fragment union tactics of our synthesis featured our three-stage Petasis-Ferrier union/rearrangement.28 For the union of complex fragments, macrolactonization to connect the western hemisphere 68 and the eastern hemisphere 69 was then achieved. The latter in turn was rapidly constructed by a multi-component Type I ARC tactic, followed by a dithiane-epoxide union (Scheme 11). Here, the Type I ARC reaction began with the deprotonation of linchpin 25. Nucleophilic addition to epoxide 70, followed by a solvent-mediated Brook rearrangement and addition of the second epoxide 71 then led to the three-component adduct 72 in 90% yield as a single diastereomer. Further elaboration of 72 furnished the backbone of eastern hemisphere 69.
Scheme 11.
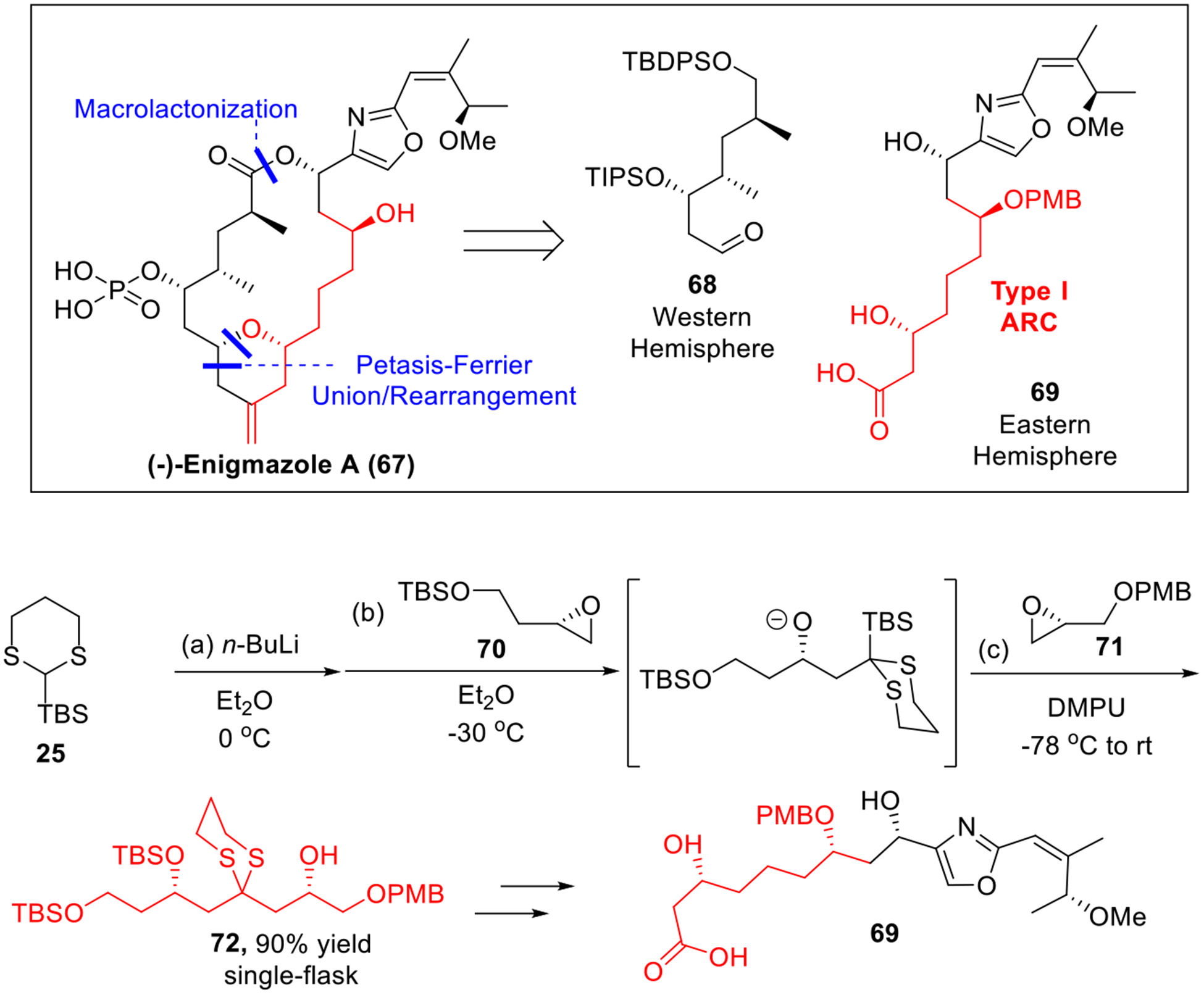
Construction of Eastern Hemisphere of Enigmazole A.
3. Evolution of Type II ARC and Synthetic Applications
In 2000, Moser and co-workers reported the synthesis and reaction of the o-trimethylsilyl benzaldehyde chromium tricarbonyl complex 73 in a multi-component union tactic (Scheme 12a).29 Addition of alkyl lithiums or lithium enolates to the aldehydic carbonyl in 73 generated alkoxides (i.e., 74a or 74b), which underwent the [1,4]-Brook rearrangement. The resulting aryl anion was then captured intermolecularly with various electrophiles (i.e., allyl bromide)29 to deliver the adducts 75a, or intramolecularly30 to deliver the indanones 75b after facile removal of the chromium tricarbonyl moiety. This innovative sequence can be viewed as the first example to employ a bifunctional linchpin in ARC. However, several drawbacks restrict the synthetic application of the Moser tactic, including difficulty in preparation of linchpin 73 (6 steps in 24% yield), the environmental hazard in the use of chromium, and low atom efficiency. Later in 2004, we employed commercially available 2-bromo-trimethylallylsilane (76) and developed what we now term a Type II multi-component ARC reaction sequence (Scheme 12b).7a A series of structurally diverse allylic alcohols 78 were readily prepared. However, due to the basicity of the allylic anion intermediate (77) generated upon Brook rearrangement, the scope of the second electrophile was limited due to potential deprotonation by the basic nature of allyl anion. The development of viable bifunctional linchpins to enhance the efficiency and diversity of this multi-component union became central to the study of Type II Anion Relay Chemistry.
Scheme 12.
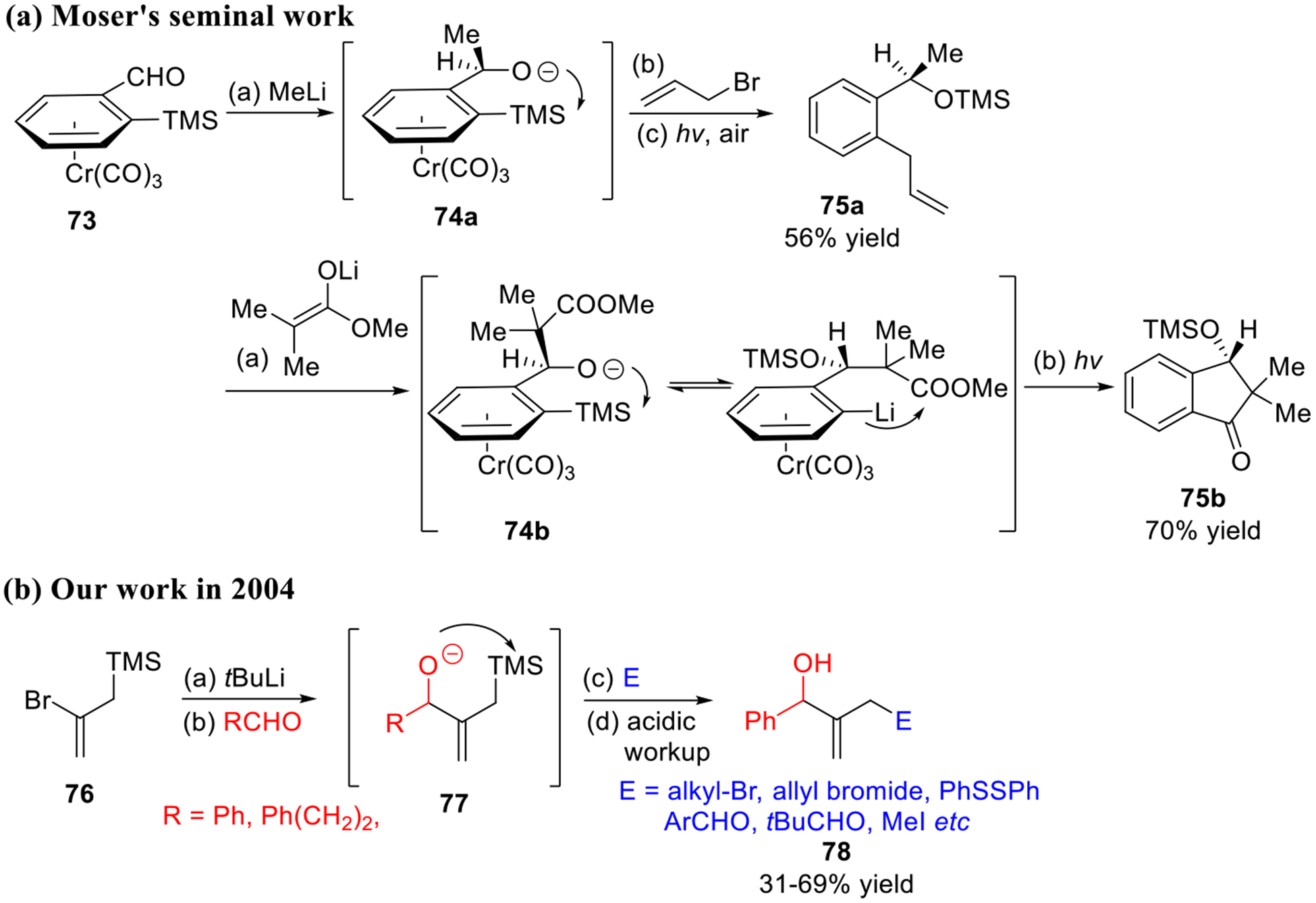
Early studies on Type II ARC.
