Abstract
The mammalian meat allergy known as the “α-Gal syndrome” relates to IgE specific for galactose-α−1,3-galactose (α-Gal), an oligosaccharide that is present in cells and tissues of nonprimate mammals. The recognition of delayed reactions to food derived from mammals in patients with IgE to α-Gal and also the association with tick bites have been increasing worldwide. In 2018, the National Institute of Allergy and Infectious Diseases, Division of Allergy, Immunology and Transplantation, sponsored a workshop on this emerging tick-related disease. International experts from the fields of tick biology, allergy, immunology, infectious disease, and dermatology discussed the current state of our understanding of this emerging medical condition. The participants provided suggestions for specific research priorities and for the development of resources to advance our knowledge of the mechanisms, diagnosis, management, and prevention of this allergic disease. This publication is a summary of the workshop and the panel’s recommendations are presented herein.
Keywords: α-Gal, anaphylaxis, IgE, oligosaccharide allergen, glycolipids, mammalian meat allergy, ticks
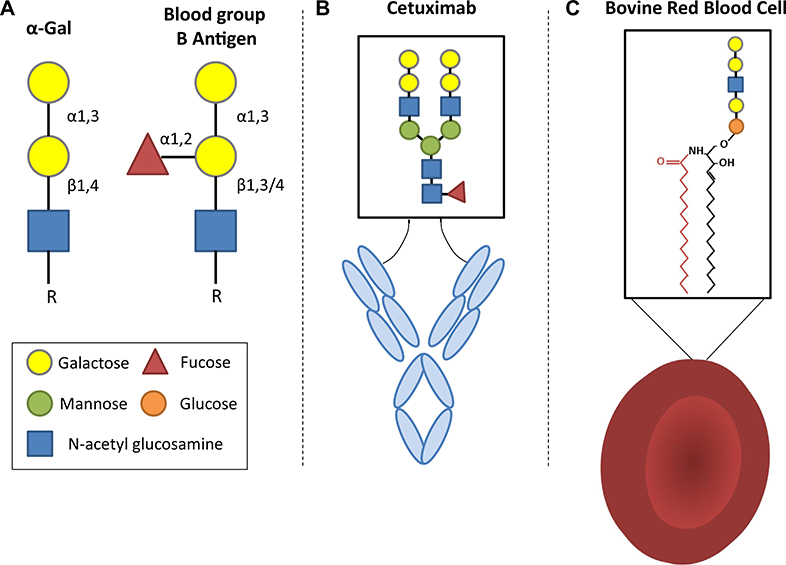
Recognition of the oligosaccharide galactose-α−1,3-galactose (α-Gal) as an important target for IgE antibodies came from the investigation of the often severe and occasionally fatal reactions during the first infusion of the mAb cetuximab.1 Those studies established that the IgE antibodies present in the patients before their first exposure were specific for the α-Gal glycan present on the Fab portion of cetuximab (Fig 1, A and B). The investigation was greatly helped by ImClone who had developed cetuximab. The company published the full glycosylation pattern of the monoclonal and also provided the molecule from an alternative cell line that did not express α-Gal.2 In addition, Bristol-Myers Squibb facilitated collaboration with the oncology group at Vanderbilt who provided the pretreatment sera and control sera from central Tennessee. Once the assay for IgE to α-Gal was available, it became clear that there were patients in allergy clinics in Charlottesville, Virginia, and also in Springfield, Missouri, who reported delayed reactions to mammalian meat and who had IgE specific for the same oligosaccharide.3 Over the first year it became clear that there were also cases in North Carolina, Tennessee, and Arkansas. This not only matched the distribution of cases of reactions to cetuximab but also resembled the map of the maximum density of Rocky Mountain spotted fever (RMSF) published by the Centers for Disease Control and Prevention (CDC).4 This provided a clue that this form of sensitization might be related to tick bites. Interestingly, 2 earlier abstracts had reported cases of mammalian meat allergy that were associated with preceding tick bites. The first was 5 cases in Athens, Georgia, reported to the Georgia Allergy Society in 1989 by Anthony Deutsch (personal communication). The second was reported by Sheryl van Nunen to the Sydney Allergy Society in 2006 on patients who had been bitten by ticks in the North Shore of Sydney.5 Dr van Nunen published her cases in 2009 and related them to the tick Ixodes holocyclus.6 Interestingly, investigators in Sweden had made another early observation that was relevant to the unfolding story. Marianne van Hage’s group reported in 2007 that some patients in Stockholm had IgE antibodies specific for an oligosaccharide on cat IgA,7 which was subsequently shown to be α-Gal.8 Finally, Uta Jappe in Germany had been investigating allergic reactions to the consumption of pork kidney as early as 2005, which in subsequent work with Tilo Biedermann were confirmed to be related to IgE to α-Gal.9–11 Thus, the discovery that α-Gal was an epitope for IgE on mammalian glycoproteins connected a series of previous observations that had not been recognized as related. It is important to remember that this glycan is also the “B-like antigen” that Karl Landsteiner recognized on the red cells of mammals nearly a century ago, that it can also be present on glycolipids, and that it is a major xenotransplantation barrier (Fig 1, A and C).12–14
FIG 1.