In general, a Type II bifunctional linchpin contains both an electrophilic and a masked nucleophilic site, the latter possessing an anion-stabilizing group (ASG). The central feature of a viable bifunctional Type II ARC linchpin therefore entails: (a) an appropriate functional group for the electrophilic site and the required stereochemistry; (b) the distance required for group transfer (i.e., [1,4]- or longer Brook rearrangement); and (c) a suitable anion-stabilizing group (ASG). Based on the distance between the electrophilic site and the masked nucleophilic site, Type II linchpins can be categorized into four classes (Scheme 13).
Scheme 13.
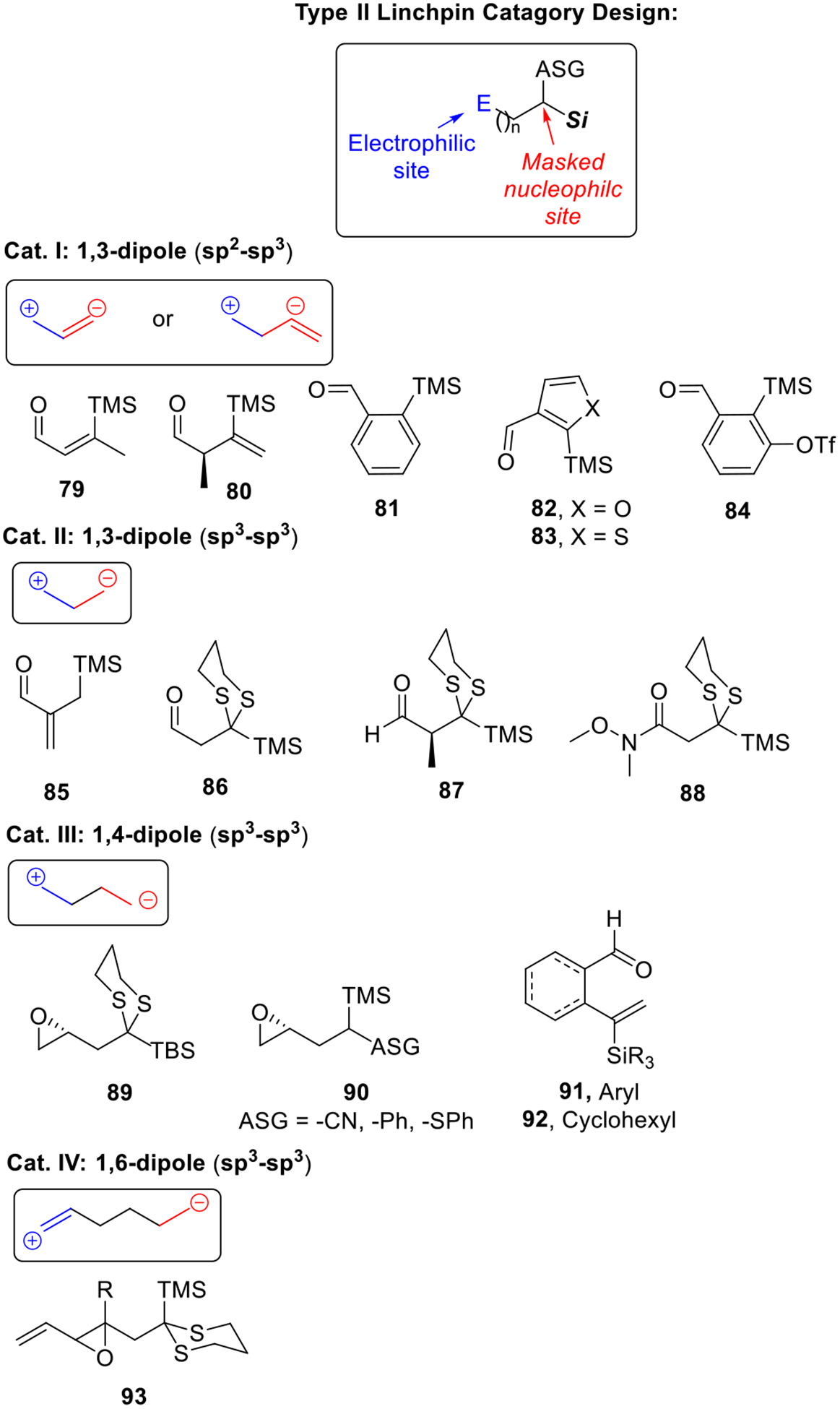
Selected Examples of Type II ARC Linchpins.
Design of Cat. I bifunctional linchpins were inspired by the Moser linchpin (73, Scheme 12a). For this class of linchpins, silyl groups are directly installed on vinyl (79-80),7f aryl (81,7d, 847h) or heteroaryl (82-83)7e groups, which also serve as the anion-stabilizing group (ASG), to generate a sp2-carbonanion after silyl transfer. The constrained structures of these linchpins permit facile [1,4]-Brook rearrangement with the resulting sp2-carbanion captured with various electrophiles or even involvement in Pd-catalyzed cross-coupling reactions (Scheme 14a)!7f It is noteworthy that linchpin 84 (Scheme 14b) is a unique benzyne precursor.7h For example, after initial nucleophilic attack of linchpin 84, a [1,4]-Brook rearrangement followed by elimination generates a benzyne intermediate 94 in situ, which is capable of undergoing a wide variety of inter- or intramolecular [2+2], [3+2] or [4+2] cycloaddition reactions.
Scheme 14.

Examples of Cross-coupling and Cyclization Reaction Employing Type II Linchpins.
The breakthrough in the development of Type II ARC was made by the rational design of Cat. II and III linchpins (Scheme 13). For example, various nucleophiles (cf., 95 in Scheme 15) reacts smoothly with linchpin 89 and then after Brook rearrangement, capture of the resultant stabilized anion 96 proceeds smoothly with a series of electrophiles, including primary epoxides, alkyl, allyl and benzyl halides to furnish in good to excellent yields tri-component adducts (97a-e) with substrate-controlled stereochemistry.7b It is noteworthy that when epichlorohydrin was used as the second electrophile, a new electrophilic epoxide site (i.e., 97e) is generated for potential further functionalization. Importantly, Cat. II and III linchpins hold great potential for the “one-pot” iterative Type II ARC tactic, that is just by replacing the terminating electrophile with a second different bifunctional linchpin permits a form of “living polymerization” (Scheme 15b).
Scheme 15.

Multi-component Union Tactics Employing Type II Linchpins.
In 2015, we developed bifunctional linchpin 87 (Scheme 16a),7k which proved to be a significant extension of our Type II ARC coupling tactic. This linchpin (87) permits the Type II ARC process to mimic Nature’s method of uniting propionate building blocks with complete stereo-induction when employing α-substituted linchpins given Felkin-Anh control. Later in 2015, a bifunctional Weinreb amide linchpin (88)7l was developed to preserve the carbonyl functionality in the ARC adducts (i.e., 99, Scheme 16b), thus permitting access to even more functionally diverse synthetic targets, including 1,3-diketones, pyran and spiroketal scaffolds. In this reaction, the Brook rearrangement was initiated by a stabilized tetrahedral intermediate 98.
Scheme 16.
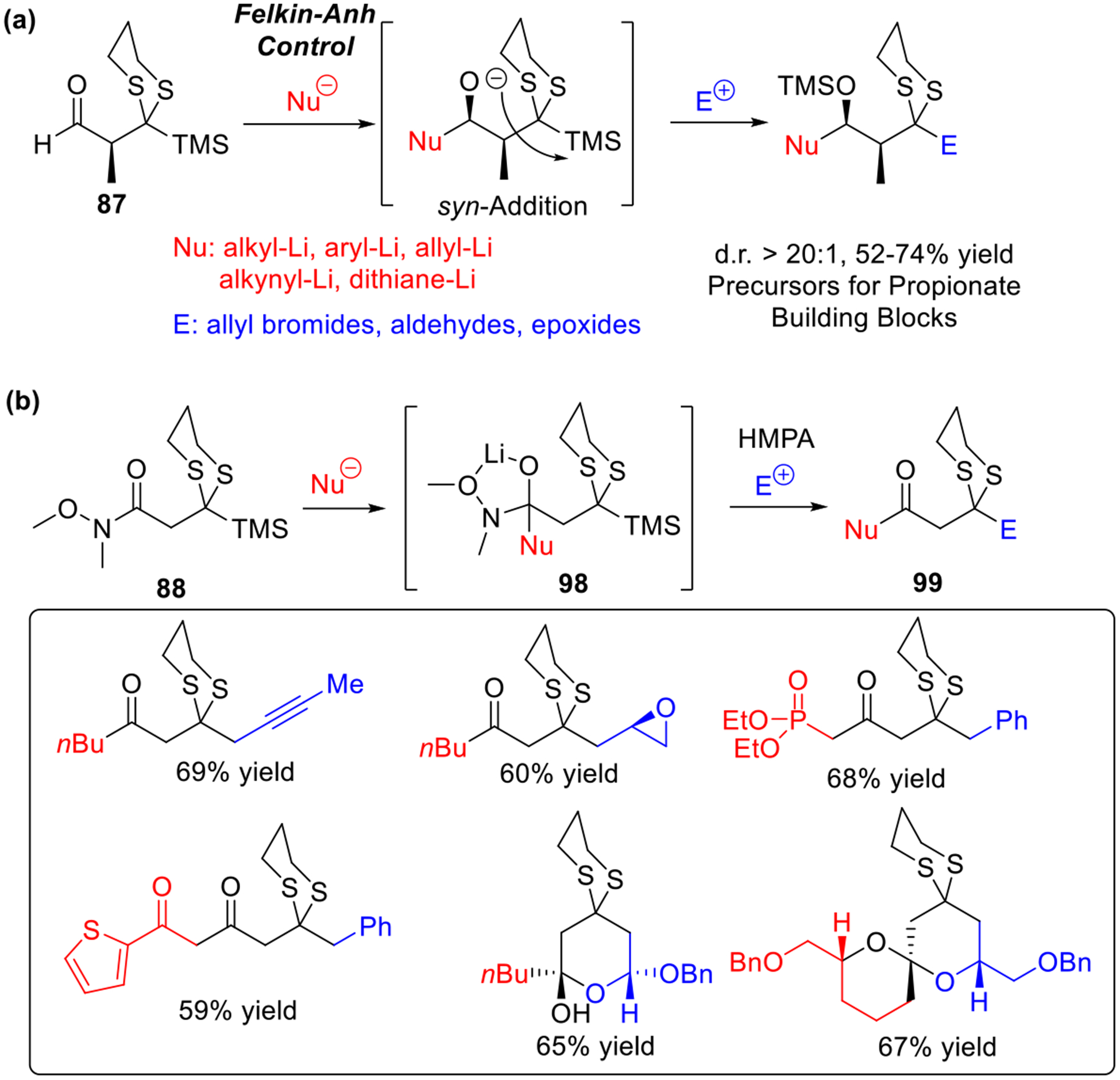
Type II Linchpins for Various Synthetic Targets.
Cat. IV bifunctional linchpins with a vinylepoxide as the initial electrophilic site (93) represent a new class of linchpins that for the first time, permit execution of the “ThroughBond/Through-Space” Type II ARC tactic.7j The propagation of negative charge during the reaction is achieved in a single flask that enables the construction of structurally complex motifs 100, not previously readily accessible (Scheme 17).
Scheme 17.
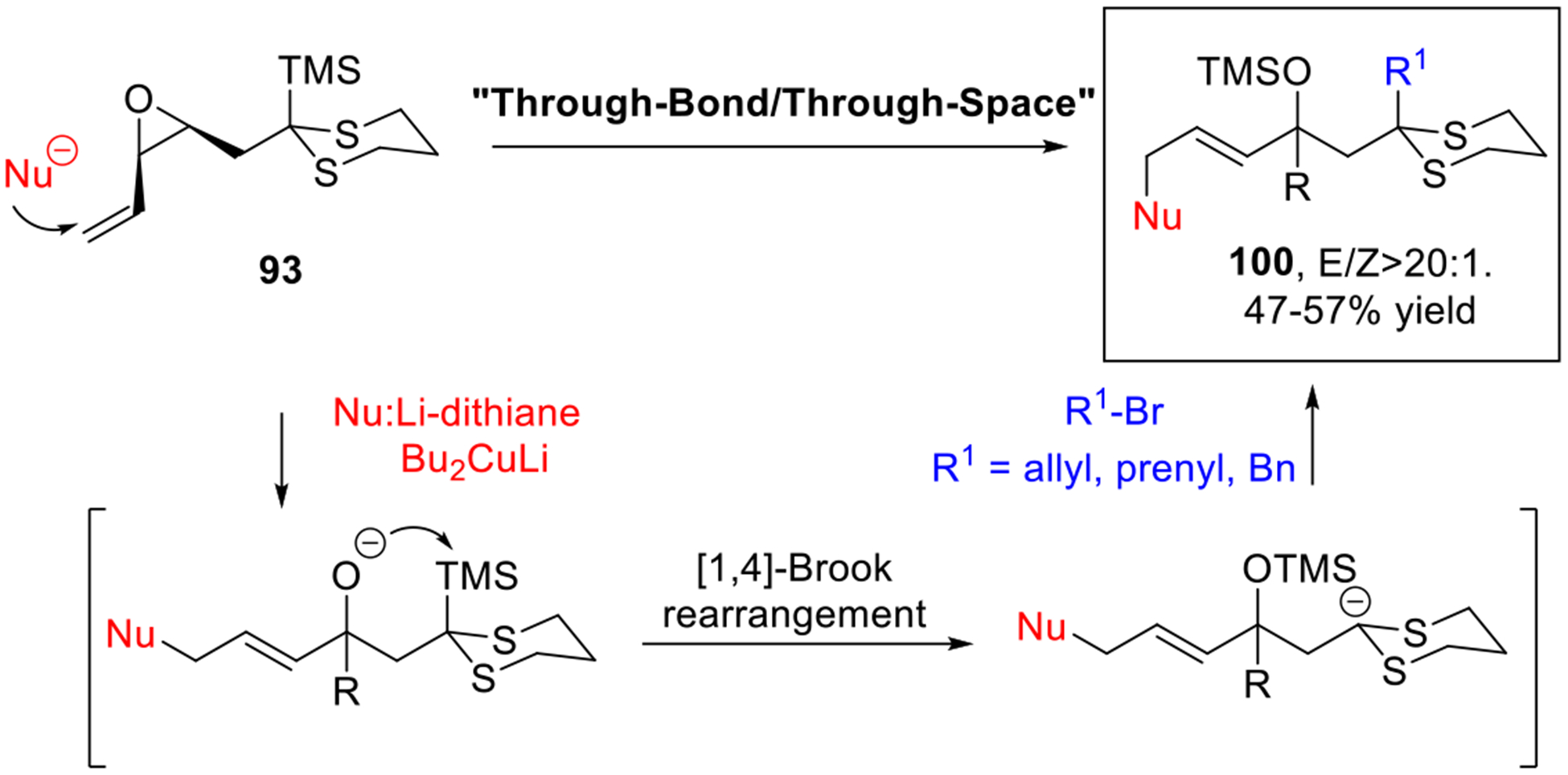
“Through-Bond/Through-Space” Type II ARC Tactic.
It is important to note that the synthetic application of Type II ARC tactics is not restricted to linear carbon frameworks. Bicyclic building blocks are also accessible by the combination of designed linchpins and certain nucleophiles. For example, in 2009 in collaboration with Xian, we reported a “one-pot” cyclization reaction employing thiophene linchpin 83 and an ester enolate 101 as the nucleophile (Scheme 18a).7e After Brook rearrangement, the resulting 2-thiophenyl anion (103) underwent intramolecular addition to the ester group and as such delivered cyclized adduct 104. These heterocycle products hold considerable promise for diversity-oriented synthesis of focused libraries of biomedical relevance. More recently, we developed a diastereoselective [3+2] Type II ARC annulation tactic exploiting “soft” ketone enolates (105) with an aldehyde linchpin (i.e., 86) to construct highly functionalized cyclic systems 108 (Scheme 18b).31 Precise stereocontrol of the initial aldol reaction, followed by a Brook rearrangement/cyclization sequence delivered various [3+2] adducts, thus providing a new strategy to access diverse [n.3.0] bicyclic building blocks.
Scheme 18.

Type II ARC Cyclization Tactic.
Longer-distance Brook rearrangements, namely [1,5]- and [1,6]-silyl group migration, have been explored in our group to expand both the scope and diversity of the multi-component ARC process.8 For example, a tri-component Type II ARC involving [1,5]-Brook rearrangement was reported in 2006 (Scheme 19a), wherein the potassium base was essential to trigger the silyl migration.8a More recently, restrained bifunctional vinyl linchpins (Scheme 19b), which are conformationally anchored on six-membered rings (phenyl 91, cis- or trans-cyclohexyl 92), were exploited in Type II ARC involving [1,5]-Brook rearrangement.8b To trigger the [1,5]-Brook rearrangement, additives including HMPA and tBuOK were required. As for cyclohexyl linchpin 92, increased temperature or even microwave irradiation is necessary to trigger the silyl transfer. DFT calculations indicated that the tether with conformational constraints in these vinyl linchpins significantly lowers the energy barrier of the [1,5]-Brook rearrangement, thus permitting both alkylations and Pd-mediated cross-coupling reaction of the in-situ generated sp2 carbanions.
Scheme 19.
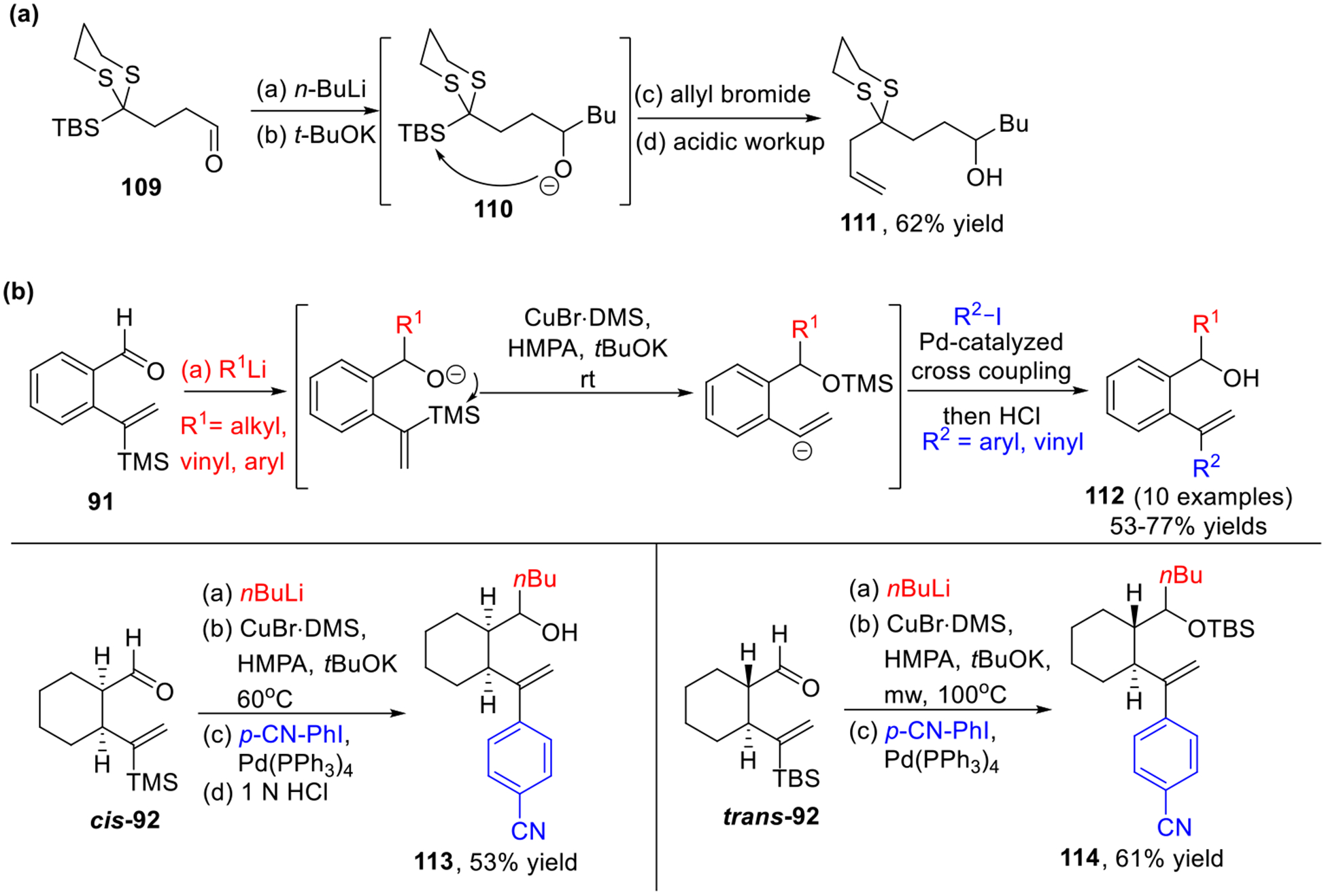
Type II ARC involving [1,5]-Brook Rearrangement.