Depiction of the structure of the α-Gal epitope and representative α-Gal–bearing glycoproteins and glycolipids. A, The structure of the complete α-Gal trisaccharide epitope (galactose-α−1,3-galactose-β1,4-GIcNAc) in comparison to the blood group B antigen. B, Cetuximab is a chimeric mouse-human monoclonal IgG that expresses α-Gal glycans. Although α-Gal can be present in the Fc domain, the strongest expression of α-Gal, and that which is recognized by IgE antibodies, occurs in the Fab domain. The dominant form of α-Gal that is present in cetuximab is a bi-antennary glycan. C, Lipid forms of α-Gal are less well studied than their glycoprotein counterparts, but it is known that nonprimate erythrocytes are an abundant source of O-linked glycosphingolipids, which can bear α-Gal epitopes.
In June of 2018 a workshop was convened in Bethesda, Maryland, under the auspices of the National Institute of Allergy and Infectious Diseases. This workshop was designed to evaluate the evidence that had been published since 2008, and in particular to lay out a background and plan for future research into the causes and consequences of IgE-mediated mammalian meat allergy and the contributions of α-Gal sensitization. The primary areas discussed included the following:
Review of the clinical syndrome in North America and comparison with the European experience and emerging work from South Africa.
Current understanding of the mechanisms by which tick bites can induce IgE responses to this particular oligosaccharide.
Factors relevant to the severity and symptoms of allergic reactions in patients who are sensitized to α-Gal as well as the mechanism and significance of the delay in clinical responses after eating mammalian meat.
Review of Clinical Syndromes Related to α-Gal-Specific IgE
As an oligosaccharide of nonprimate mammals and New World monkeys, α-Gal can be present on glycoproteins and glycolipids of a multitude of products derived from mammals. This can include skeletal muscle, organs, milk, and gelatin, but also other products such as biological drugs and vaccines that are prepared with mammalian cells or constituents.15 As such the term “α-Gal syndrome” (AGS) is often used to describe allergic reactions to mammalian meat and other α-Gal–containing products derived from mammals.9,16 In patients with an appropriate clinical history, the main method of diagnosis is the measurement of IgE to α-Gal. Several α-Gal–bearing glycoproteins have been used on the solid phase of the assay, but the 2 most widely reported have been beef thyroglobulin and cetuximab. Importantly, the performance characteristics of the assay have been similar with both glycoproteins.3,17,18 It is also important to highlight that not all subjects who are sensitized to α-Gal report allergic reactions to mammalian meat. For example, among 300 hunters and forest workers in Southwest Germany, the prevalence of IgE to α-Gal was 19.3%, but among the 58 subjects who were positive (cutoff, 0.35 IU/mL) only 5 had allergic symptoms to mammalian meat or innards.19 Thus, as with other allergic diseases, a good clinical history plays an important role in the diagnosis of the syndrome.
Anaphylaxis during treatment with cetuximab
These reactions that occur during the first infusion of the drug can be very severe and rapid and clearly relate to preexisting IgE antibodies to α-Gal.1,2 Screening patients with an IgE assay could reduce the risk of reactions; however, severe reactions to cetuximab can also occur in subjects who are not sensitized to α-Gal. The predictive value of the test is expected to vary in different patient populations, but the α-Gal IgE test has shown a sensitivity of 75% to 92% and a specificity of 90% to 92%.1,20 To date, use of an IgE assay specific for α-Gal has not been recommended as a screening procedure for use of this drug.20 There have been several consequences following the initial description of the syndrome. The most important is the increased awareness of the possible relevance of glycosylation on recombinant molecules to allergic or other reactions during treatment, including consideration given to the cell line used for the generation of the molecules.21–23 In keeping with that, many recently developed mAbs have the α-Gal glycosylation site on the Fab region engineered out. A related question is whether the immune response to tick bites can induce significant IgE to other relevant oligosaccharides.24 To date, limited studies in subjects who are positive for IgE to α-Gal (and had histories of tick bites) have not revealed significant IgE to other oligosaccharides.25,26
Allergic reactions to gelatin-containing vaccines and other mammalian-related pharmaceuticals
Recent evidence suggests that some patients can react to vaccines that are prepared in culture systems or with excipients that are derived from nonprimate mammals. For example, Stone et al27 reported a case with a history of delayed anaphylaxis to mammalian meat who experienced anaphylaxis upon receipt of Zostavax. In a subsequent report, the same group described a similar pediatric case who reacted to the measles, mumps, and rubella vaccine.28 In both cases, the patient’s serum had IgE that bound to preparations of the relevant vaccine and, importantly, the in vitro activity was lost when α-Gal–specific IgE was depleted. Further investigation suggested that gelatin in the vaccine preparation was likely the source of the α-Gal. Taken together, individuals with histories of severe reactions to mammalian meat or gelatin could be good candidates for allergy consultation and consideration to graded administration of relevant vaccines. However, it is important to realize that many individuals who are sensitized to α-Gal have tolerated gelatin-containing vaccines (which in the United States currently include measles, mumps, and rubella vaccine, Zostavax, yellow fever, rabies, oral typhoid, and FluMist).29,30 It is possible that measuring IgE to gelatin could be useful for stratifying risk to gelatin-containing vaccines, but we are unaware of studies that have addressed this.
In addition to vaccines there are other pharmaceutical products and devices that have the potential to contain α-Gal epitopes. For example, Fischer et al31 and others32 have reported on a putative link between α-Gal IgE and immediate reactions to antivenom. As addressed in a recent review, there have also been case reports relating AGS to heparin, magnesium stearate, pancreatic enzyme replacement (eg, pancrelipase), gelatin-containing vaginal capsules, and porcine-derived heart valves.15,33–38 Further research is needed to determine whether these products carry α-Gal, either consistently or sometimes, and whether the concentration is sufficient to cause clinical reactions.