In connection with the evolution of Anion Relay chemistry, a unification of Type II ARC with the Takeda and Hiyama cross-coupling tactic was also achieved, inspired by the pentacoordinate silicate intermediates encountered during the Brook rearrangement (Scheme 20; 116 ⇌ 117).32 This “green” protocol permits room-temperature cross-coupling of aryl and heteroaryl halides with aryl lithium reagents by in-situ generation of a pentacoordinate silicate intermediate (116), and as such circumvents preparation and isolation of organometallic coupling partners required for examples in the Negishi Zn intermediate. Four generations of siloxane transfer agents have been developed,33 including recoverable siloxanes 115b-e, which can be easily recycled during work-up procedure; polystyrene-supported siloxanes 115f-g, which can be recycled by simple filtration and reused in repeated reaction cycles without the loss of efficiency and stability; and bench-stable electron-withdrawing siloxane (115h), which enables the room temperature cross-coupling reaction with aryl chlorides. It is noteworthy that in the presence of these recyclable siloxanes, undesired homocoupled products of organolithium were not detected, and most importantly, the only stoichiometric waste product in this cross-coupling protocol is LiCl!
Scheme 20.
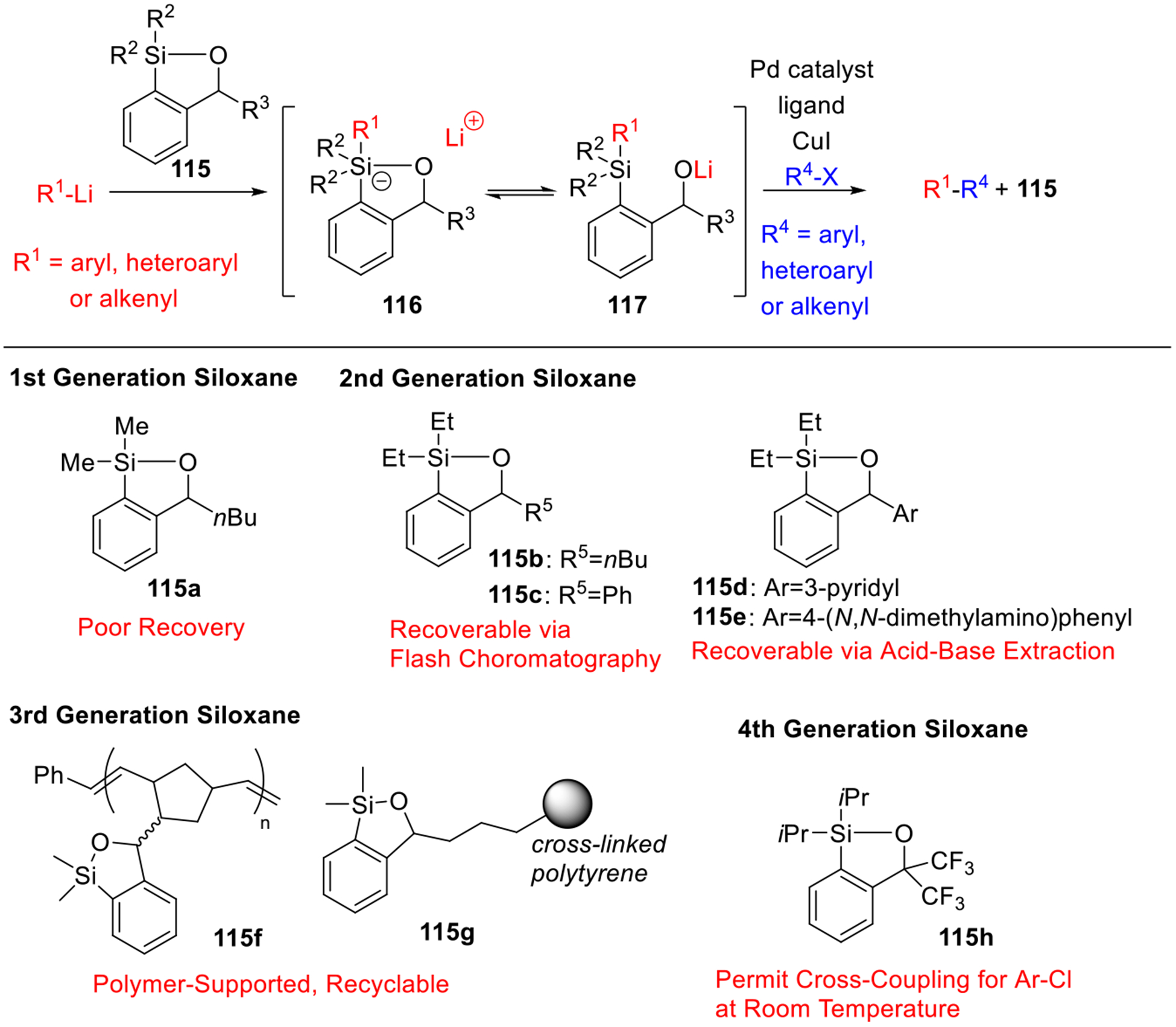
Unification of Type II ARC with Cross-Coupling Reaction.
Recognizing the synthetic potential of the Type II ARC multi-component process and diversity of bifunctional ARC linchpins shown above, the construction of several architecturally complex natural products or fragments thereof have been achieved employing this effective fragment union protocol. Selected examples are presented as followed.
i. The Spirastrellolides: C(1)-C(25) Southern Hemisphere
Spirastrellolides A (118a) and B (118b) comprise two architecturally unique polyketide natural products with selective protein phosphatase inhibition.34 The scarcity of these compounds and the architectural complexity attracted considerable attention in synthetic community.35 Effective syntheses of the C(1)-C(25) southern hemisphere of spirastrellolides A and B have been achieved by our group (Scheme 21),36 featuring four dithiane unions, including a three-component Type II ARC tactic to access the linear precursor of the BC spiroketal (119).
Scheme 21.
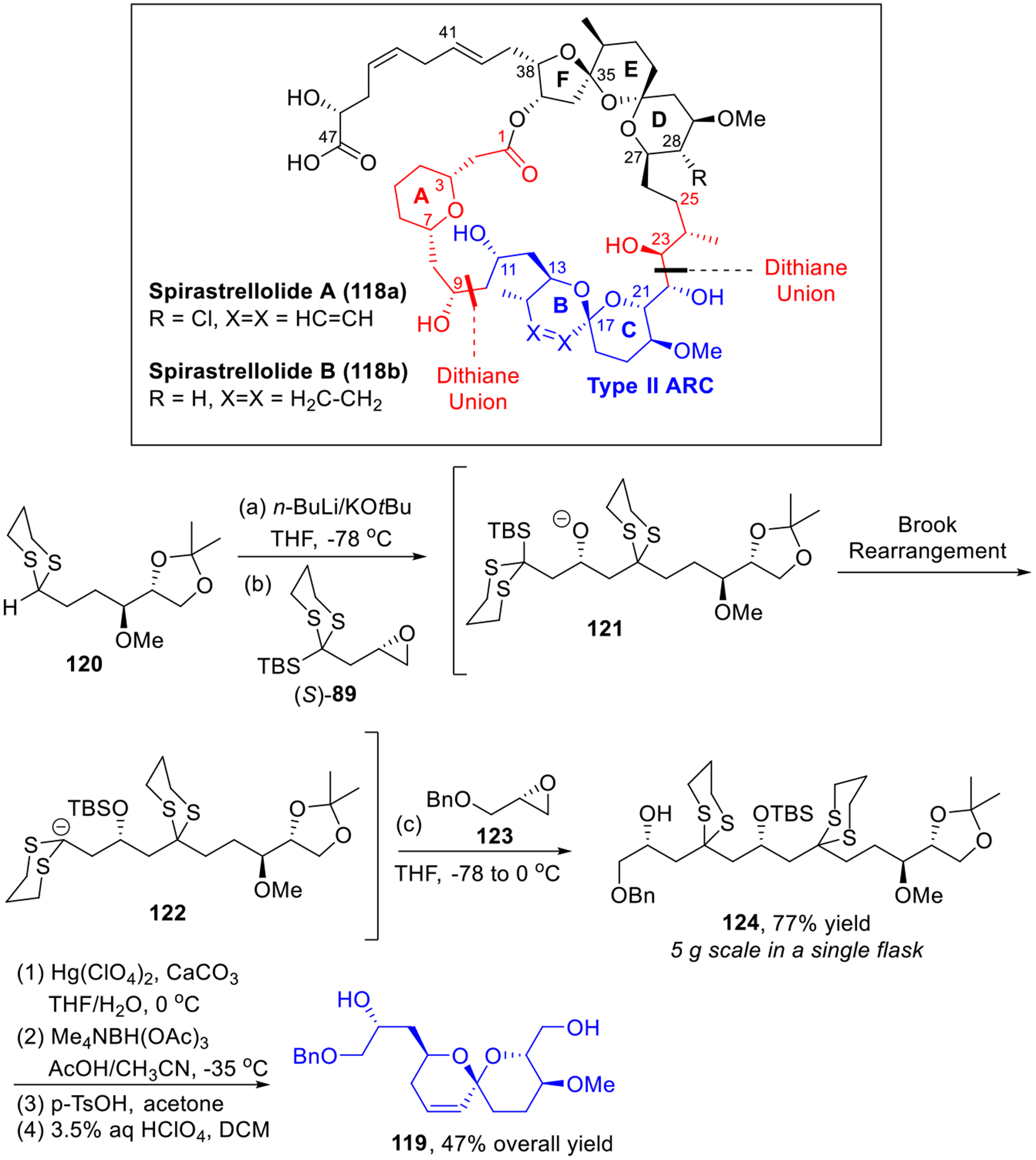
Construction of BC Spiroketal Fragment of Spirastrellolides.