Reactions related to meat and other mammalian products that are ingested
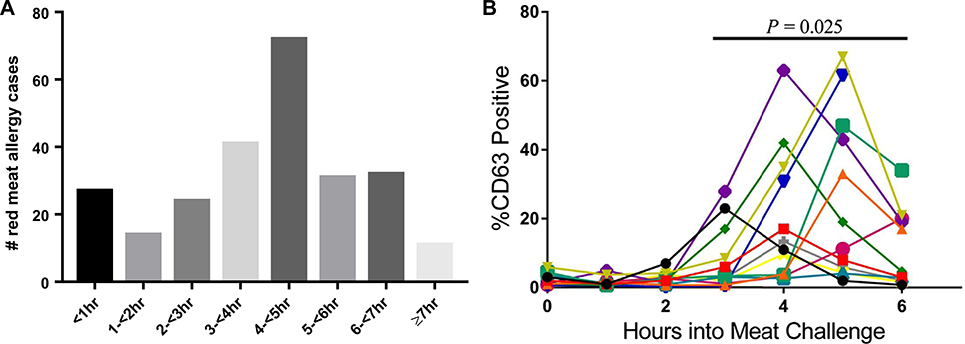
There are multiple surprising features of AGS, but the 2 most important are that it can develop at any age and, in contrast to the immediate reactions to infused cetuximab, there is almost always some delay after ingestion before symptoms start. In a recent analysis of 261 adults and children presenting for evaluation of allergic symptoms to mammalian meat in central Virginia, 248 cases consistent with AGS were identified.39 Patients’ age ranged from 5 to 82 years, and 84% reported symptoms beginning at least 2 hours after ingestion of mammalian meat (median, 240 minutes). This delay in symptom onset, which has also been reported from Europe,40 was consistent with the results of challenge studies that Commins et al41 reported in 2014 on a similar population where all patients had a delay of at least 150 minutes. The extent of delay has not been unanimous across all studies, however. Mabelane et al42 described a median onset of symptoms of 100 minutes in challenges conducted in a cohort in rural South Africa. In this cohort, the likely cause of the sensitization was locally prevalent ticks, but the study was also notable for a large proportion of children who reacted to challenge. Tilo Biedermann’s group in Germany reported similar kinetics when using pork kidney for the challenges.10 The explanation for the differences in timing between the studies could relate to the specific patient populations investigated or the type of product used for challenge (ie, beef muscle vs pork kidney). Regardless, we would highlight that most reactions occurred more than 1 hour after ingestion in all the aforementioned studies (Fig 2, A). The allergic reactions have not differed by age, with children as likely as adults to report urticaria, gastrointestinal distress, and/or anaphylaxis with an equally delayed response.39,42,43
FIG 2.
A, Time course of self-reported symptom onset of allergic symptoms in 261 patients evaluated for mammalian meat allergy, of which 248 had IgE testing consistent with AGS, from Wilson et al.39 B, Results of basophil activation as assessed by CD63 expression in PBMCs drawn sequentially following mammalian meat challenges in individuals with known AGS from Commins et al.41
Most α-Gal meat allergy reactions reported in the literature focus on the presentation of delayed pruritus, urticaria, angioedema, and anaphylaxis.3 More recently, and at the workshop, researchers have described reactions that manifest with abdominal pain—both in conjunction with skin reactions and as isolated gastrointestinal reactions. For example, Marianne van Hage reported that 74% of the 128 participants in her Swedish cohort reported gastrointestinal symptoms (unpublished data, 2020), so did 64% in the Virginia cohort.39 Mabelane et al42 reported a large case series of challenge-proven α-Gal allergy with high prevalence of isolated abdominal reactions (~ 20%); surprisingly, the time to onset of symptoms did not differ between individuals with isolated abdominal symptoms and those with skin manifestations and there were no differences in α-Gal IgE levels between the groups. Anecdotally, there are patients in whom the initial symptoms were restricted to the gastrointestinal tract but over a period of weeks or months, in some cases following additional tick bites, the symptomatology expanded to include the skin and/or anaphylaxis. It is, therefore, possible that isolated abdominal pain is an underreported and underdiagnosed feature of AGS.44
Patients with α-Gal food allergy report marked intraindividual variability in the dose that causes a reaction.45 Many describe mild or no reactions with some exposures, yet have severe symptoms on repeat exposure to the same food served in a similar amount and preparation. This variation in susceptibility, at least in part, appears to be modulated by cofactors.46 In keeping with many other forms of food allergy, the most prominent cofactors associated with α-Gal consumption reactions are exercise, alcohol, and nonsteroidal anti-inflammatory medications.10,41,45 Theoretically, exposure to these cofactors may enhance absorption of the antigen, may decrease the threshold of response, and/or contribute to the severity of the allergic responses to α-Gal.47 Another possible explanation for the variability is that the amount of α-Gal varies in different foods. Unfortunately, this is a topic that has not been systematically examined; however, α-Gal content appears to be particularly high in foods derived from internal organs.48 In addition, consumption of lipid-laden mammalian meat has been associated with more consistently severe hypersensitivity responses in subjects allergic to α-Gal.49 There are also marked interindividual differences in susceptibility to α-Gal. This runs the gamut from patients who are sensitized to α-Gal but are consistently asymptomatic when ingesting mammalian meat to those who react to minor quantities of α-Gal present in milk and gelatin-containing medications and sweets.19,50,51 For example, α-Gal patients with concomitant mastocytosis can react to very small amounts of pork kidney (3 g).52
What explains the delay in symptom onset after ingestion of mammalian meat?
The observations about the symptom delay from patient reports and challenge studies are reinforced by a study of basophils collected from patients who underwent mammalian meat challenge. In this study, in which the basophils did not receive any ex vivo stimulation, basophils collected at early time points did not exhibit appreciable activation but the activation marker CD63 increased significantly over baseline in blood that was drawn between 3 and 5 hours after challenge (Fig 2, B).41 Understanding the mechanism that explains the delay remains an important objective. Differences in timing following the ingestion of mammalian meat and organs, as reported by Tilo Biedermann’s group, suggests that differences in the quantity or “quality” of α-Gal present in different tissues could be an important element in explaining the delay.10 An important qualitative consideration is that α-Gal can be present on not only glycoproteins but also glycolipids. It is well established that α-Gal–bearing glycosphingolipids are an important source of the glycan in nonprimate mammalian cells, tissues, and organs (including kidneys).53–55 The argument for this “glycolipid hypothesis” revolves around the fact that the digestion, absorption, and processing of lipids occurs with kinetics very similar to the delay in symptom onset.47 Lipid processing relies on transit via chylomicrons through the thoracic duct before arriving into the systemic circulation at the left subclavian vein. The lipid cargo subsequently transitions to progressively smaller lipoprotein particles, including low-density lipoprotein particles, which are sufficiently small to passively filter through the vasculature into the interstitium (ie, ~20 nM).56 Studies with radiolabeled lipids have demonstrated that the peak lipid level at the thoracic duct occurs 4 hours after a meal, and the peak level in tissue (eg, muscle) 5 hours after the meal.57 This kinetic pattern corresponds with the clinical experience and the result of the ex vivo basophil testing. Recent studies using an in vitro Caco-2 gut barrier model have provided additional support for this hypothesis because beef-derived α-Gal glycolipids, but not glycoproteins, passed through the epithelial cells and were packaged into chylomicrons.58
Relevance beyond traditional allergic disease?
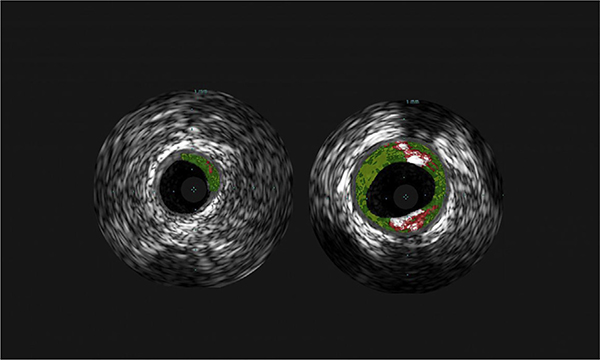
In addition to the isolated gastrointestinal symptoms that were previously discussed, preliminary evidence reported by Wilson et al59 suggests that α-Gal sensitization could be a risk factor for coronary artery disease. Among a population of 118 adults in Virginia who underwent coronary catheterization at the discretion of their cardiologist, 26% had measurable IgE to α-Gal (cutoff, 0.1 IU/mL). Using highly sensitive intravascular ultrasound imaging, the sensitized group was found to have a greater burden of atherosclerotic plaques and their plaques had high-risk features, as assessed by the extent of atheroma calcification and necrosis (Fig 3). Importantly, the association was significant in multiple variable regression analyses accounting for traditional cardiovascular risk factors. Although a connection between allergic disease and atherosclerosis may not be obvious to most physicians, there is already good evidence in the cardiology literature for a link between both mast cells and IgE with atherosclerosis development and severity.60,61 Cardiac anaphylaxis, commonly known as Kounis syndrome, further supports a link between allergic disease and coronary artery disease.62,63 In thinking about a putative mechanism, it may also be relevant to consider that α-Gal can exist as a glycolipid antigen. Although dietary and allergic history of the study participants in the report by Wilson et al was not available, it is expected that many and possibly most study participants were asymptomatic and, thus, in all likelihood were continuing to consume mammalian products. This inference is based on our understanding of differences in the prevalence of α-Gal sensitization in the community (and high-risk populations) as compared with the prevalence of subjects who report symptoms to mammalian meat.19 The association between α-Gal IgE and coronary artery disease does not offer evidence of causality and requires confirmation in larger populations.64
FIG 3.
Representative cross-sectional imaging of coronary arteries of a subject without (left) or with (right) IgE to α-Gal.59 Images obtained by intravascular ultrasound, with central black area representing the lumen of the coronary artery and surrounding colored areas indicative of atherosclerotic lesions with fibrous (dark green), fibrofatty (light green), necrotic (red), and calcified plaques (white) evident. Images courtesy of Angela Taylor, MD, University of Virginia Health System and the National Heart, Lung, and Blood Institute.
The Tick Connection: What Controls The Distribution of Cases of The Ags And How Is It Likely to Change?