The ARC reaction begins with the deprotonation of 120 with Schlosser base. Addition of linchpin (S)-89 then results intermediate 121, which after Brook Rearrangement is alkylated with epoxide 123 to furnish the expected three-component adduct 124 in 77% yield. It is important to note that this reaction could be conveniently run on a 5-gram scale in a single flask! At this stage, a four-step reaction sequence of dithiane removal, selective anti-reduction followed by spirocyclization/Ferrier rearrangement completed the synthesis of the key BC spiroketal fragment (119, Scheme 21).
ii. The Cryptocarya Family of Natural Products
The cryptocarya family (125–128) represents one kind of polyhydroxylated dihydropyrone natural products (Scheme 22).37 Because of their unique polyol structure, both Type I and Type II ARC have been demonstrated as powerful tools in the syntheses of the cryptocarya family members. Type I ARC was employed by the She group in 2009 in their syntheses of cryptocarya triacetate (125), cryptocaryolones (126-127).38 In 2013, we developed a divergent total synthesis to access four members of cryptocarya family including 125-127 and polyrhacitide A (128) by the common intermediate 129 (Scheme 22).39 Pleasingly, an efficient four-component Type II ARC protocol was exploited, wherein the common intermediate 129 was rapidly assembled in one step. To this end, nucleophilic attack of bifunctional linchpin 89 by Li-130a generates alkoxide 131, which after [1,4]-Brook rearrangement is captured by the second electrophile 132. Upon warm-up, epoxide 133 is formed. Addition of vinylmagnesium bromide and copper bromide then delivers the desired adduct 129a in 52% yield. Of interest, common intermediate 129a possesses the shared linear carbon skeleton of 125-127. Changing the initial nucleophile 130a to 130b and employing the enantiomers of 89 and 132 furnishes another adduct 129b, albeit in a lower yield. Elaboration of adduct 129b then completed the total synthesis of polyrhacitide A (128).
Scheme 22.

Total Synthesis of Cryptocarya Family.
iii. (−)-Secu’amamine A
In 2008, the first total synthesis of (−)-secu’amamine A (134), an architecturally intricate member of the Securinega alkaloids family,40 was achieved by the Weinreb group,41 employing advanced tetracyclic intermediate 135 to construct the critical diene motif at late stage. In 2015, we explored the Type II ARC tactic for the total synthesis of (−)-secu’amamine A (Scheme 23).42 In our total synthesis, a more concise synthesis of the advanced intermediate 135 could be achieved that showcased a Type II ARC union of four components (i.e., dithiane 136, linchpin 89, electrophile 139 and MOMBr) that rapidly permits access to the carbon and nitrogen linear backbone of (−)-secu’amamine A in a single flask.
Scheme 23.
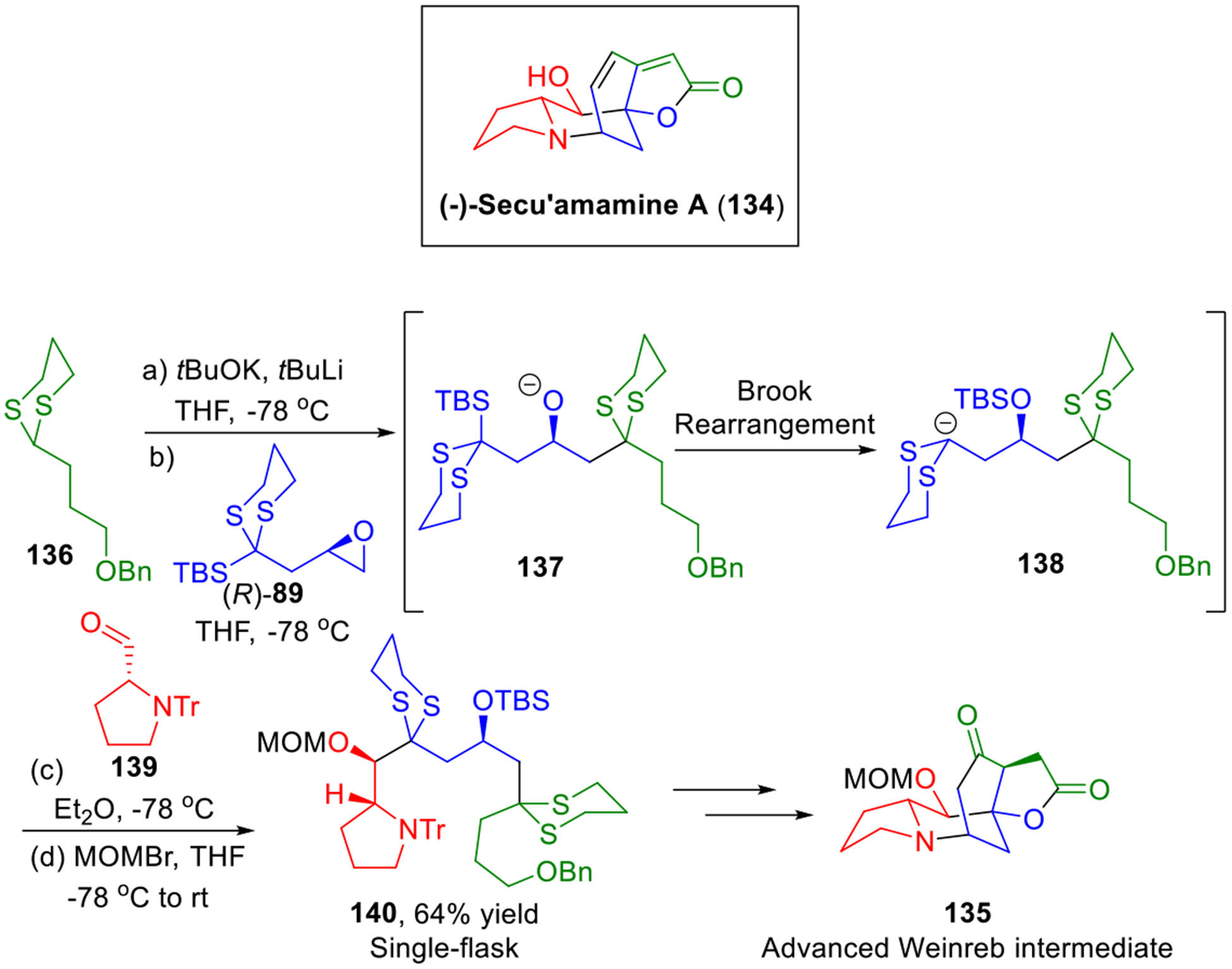
Synthesis of the Weinreb Intermediate of (−)-Secu’amamine A.
To this end, deprotonation of dithiane 136 followed by addition of linchpin (R)-89 produced intermediate alkoxide 137. A solvent-mediated [1,4]-Brook rearrangement involving Felkin-Anh controlled nucleophilic attack on aldehyde 139 and capture with MOMBr then completed construction of the four-component adduct 140 in a single flask. The yield for the overall reaction sequence was 64%. Pleasingly, adduct 140 was further carried forward to the advanced Weinreb intermediate 135 in 7 steps in 24% overall yield. Completion of the synthesis of (−)-secu’amamine A (134) was then achieved following the Weinreb five-step sequence.
iv. (−)-Mandelalide A
The mandelalides comprise a family of glycosylated polyketide macrolides that exhibit nanomolar cytotoxicity.43 In 2016, we reported a convergent, modular synthesis of (−)-mandelalide A (141), the most potent member of this family, as an illustration of the strategic value of both Type I and Type II ARC tactics (Scheme 24).44
Scheme 24.

Total Synthesis of Mandelalide A by ARC.
Recognizing that the backbone of northern hemisphere (142) could be constructed from advanced intermediate 150, we developed for the first time a combination of Type II ARC/CuCN-mediated cross-coupling strategy. Nucleophilic attack of the linchpin 146 with 2-lithio-1,3dithiane 145, is presumed to result in pentacoordinate silicate intermediate 148 derive from an adjacent alkoxide group (147) by [1,4]-Brook rearrangement. A cross-coupling reaction mediated by CuCN between vinyl iodide 149 and the silicate 148 then furnishes adduct 150 in excellent yield (89%) on a gram scale. 150 was then elaborated to the northern hemisphere (142) in 10 steps and in 42% yield.
The construction of the southern hemisphere aglycon (144), on the other hand, relied on a four-component Type I ARC tactic. Nucleophilic attack to epoxide 152, followed by a solvent-triggered Brook rearrangement generated the dithiane anion (154). To the same flask, the second electrophile 132 was added to form a new epoxide 155. Addition of vinylmagnesium bromide and CuI then furnished the requisite homoallylic alcohol 156 on half-gram scale in 87% yield. Further elaboration of 156 completed the construction of 144 in 32% yield in 10 steps.
v. (−)-Nahuoic Acid Ci (Bii)
The nahuoic acids comprise the first class of natural products that display selective (S)-adenosylmethionine (SAM)-competitive inhibitory activity against the histone lysine methyltransferase SETD8 enzyme.45 In 2017, we completed the first total synthesis of (−)-nahuoic acid Ci (Bii) (157, Scheme 25).46 In this total synthesis, we exploited the efficiency of the Type II ARC tactic to construct the 1,3,5-trialkoxy side chain (158). Starting with linchpin 87, addition of 1.2 equivalent of isopropyl lithium, followed by a solvent-mediated [1,4]-Brook rearrangement and addition of the second electrophile (R)-132, led to the three-component adduct 160 in a 76% yield on a 5-gram scale. The latter was then carried forward to the sidechain 158 in five steps in 39% overall yield. It is noteworthy that the key fragment union of the side chain 158 and cis-decalin 161 was then achieved by the beautiful Micalizio union protocol47 to furnish the adduct 162 in 61% yield. The completion of the synthesis of nahuoic acid Ci (Bii) (157) was then achieved by olefin metathesis and global deprotection as outlined in Scheme 26.
Scheme 25.
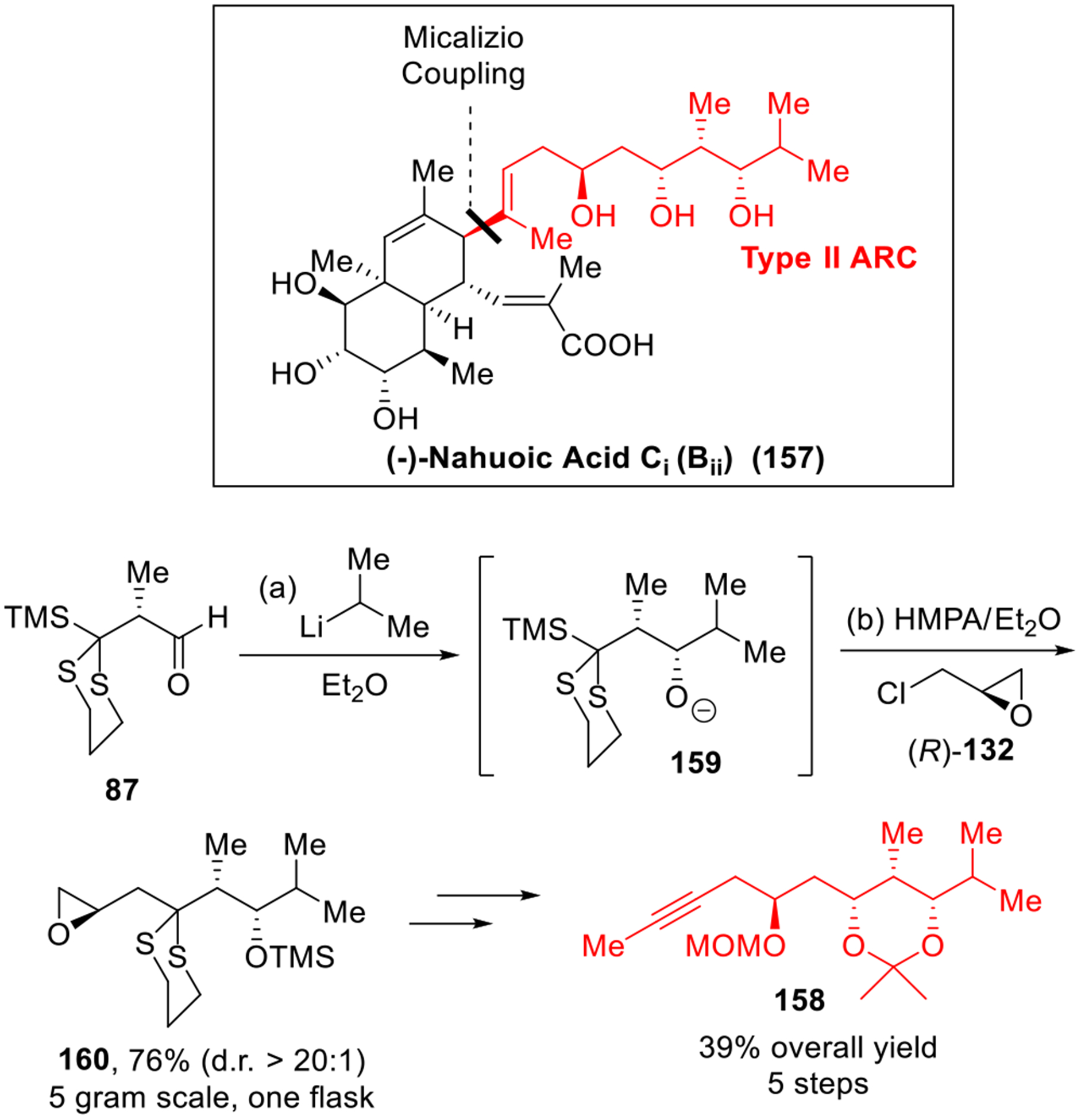
Construction of Polyol Sidechain of Nahuoic Ci (Bii).
Scheme 26.
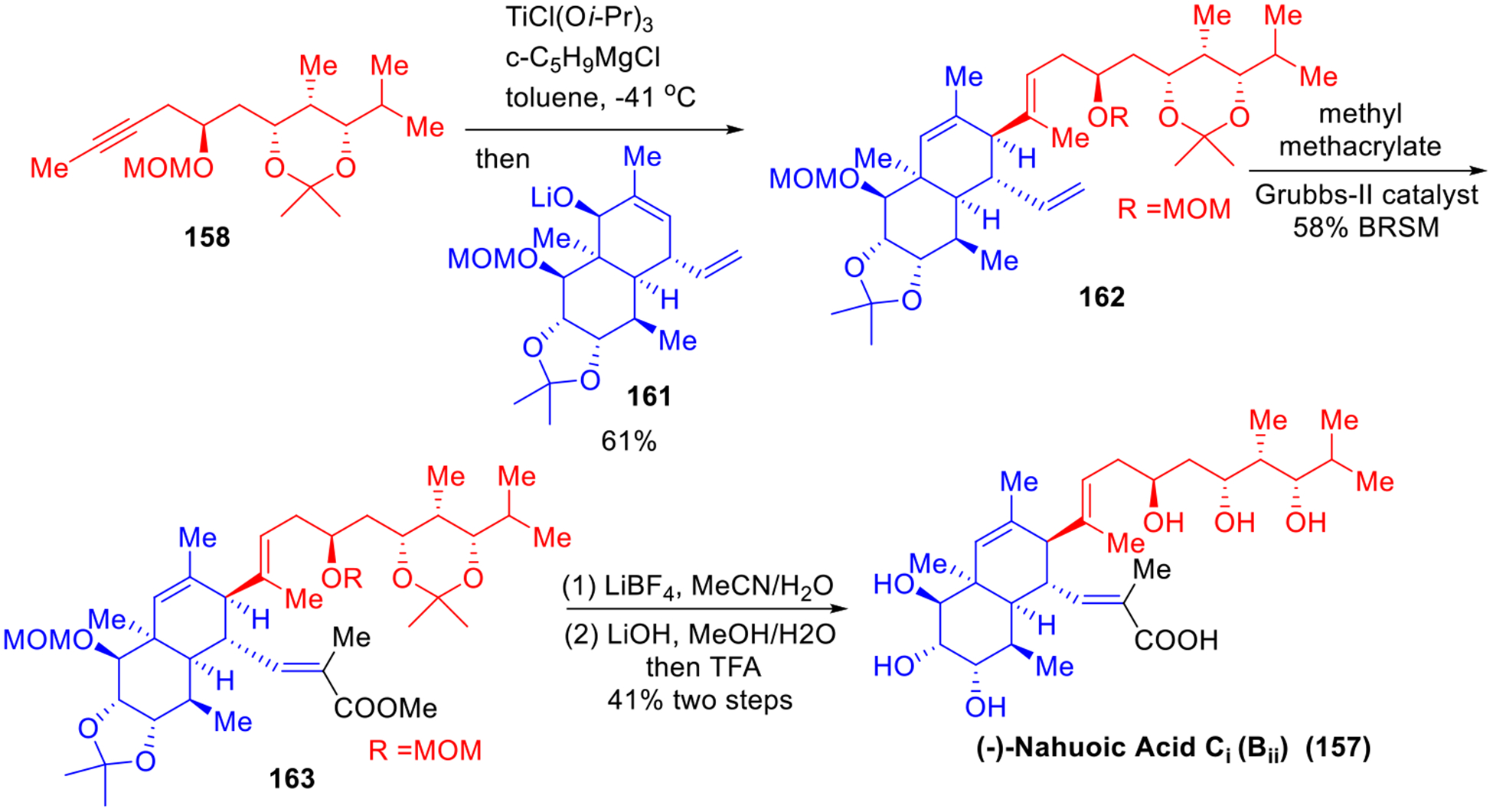
Final Elaboration to Nahuoic Ci (Bii).
4. Conclusion
Significant achievements have been made in the past 20 years with the Type I and II multi-component Anion Relay Chemistry (ARC) tactics. Specifically, the ability to harness the Brook rearrangement in a controllable manner with the development of bifunctional linchpins proved critical to these advances. In this Account, the evolution of the ARC protocols and their application in the total synthesis of complex natural products have been highlighted. However, in today’s society, with urgent demand on syntheses that are step-efficient, diversity-oriented and environmentally friendly, there remains a great need to design and exploit new bifunctional linchpins for effective protocols for multi-component union tactics. Anion Relay Chemistry, with the ability to unite multiple, structurally complex starting materials in a “one-pot” and stereocontrolled manner, thus holds great potential as we move closer to that goal.
ACKNOWLEDGMENT
The authors thank all previous co-workers who have helped advance this project. The research presented in this Account has been primarily supported by the NIH through Grants CA-19033 and GM-29028.
Biographies
Yifan Deng, born in Ningbo, China (1992), received his B. S. degree from Peking University in 2015. He is currently pursuing his Ph. D. in Chemistry at the University of Pennsylvania under the guidance of Prof. Amos B. Smith, III. In 2019, he was awarded the Bader Fellowship.