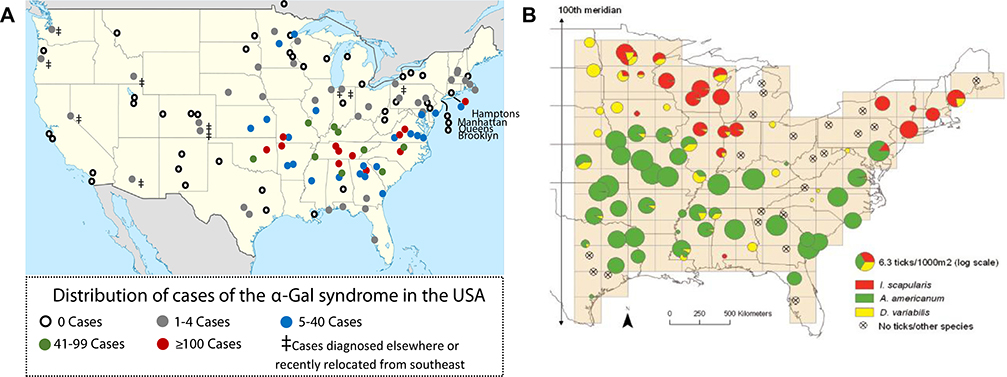
In the United States, cases of immediate reactions to cetuximab and delayed anaphylaxis to mammalian meat have been strikingly regional, and it was the overlap of those cases with the CDC map of cases of RMSF that helped establish the initial tick connection. Ten years later, it is now clear that the CDC map is actually most consistent with a family of spotted fever-group rickettsiosis, and not with cases of bona fide RMSF.65 In keeping with this, the tick Amblyomma americanum, commonly known as the lone star tick, rarely carries Rickettsia rickettsii (the cause of RMSF), but commonly carries a nonpathogenic or minimally pathogenic spotted fever-group rickettsiosis called Rickettsia amblyommatis.66–69 The abundance of α-Gal cases in Virginia, North Carolina, Tennessee, Arkansas, Oklahoma, and Missouri, and also the eastern tip of Long Island, NY, is highly consistent with the area where A americanum is endemic.70,71 The connection between α-Gal sensitization and tick bites is further supported by a number of experimental studies and also the observation that subjects with AGS who avoid recurrent tick bites, but not those who report ongoing tick bite(s), experience a decline in their α-Gal IgE titers (see Box 1).72–74 In Sweden, AGS cases are also regional and match the distribution of Ixodes ricinus.40 In particular, cases are not reported in or north of Umeå. In France, Germany, and Spain, cases have been identified in wide areas and may relate primarily to outdoor activity.19 In these areas, the evidence also relates to I ricinus.9 In Australia, the cases of delayed anaphylaxis to mammalian meat are restricted to a region north and south of Sydney extending as far inland as Canberra, an area that is the primary distribution of the tick I holocyclus.75 Formal studies characterizing the incidence and prevalence of AGS have been lacking, but it is clear from reports in the United States and Europe that sensitization to α-Gal can reach 10% to 20% of the population in some regions.4,19,76 Notably, these levels of community sensitization (ie, 10%−20%) occur only in those areas where ticks are common and are much higher than the prevalence found in areas where ticks are rare.1
Box 1. Evidence supporting a role for tick bites in the induction of IgE to α-Gal4,6,19,72,73,89,102.
Epidemiology: Overlap in cases of the AGS with the distribution of the lone star tick (in North America)
Cases: Prospective investigation of a small number of subjects who have had tick bites
Many subjects who make IgE to α-Gal also make IgE directed to other elements of tick extract
Identification of the epitope in tick midgut and saliva
Models using α-Gal knockout mice where saliva or salivary gland extract is sufficient to recapitulate the syndrome
Ongoing efforts to determine the distribution of AGS in the United States have generally confirmed that cases are most common in areas where A americanum (but not Ixodes scapularis, the primary vector for Lyme disease) is established; nonetheless, unpublished evidence presented at the workshop by Thomas Platts-Mills has suggested 2 anomalies (see Fig 4, A and B).77 First is the presence of a cluster of more than 20 cases reported in northern Minnesota. This is interesting because A americanum is not known or expected to be established in this region because of climatic conditions.78,79 These cases suggest that North American ticks beside A americanum could be relevant to α-Gal sensitization and/or that agents other than ticks could contribute to α-Gal sensitization.80 A second anomaly is the lack of AGS cases in the Gulf Coast and Texas, an area where the CDC reports that A americanum is established.78,79 This finding, although preliminary, suggests that lone star tick populations could be decreasing in abundance in the Gulf Coast and Texas. There are some predictions that such a pattern could be expected as part of future climate change, but it is not clear this has been a factor to date.78 Dr Platts-Mills suggested an alternative possibility, which relates to the steady expansion of an established tick predator in the Gulf Coast region—the imported fire ant.81 In support of this idea, evidence was presented for a negative correlation between cases of fire ant anaphylaxis and cases of the AGS in the Deep South. Relatedly, during the workshop, there was discussion about a connection between rising tick populations and increasing numbers of white-tailed deer in urban and suburban environments. Indeed, the significance of this increase in deer populations in relation to tick-borne zoonoses associated with the lone star tick was described as “ecological havoc” by Paddock and Yabsley82 in 2007.
FIG 4.
A, Cases of the AGS based on an ongoing survey of allergy practices in the United States (unpublished results, 2018). B, Density of the most abundant tick species in eastern North America based on a sampling of 95 sites, from Diuk-Wasser et al.77 Of note, there is a striking overlap of α-Gal cases with the presence of A americanum (the lone star tick), but not I scapularis (the black-footed deer tick) or Dermacentor variabilis (the American dog tick).
How do bites from ticks of many species induce IgE to α-Gal?
Evidence has been published relating mammalian meat allergy to bites of A americanum,4 l ricinus,19,72 l holocyclus,6 Haemophysalis longicornis,83 and most recently Amblyomma testudinarium.84 In each case, there was a dominant IgE response specific for α-Gal. However, the evidence does not exclude other ticks. Most importantly, we do not know the mechanism whereby a tick bite can lead to induction of IgE specific to α-Gal. Speaking to the question of why only certain ticks have been associated with α-Gal IgE sensitization, Maria Diuk-Wasser discussed the possibility that differences in tick habitat, questing behavior, and/or anatomy could be relevant. She also highlighted the unexplained observation that although I ricinus is the dominant cause of sensitization to α-Gal in Europe, its phylogenetic North American relative I scapularis is almost certainly not a major cause of sensitization in the United States.
In regard to mechanism, tick saliva is suspected to play an important role, which is consistent with the remarkable complexity of tick saliva and the view that ticks represent venomous ectoparasites.85–87 Despite the fact that α-Gal has been identified in the midgut and saliva of several species of hard ticks, the source of the α-Gal remains uncertain. A possibility is that ticks can intrinsically produce the oligosaccharide, but this has been questioned because the enzyme that catalyzes the generation of α-Gal in vertebrates (α1,3-galactosyltransferase) has not been identified in ticks.88 Two alternative possibilities are that (1) α-Gal could be present in ticks because of a prior blood meal on a nonprimate mammal, or (2) Rickettsiales or other symbionts present in tick saliva could express α-Gal and be transferred to a human host during a tick bite. Evidence for these competing hypotheses are detailed in Table I, though notably emerging evidence increasingly suggests that ticks can intrinsically generate the α-Gal glycan.88–91 Crispell et al90 reported that α-Gal was not detectable in the salivary glands of unfed adult A americanum; however, the α-Gal glycan was detectable after the ticks were fed using an artificial feeding model where a silicone membrane covered human blood. Furthermore, Apostolovic et al89 have reported that larval ticks contain α-Gal–carrying proteins and these larvae were not fed on the host, which supports the view that α-Gal–carrying proteins originate from ticks.
TABLE I.
Description of the 3 main hypotheses to explain the source of the glycan that leads to an IgE response directed to the α-Gal epitope
| Hypothesis | Supporting evidence | Conflicting evidence |
|---|---|---|
| 1. Certain ticks can intrinsically produce α-Gal, either at baseline or during feeding | • Work from the groups of Marianne van Hage and Shahid Karim investigating tick extracts by Western blot and mass spectrometry • Cabezas-Cruz et al88 reported that galactosyltransferase enzymes other than the α1,3-galactosyltransferase can generate α-Gal (ie, α1,4- and β1,4 galactosyltransferases, which have been identified in ticks) |
• No evidence that ticks express the specific enzyme that is thought to be necessary to catalyze the terminal α-1,3 bond |
| 2. The α-Gal present in tick saliva is residual from a prior blood meal of a nonprimate mammal | • Hard ticks have 3 life stages and their natural hosts are often mammals that would have α-Gal | • Does not explain how larval (ie, seed) ticks can acquire the epitope • Unclear whether α-Gal and other constituents of blood meal would persist inside tick for the duration, often many months, between different blood feeds |
| 3. The α-Gal present in tick saliva is produced by a microbial symbiont of ticks | • Multiple bacteria and parasites can express the glycan | • Lack of any established association with known tick-borne diseases • No published reports have demonstrated that microbes common to relevant ticks express α-Gal |
Another important question that was the focus of comments by Lisa Beck relates to why tick bites are so effective at inducing IgE responses in humans and whether the skin plays an important role in facilitating this response. For example, we recognize that the skin is an important route for IgE responses to parasites, venomous insects, and also to foods such as peanut.92–94 Hightiter IgE is also a hallmark of atopic dermatitis.95 Interestingly, however, in the experience of the panel, AGS does not appear to be overrepresented among subjects with atopic dermatitis. This suggests that intrinsic barrier defects in the skin are not a prerequisite for the syndrome. Perhaps this is not surprising given that tick “mouthparts” can penetrate through the epidermis and generate a local inflammatory response in the dermis.96 Nonetheless, the skin may represent a tissue microenvironment that strongly favors the development of IgE (and type 2 immunity generally), a position that is supported by recent work in mice that demonstrated that GATA-3, a “master” regulator of type 2 immunity, is particularly abundant in effector and regulatory T cells of the skin.97 The idea that IgE responses to tick depend on presentation via the skin is also supported by a recent report that showed that subcutaneous but not intraperitoneal inoculation of tick extracts elicited a robust specific IgE response.98
Animal models and investigations of the tick-host interface and host immune response
Despite increased awareness, the mechanism of several aspects of mammalian meat allergy remain unknown. The link between tick bites and the development of IgE to α-Gal is strong, though notably the IgE response does not develop in all individuals bitten by ticks.4,74,84,99,100 To investigate the mechanisms driving IgE responses to α-Gal as well as the delayed reaction, investigators recognize the need for an animal model of mammalian meat allergy. Barriers to a mouse model include the fact that mice naturally remove ticks and also that mice express α-Gal (hence they do not naturally make anti-Gal antibodies). To overcome the first obstacle, Susan Little and Brian Herrin at Oklahoma State University developed a small chamber to allow prolonged exposure and tick feeding of mice. In multiple experiments, A americanum ticks feeding on CH3/HeN mice for 10 to 14 days induced a significant increase in total IgE compared with control mice. The magnitude of the IgE response appeared to be dose-dependent because mice with greater numbers of ticks that were attached and feeding had higher total IgE levels. Although these experiments demonstrated the feasibility of using mice to examine the IgE response to tick bites, the inherent variability in dosing associated with live tick feedings has led to the development of tick-independent methods for delivery of tick-associated factors to mice. The second barrier has been addressed with the use of α-Gal “knockout mice,” in which the gene that encodes for α1,3-galactosyltransferase has been genetically modified. An initial report by Araujo et al73 demonstrated that subcutaneous inoculation of saliva from Amblyomma sculptum, or direct feeding of the same ticks, induced an IgE response in α-Gal–deficient mice that bound α-Gal epitopes presented on virus-like particles. Similarly, Scott Commins presented studies at the workshop that used a model where salivary gland extract from partially fed A americanum was repeatedly inoculated into a different transgenic model of α-Gal–deficient mice. These mice produced an α-Gal–directed IgE response and also demonstrated systemic allergic reactions, as measured by a decrease in body temperature, following injection of α-Gal–containing antigen (cetuximab) or gavage with pork.101,102 As stated above, a recent article by Chandrasekhar et al98 provides additional evidence that the skin plays an important role in sensitization to tick proteins. Subcutaneous inoculation of A americanum larval extracts (whole body), but not intraperitoneal injection of the same extracts, elicited a robust tick-specific IgE response in wild-type mice.98 The same investigators also looked at α-Gal–specific IgE responses using the α-Gal knockout mice. Cutaneous sensitization using a tick extract that was modified to include α-Gal-BSA led to an induction of IgE specific to α-Gal as well as an increase in the frequency of activated basophils following beef thyroglobulin challenge.98