Amos B. Smith, III, born in Lewisburg, PA (1944), completed Bucknell University’s inaugural B.S.-M.S. degree in chemistry (1966) with Professor H. W. Heine. After a year at the University of Pennsylvania School of Medicine, he entered The Rockefeller University, completing his Ph.D. degree in 1972, followed by a year as a Research Associate, both with Professor W. C. Agosta. In 1973 joined the Department of Chemistry at University of Pennsylvania, where he is currently the Rhodes-Thompson Professor of Chemistry and a Full Member of the Monell Chemical Senses Center. From 1988 to 1996, he served as Chair of the Department of Chemistry. In addition, he holds Honorary Memberships in the Kitasato Institute, the Pharmaceutical Society of Japan, the American Academy of Arts Sciences and served as the inaugural Editor-in-Chief of Organic Letters (1998–2018). He has been awarded the Centenary Medal-Royal Society of Chemistry, the Order of the Rising Sun-Government of Japan, a D.Sc. (honoris causa) Queens University Belfast (Northern Ireland), the Paul Gassman Award of the ACS Organic Division, the William H. Nichols Medal, the Allan R. Day Award, the Perkin Prize for Organic Chemistry by Royal Society of Chemistry, recipient of an Honorary Issue of the Journal of Antibiotics, and Inventor of The Year at Penn.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Bertz SH Complexity of Synthetic Routes: Linear, Convergent and Reflexive Syntheses. New. J. Chem 2003, 27, 870–879. [Google Scholar]
- (2).Smith AB III; Wuest WM Evolution of Multi-Component Anion Relay Chemistry (ARC): Construction of Architecturally Complex Natural and Unnatural Products. Chem. Commun 2008, 5883–5895 and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Graaff C; Ruijter E; Orru RVA Recent Developments in Asymmetric Multicomponent Reactions. Chem. Soc. Rev 2012, 41, 3969–4009 and references therein. [DOI] [PubMed] [Google Scholar]
- (4).Brook AG Isomerism of Some α-Hydroxysilanes to Silyl Ethers. J. Am. Chem. Soc 1958, 80, 1886–1889. [Google Scholar]
- (5).Gilman H; Wu TC Reactions of Triphenylsilylpotassium with Benzophenone and with 4,4’-Dimethylbenzophenone. J. Am. Chem. Soc 1953, 75, 2935–2936. [Google Scholar]
- (6).Naganuma K; Kawashima T; Okazaki R Control Factors of Two Reaction Modes of Pentacoordinate 1,2-Oxasiletanides, the Peterson Reaction and Homo-Brook Rearrangement. Chem. Lett 1999, 28, 1139–1140. [Google Scholar]
- (7).(a) Smith AB III; Duffey MO Bifunctional Molecular Linchpins: A Three-Component Coupling Protocol Employing 2-Bromoallyltrimethylsilane. Synlett 2004, 8, 1363–1366. [Google Scholar]; (b) Smith AB III; Xian M Anion Relay Chemistry: An Effective Tactic for Diversity Oriented Synthesis. J. Am. Chem. Soc 2006, 128, 66–67. [DOI] [PubMed] [Google Scholar]; (c) Smith AB III; Kim D-S ; Xian M Anion Relay Chemistry Extended. Synthesis of a Gorgonian Sesquiterpene. Org. Lett 2007, 9, 3307–3309. [DOI] [PubMed] [Google Scholar]; (d) Smith AB III; Kim W-S; Wuest WM Ortho-TMS Benzaldehyde: An Effective Linchpin for Type II Anion Relay Chemistry (ARC). Angew. Chem. Int. Ed 2008, 47, 7082–7086. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Devarie-Baez NO; Kim W-S; Smith AB III; Xian M Multicomponent Type II Anion Relay Chemistry (ARC): One-Pot Syntheses of 2,3-Disubstituted Furans and Thiophenes. Org. Lett 2009, 11, 1861–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Smith AB III; Kim W-S; Tong R Uniting Anion Relay Chemistry with Pd-Mediated Cross Coupling: Design, Synthesis and Evaluation of Bifunctional Aryl and Vinyl Silane Linchpins. Org. Lett 2010, 12, 588–591. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Smith AB III; Tong R Benzyl and Phenylthiomethyl Silanes: A New Class of Bifunctional Linchpins for Type II Anion Relay Chemistry (ARC). Org. Lett 2010, 12, 1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Smith AB III; Kim W-S Diversity-Oriented Synthesis Leads to an Effective Class of Bifunctional Linchpins Uniting Anion Relay Chemistry (ARC) with Benzyne Reactivity. Proc. Nat. Acad. Sci 2011, 108, 6787–6792. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Sokolsky A; Smith AB III Validation of Diethoxyphosphonate as an Effective Agent for Charge Transfer in Anion Relay Chemistry (ARC). Org. Lett 2012, 14, 4470–4473. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Chen MZ; Gutierrez O; Smith AB III Through-Bond/Through-Space Anion Relay Chemistry Exploiting Vinylepoxides as Bifunctional Linchpins. Angew. Chem. Int. Ed 2014, 53, 1279–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Melillo B; Chen MZ; Forestieri R; Smith AB III An Effective Bifunctional Aldehyde Linchpin for Type II Anion Relay Chemistry: Development and Application to the Synthesis of a C16-C29 Fragment of Rhizopodin. Org. Lett 2015, 17, 6242–6245. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Farrell M; Melillo B; Smith AB III Type II Anion Relay Chemistry: Exploiting Bifunctional Weinreb Amide Linchpins for the One-Pot Synthesis of Differentiated 1,3-Diketones, Pyrans and Spiroketals. Angew. Chem. Int. Ed 2016, 55, 232–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).(a) Smith AB III; Xian M; Kim W-S; Kim D-S The [1,5]-Brook Rearrangement: An Initial Application in Anion Relay Chemistry. J. Am. Chem. Soc 2006, 128, 12368–12369. [DOI] [PubMed] [Google Scholar]; (b) Liu Q; Chen Y; Zhang X; Houk KN; Liang Y; Smith AB III Type II Anion Relay Chemistry: Conformational Constraints to Achieve Effective [1,5]-Vinyl Brook Rearrangements. J. Am. Chem. Soc 2017, 139, 8710–8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Matsuda I; Murata S; Ishii Y A Simple Preparation of Trimethylsilylacetonitrile and a Novel Ring-Opening of Epoxides with Trimethylsilylacetonitrile Anion. J. Chem. Soc., Perkin Trans 1 1979, 26–30. [Google Scholar]
- (10).Tietze LF; Geissler H; Gewert JA; Jakobi U Tandem-Bisalkylation of 2-Trialkylsilyl-1,3-Dithiane: a Sequential Transformation for the Synthesis of C2-Symmetrical Enantiopure 1,5-Diols and β, β’-Dihydroxyketones as well as of Enantiopure 1,3,5-Triols. Synlett 1994, 7, 511–512. [Google Scholar]
- (11).Fischer M-R; Kirschning A; Michel T; Schaumann E Syntheses of Cyclopentanols by a Silicon-Induced Cascade Reaction. Angew. Chem. Int. Ed. Engl 1994, 33, 217–218. [Google Scholar]
- (12).Hale KJ; Hummersone MG; Bhatia GS Control of Olefin Geometry in the Bryostatin B-Ring through Exploitation of a C2-Symmetry Breaking Tactic and a Smith-Tietze Coupling Reaction. Org. Lett 2000, 2, 2189–2192. [DOI] [PubMed] [Google Scholar]
- (13).(a) Shinokubo H; Miura K; Oshima K; Utimoto K tert-Butyldimethylsilyldihalo-methyllithium as a Dihalomethylene Dianion Synthon. 1,3-Rearrangement and 1,4-Rearrangement of Silyl Group from Carbon to Oxide. Tetrahedron 1996, 52, 503–514. [Google Scholar]; (b) Takaku K; Shinokubo H; Oshima K Sequential Carbon-Carbon Bond Formation Reaction Using 1-Silyl-2-Propenyllithium. Tetrahedron Lett. 1998, 39, 2575–2578. [Google Scholar]
- (14).Smith AB III; Boldi AM Multicomponent Linchpin Couplings of Silyl Dithianes via Solvent-Controlled Brook Rearrangement. J. Am. Chem. Soc 1997, 119, 6925–6926. [Google Scholar]
- (15).Smith AB III; Pitram SM Multicomponent Linchpin Couplings of Silyl Dithianes: Synthesis of the Schreiber C(16–28) Trisacetonide Subtarget for Mycoticins A and B. Org. Lett 1999, 1, 2001–2004. [DOI] [PubMed] [Google Scholar]
- (16).Poss CS; Ryychnovsky SD; Schreiber SL Two-Directional Chain Synthesis: An Application to the Synthesis of (+)-Mycoticin A. J. Am. Chem. Soc 1993, 115, 3360–3361. [Google Scholar]
- (17).Zonder JA; Shields AF; Zalupski M; Chaplen R; Heilbrun LK, Arlauskas P; Philip PA A Phase II Trial of Bryostatin 1 in the Treatment of Metastatic Colorectal Cancer. Clin. Cancer Res 2001, 7, 38–42. [PubMed] [Google Scholar]
- (18).Pettit GR; Chicacz ZA; Gao F; Herald CL; Boyd MR; Schmidt JM; Hooper JNA Antineoplastic Agents. 257. Isolation and Structure of Spongistatin 1. J. Org. Chem 1993, 58, 1302–1304. [Google Scholar]
- (19).For examples:; (a) Evans DA; Trotter BW; Cǒté B; Coleman PJ; Dias LC; Tyler AN Enantioselective Synthesis of Altohyrtin C (Spongistatin 2): Fragment Assembly and Revision of the Spongistatin 2 Stereochemical Assignment. Angew. Chem. Int. Ed. Engl 1997, 36, 2744–2747. [Google Scholar]; (b) Paterson I; Chen Y-K ; Coster MJ; Aceña JL; Bach J; Gibson KR; Keown LE; Oballa RM; Trieselmann T; Wallace DJ; Hodgson AP; Norcross RD Stereocontrolled Total Synthesis of (+)-Altohyrtin A/Spongistatin 1. Angew. Chem. Int. Ed 2001, 40, 4055–4060. [DOI] [PubMed] [Google Scholar]; (c) Crimmins MT; Katz JD; Washburn DG; Allwein SP; McAtee LF Asymmetric Total Synthesis of Spongistatins 1 and 2. J. Am. Chem. Soc 2002, 124, 5661–5663. [DOI] [PubMed] [Google Scholar]