It is anticipated that further development and investigation using mouse models will help identify specific constituents of the tick “sialome” that act as adjuvants to promote IgE and type 2 immune responses.85 Putative candidates within the tick that could have this activity include prostaglandins, phospholipases, and lipocalins.85,86,103,104 Animal models will also be helpful to characterize the nature of the host immune response that contributes to the generation of IgE to α-Gal. There are reasons to think that basophils may play an important role in promoting IgE induction to α-Gal. For several decades it has been known that mammalian hosts can develop acquired immunity that protects against subsequent tick infestation, and it is becoming increasingly clear that basophils, as well as IgE, play an important role in this process.105–107 In an area of Japan where Amblyomma testudinarium is common, a recent publication demonstrated that IgE to α-Gal was common in subjects with recurrent tick bites and that the number of basophils at the site of tick penetration increased with repeated tick exposures.84 Tilo Biedermann reported that in his cohort in Germany he and his colleagues have also observed elevated levels of basophils at the site of tick bites. Interestingly, his group has also recently reported that urticaria at the site of a prior tick bite can be an early sign of an allergic reaction in individuals with AGS.108 Although basophils are not expected to remain in the skin long after a tick exposure, this finding of “recall urticaria” suggests the presence of long-lived mast cells with specific IgE on them which had accumulated at the site of a prior bite.
The role of B cells in the AGS is another area that would benefit from further research. It has long been appreciated that “natural” anti–α-Gal antibodies of the IgM, IgG, and IgA isotype are abundant in immunocompetent humans.109–112 In fact, Galili14 has estimated that up to approximately 1% of the IgG repertoire (and B cells in circulation) was specific for α-Gal. The source of α-Gal–specific natural antibodies in mouse models has been reported to be B-1b B cells, although in humans the lineage of the B cell is less clear.113,114 An interesting possibility, and one that could distinguish the allergic response to α-Gal from the response to traditional environmental allergens, is that induction of α-Gal IgE could involve class switch of existing memory B cells rather than a de novo humoral immune response. It is anticipated that advanced tools such as polychromatic flow cytometry and deep sequencing of antibody VDJ will provide useful insights into this question.
Another question is whether the IgE response to α-Gal requires T-cell help, or whether the class switch occurs independent of T cells.115 Because the α-Gal epitope is a nonzwitterion carbohydrate, it is unlikely that it can directly participate in MHC II priming of CD4 T cells. Nonetheless, there are reports from animal models that suggest that antibody responses to α-Gal depend on CD4+ T-cell help.116,117 This is in keeping with the fact that the immune response to α-Gal that occurs in subjects with mammalian meat allergy has some TH2-related features in addition to IgE class switch. For example, IgG1 specific to α-Gal is consistently elevated in subjects with AGS. IgG4 specific to α-Gal has not been consistently reported, but this could relate to the fact that the exposure that causes α-Gal sensitization (ie, tick bites) is usually an intermittent and not a chronic event.118–121 Marianne van Hage presented evidence that dendritic cell processing and presentation of α-Gal–bearing glycoproteins was delayed compared with nonglycosylated proteins in an in vitro model, a finding that could also have implications for T-cell activation.122 An interesting possibility is that the glycolipid form of α-Gal could be present in ticks and be recognized as an antigen by noncanonical T cells, such as natural killer T cells. There is evidence that α-Gal–bearing lipids can be presented by CD1d on antigen-presenting cells, that blockade of CD1d impairs anti–α-Gal antibody formation, and that invariant natural killer T cells are present in higher numbers in subjects with AGS than in healthy controls.123–125
An additional area of interest that is relevant to understanding the host response relates to the observation that subjects who have ABO blood group B (ie, have B or AB blood) may be partially protected from developing the syndrome.39,99,120,126 This is likely explained by mechanisms governing tolerance: (1) the B antigen and α-Gal are structurally similar (see Fig 1), (2) subjects with A and O blood groups have antibodies that recognize both the B antigen and α-Gal, and (3) subjects with group B blood have lower titers of antibodies that recognize α-Gal than subjects with group A or O blood.39,120,127
Workshop Recommendations
It has now been 10 years since the initial publications describing the oligosaccharide α-Gal as an IgE-binding epitope and a causal antigen in anaphylaxis to mammalian products.1,3 The “AGS” can involve immediate reactions to drugs that are delivered intravascularly, but characteristically manifests with a delay of 3 to 6 hours upon oral ingestion of mammalian products such as meat, organs, and/or dairy.128 Anti–α-Gal IgM, IgG, and IgA, which arguably represent natural antibodies, are produced by all immunocompetent humans, but it is increasingly clear that bites from certain species of hard ticks are the dominant cause of IgE sensitization to α-Gal. The National Institute of Allergy and Infectious Diseases Workshop on Understanding IgE-Mediated Mammalian Meat Allergy recommends that future research focuses on the following topics:
- Epidemiologic and observational research to
- determine the prevalence of α-Gal IgE sensitization in the general population and its association with (1) mammalian meat allergy, (2) other allergic reactions (eg, vaccine reactions), and (3) other medical conditions (eg, atherosclerotic disease),
- confirm the association between α-Gal IgE sensitization and tick bites and identify specific tick species that can induce sensitization and environmental conditions that modify the sensitization process, and
- describe the natural history of α-Gal IgE sensitization and determine whether early symptomatology can be identified that develops over time into more severe, anaphylactic reactions.