- (20).(a) Smith AB III; Doughty VA; Lin Q; Zhuang L; McBriar MD; Boldi AM; Moser WH; Murase N; Nakayama K; Sobukawa M The Spongistatins: Architecturally Complex Natural Products – Part One: A Formal Synthesis of (+)-Spongistatin 1 by Construction of an Advanced ABCD Fragment. Angew. Chem. Int. Ed 2001, 40, 191–195. [DOI] [PubMed] [Google Scholar]; (b) Smith AB III; Lin Q; Doughty VA; Zhuang L; McBriar MD; Kerns JK; Brook CS; Murase N; Nakayama K The Spongistatins: Architecturally Complex Natural Products – Part Two: Synthesis of the C(29–51) Subunit, Fragment Assembly, and Final Elaboration to (+)-Spongistatin 2. Angew. Chem. Int. Ed 2001, 40, 196–199. [DOI] [PubMed] [Google Scholar]; (c) Smith AB III; Tomioka T; Risatti CA; Sperry JB; Sfouggatakis C Gram-Scale Synthesis of (+)-Spongistatin 1: Development of An Improved, Scalable Synthesis of the F-Ring Subunit, Fragment Union, and Final Elaboration. Org. Lett 2008, 10, 4359–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Sowinski P; Pawlak J; Borowski E Gariboldi P Stereostructure of Rimocidin J. Antibiot 1995, 48, 1288–1291. [DOI] [PubMed] [Google Scholar]
- (22).Smith AB III; Pitram SM; Fuertes MJ (+)-Rimocidin Synthetic Studies. Construction of an Advanced C(1–18) Polyol Fragment. Org. Lett 2003, 5, 2751–2754. [DOI] [PubMed] [Google Scholar]
- (23).Smith AB III; Foley MA; Dong S; Orbin A (+)-Rimocidin Synthetic Studies: Construction of the C(1–27) Aglycone Skeleton. J. Org. Chem 2009, 74, 5987–6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Tokuyama T; Nishimori N; Karle IL; Edwards MW; Daly JW; Alkaloids from Dendrobatid Poison Frogs: trans-Decahydroquinolines and Indolizidines. Tetrahedron 1986, 42, 3453–3460 [Google Scholar]
- (25).Smith AB III; Kim DS A General, Convergent Strategy for the Construction of Indolizidine Alkaloids: Total Synthesis of (−)-Indolizidine 223AB and Alkaloid (−)-205B. J. Org. Chem 2006, 71, 2547–2557. [DOI] [PubMed] [Google Scholar]
- (26).Ai Y; Kozytska MV; Zou Y; Khartulyari AS; Smith AB III Total Synthesis of (−)-Enigmazole A. J. Am. Chem. Soc 2015, 137, 15426–15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Oku N; Takada K; Fuller RW; Wilson JA; Peach ML; Pannell LK; McMahon JB; Gustafson K Isolation, Structural Elucidation, and Absolute Stereochemistry of Enigmazole A, a Cytotoxic Phosphomacrolide from the Papua New Guinea Marine Sponge Cinachyrella enigamatica. J. Am. Chem. Soc 2010, 132, 10278–10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Smith AB III; Fox RJ; Razler TM Evolution of the Petasis-Ferrier Union/Rearrangement Tactic: Construction of Architecturally Complex Natural Products Possessing the Ubiquitous cis-2,6-Substituted Tetrahydropyran Structural Element. Acc. Chem. Res 2008, 41, 675–687 and references therein. [DOI] [PubMed] [Google Scholar]
- (29).Moser WH; Endsley KE; Colyer JT Stereoseletive Bis-Functionalizations of Arene Chromium Tricarbonyl Complexes via Brook Rearrangements. Org. Lett 2000, 2, 717–719. [DOI] [PubMed] [Google Scholar]
- (30).Moser WH; Zhang J; Lecher CS; Frazier TL; Pink M Stereocontrolled [3+2] Annulations with Arene Chromium Tricarbonyl Complexes: Construction of Spirocyclic Compounds Related to Fredericamycin A. Org. Lett 2002, 4, 1981–1984. [DOI] [PubMed] [Google Scholar]
- (31).Han H; Smith AB III Anion Relay Chemistry: Development of an Effective Diastereoselective [3+2] Annulation Tactic Exploiting an Aldol/Brook Rearrangement/Cyclization Cascade. Angew. Chem. Int. Ed 2017, 56, 14102–14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Smith AB III; Hoye AT; Martinez-Solorio D; Kim W-S; Tong R Unification of Anion Relay Chemistry with the Takeda and Hiyama Cross-Coupling Reactions: Identification of an Effective Silicon-Based Transfer Agent. J. Am. Chem. Soc 2012, 134, 4533–4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).For examples:; (a) Martinez-Solorio D; Hoye AT; Nguyen MH; Smith AB III The Design, Synthesis and Validation of Recoverable and Readily Reusable Siloxane Transfer Agents for Pd-Catalyzed Cross-Coupling Reactions. Org. Lett 2013, 15, 2454–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Martinez-Solorio D; Melillo B; Sanchez L; Liang Y; Houk KN; Smith AB III Design, Synthesis and Validation of an Effective, Reusable Silicon-Based Transfer Agent for Room-Temperature Pd-Catalyzed Cross-Coupling Reactions of Aryl and Heteroaryl Chlorides with Readily Available Aryl Lithium Reagents. J. Am. Chem. Soc 2016, 138, 1836–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Nguyen MH; O’Brien KT; Smith AB III Design, Synthesis, and Application of Polymer-Supported Silicon-Transfer Agents for Cross-Coupling Reactions with Organolithium Reagents. J. Org. Chem 2017, 82, 11056–11071 and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).(a) Williams DE; Roberge M; Soest RV; Andersen RJ Spirastrellolide A, An Antimitotic Macrolide Isolated from the Caribbean Marine Sponge Spirastrella coccinea. J. Am. Chem. Soc 2003, 125, 5296–5297. [DOI] [PubMed] [Google Scholar]; (b) Warabi K; Williams DE; Patrick BO; Roberge M; Andersen RJ Spirastrellolide B Reveals the Absolute Configuration of the Spirastrellolide Macrolide Core. J. Am. Chem. Soc 2007, 129, 508–509. [DOI] [PubMed] [Google Scholar]
- (35).For examples:; (a) Fürstner A; Fenster MDB; Fasching B; Godbout C; Radkowski K Toward the Total Synthesis of Spirastrellolide A. Part 2: Conquest of the Northern Hemisphere. Angew. Chem. Int. Ed 2006, 45, 5510–5515. [DOI] [PubMed] [Google Scholar]; (b) Paterson I; Anderson EA; Dalby SM; Lim JH; Genovino J; Maltas P; Moessner C Total Synthesis of Spirastrellolide A Methyl Ester – Part 2: Subunit Union and Completion of the Synthesis. Angew. Chem. Int. Ed 2008, 47, 3021–3025. [DOI] [PubMed] [Google Scholar]
- (36).Smith AB III; Smits H; Kim D-S Spirastrellolide Studies. Synthesis of the C(1)-C(25) Southern Hemispheres of Spirastrellolides A and B, Exploiting Anion Relay Chemistry. Tetrahedron 2010, 66, 6597–6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Drewes SE; Sehlapelo BM; Horn MM; Scott-Shaw R; Sandor P 5,6-Dihydro-α-Pyrones and Two Bicyclic Tetrahydro-α-Pyrone Derivatives from Cryptocarya latifolia. Phytochemistry 1995, 38, 1427–1430. [Google Scholar]
- (38).Wang X; Wang W; Zheng H; Su Y; Jiang T; He Y; She X Efficient Asymmetric Total Syntheses of Cryptocarya Triacetate, Cryptocaryolone, and Cryptocaryolone Diacetate. Org. Lett 2009, 11, 3136–3138. [DOI] [PubMed] [Google Scholar]
- (39).Melillo B; Smith AB III A Unified Synthetic Strategy to the Cryptocarya Family of Natural Products Exploiting Anion Relay Chemistry. Org. Lett 2013, 15, 2282–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Ohsaki A; Ishiyama H; Yoneda K; Kobayashi J Secu’amamine A, a Novel Indolizidine Alkaloid from Securinega suffruticosa var. amamiensis. Tetrahedron Lett. 2003, 44, 3097–3099. [Google Scholar]
- (41).Liu P; Hong S; Weinreb SW Total Synthesis of the Securinega Alkaloid (−)-Secu’amamine A. J. Am. Chem. Soc 2008, 130, 7562–7563. [DOI] [PubMed] [Google Scholar]
- (42).Han H; Smith AB III Total Synthesis of (−)- Secu’amamine A Exploiting Type II Anion Relay Chemistry. Org. Lett 2015, 17, 4232–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Sikorska J; Hau AM; Anklin C; Parker-Nance S; Davies-Coleman MT; Ishmael JE; McPhail KL Mandelalides A-D, Cytotoxic Macrolides from a New Lissoclinum Species of South African Tunicate. J. Org. Chem 2012, 77, 6066–6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Nguyen MH; Imanishi M; Kurogi T; Smith AB III Total Synthesis of (−)-Mandelalide A Exploiting Anion Relay Chemistry (ARC): Identification of a Type II ARC/CuCN Cross-Coupling Protocol. J. Am. Chem. Soc 2016, 138, 3675–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Williams DE; Izard F; Arnould S; Dalisay DS; Tantapakul C; Maneerat W; Teatulohi M; Julien E; Andersen RJ Structures of Nahuoic Acids B-E Produced in Culture by a Streptomyces sp. Isolated from a Marine Sediment and Evidence for the Inhibition of the Histone Methyl Transferase SETD8 in Human Cancer Cells by Nahuoic Acid A. J. Org. Chem 2016, 81, 1324–1332. [DOI] [PubMed] [Google Scholar]
- (46).Liu Q; Deng Y; Smith AB III Total Synthesis of (−)-Nahuoic Acid Ci (Bii). J. Am. Chem. Soc 2017, 139, 13668–13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).O’Rourke NF; Kier MJ; Micalizio GC Metallacycle-Mediated Cross-Coupling in Natural Product Synthesis. Tetrahedron 2016, 72, 7093–7123. [DOI] [PMC free article] [PubMed] [Google Scholar]