- Pathophysiologic research using human samples and animal models to
- understand the mechanism(s) of the delayed allergic reactions to α-Gal and the factors that modify the time interval between oral ingestion and reaction,
- determine the nature of the host immune response that contributes to the induction of α-Gal–specific IgE,
- identify the source of the α-Gal in tick saliva,
- identify possible contributions of other tick-associated factors that may act as type 2 adjuvants, and
- investigate efficacy of biologics in mouse models.
Although there are many ways that α-Gal could be considered to “break the rules,” investigation into this unusual allergen is likely to reveal novel insights into the causes and consequences of all allergic diseases. It is hoped that these proceedings can be used as a guide for research proposals that will move this field forward.
Acknowledgments
Disclosure of potential conflict of interest: T. A. E. Platts-Mills was supported by the National Institutes of Health (NIH) (grant no. R37-AI-20565); has received assay support from Thermo Fisher/Phadia; and has a patent on an IgE assay to α-Gal. S. P. Commins was supported by the NIH (grant nos. R01AI135049, R56AI113095, and K08AI85190) and the Centers for Disease Control and Prevention (grant no. IPA1908943); has been on the speaker’s bureau at Genentech; and has received author royalties from Up-to-Date. M. van Hage reports grants from H2020 FoodEnTwin GA (no. 810752), the Swedish Research Council, the Stockholm County Council (ALF project), the Swedish Asthma and Allergy Association’s Research Foundation, the King Gustaf V 80th Birthday Foundation, the Swedish Heart-Lung Foundation, the Hesselman Foundation, the Swedish Cancer and Allergy Foundation, and the Swedish Association for Allergology; personal fees from Biomay AG, Vienna, Austria, and Hycor Biomedical LLC, Calif; and personal fees from Thermo Fisher Scientific and ALK, outside the submitted work; L. A. Beck is a consultant or Advisory Board Member for Abbvie, Allakos, Arena Pharma, Astra-Zeneca, Connect Biopharma, LEO Pharma, Lilly, Novan, Novartis, Pfizer, Regeneron, Sanofi, UCB, and Vimalan; is or has recently been an investigator for an Abbvie, LEO Pharma, Pfizer, and/or Regeneron clinical trial; and owns stock in Pfizer and Medtronics. D. Apostolovic reports grants from H2020 FoodEnTwin GA (no. 810752) and from Konsul Th C Berg Foundation, outside the submitted work.
Abbreviations used
- AGS
α-Gal syndrome
- α-Gal
Galactose-α−1,3-galactose
- CDC
Centers for Disease Control and Prevention
- RMSF
Rocky Mountain spotted fever
Footnotes
The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med 2008;358:1109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian J, Liu T, Yang L, Daus A, Crowley R, Zhou Q. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal Biochem 2007;364:8–18. [DOI] [PubMed] [Google Scholar]
- 3.Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol 2009;123:426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol 2011;127:1286–93.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Nunen S, O’Connor K, Fernando SL, Clarke LR, Boyle RX. The association between Ixodes holocyclus tick bite reactions and red meat allergy. Intern Med J 2007;39:A132. [DOI] [PubMed] [Google Scholar]
- 6.Van Nunen SA, O’Connor KS, Clarke LR, Boyle RX, Fernando SL. An association between tick bite reactions and red meat allergy in humans. Med J Aust 2009;190:510–1. [DOI] [PubMed] [Google Scholar]
- 7.Adedoyin J, Gronlund H, Oman H, Johansson SG, van Hage M. Cat IgA, representative of new carbohydrate cross-reactive allergens. J Allergy Clin Immunol 2007;119:640–5. [DOI] [PubMed] [Google Scholar]
- 8.Gronlund H, Adedoyin J, Commins SP, Platts-Mills TA, van Hage M. The carbohydrate galactose-alpha-1,3-galactose is a major IgE-binding epitope on cat IgA. J Allergy Clin Immunol 2009;123:1189–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer J, Yazdi AS, Biedermann T. Clinical spectrum of alpha-Gal syndrome: from immediate-type to delayed immediate-type reactions to mammalian innards and meat. Allergol J Int 2016;25:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer J, Hebsaker J, Caponetto P, Platts-Mills TA, Biedermann T. Galactose-alpha-1,3-galactose sensitization is a prerequisite for pork-kidney allergy and cofactor-related mammalian meat anaphylaxis. J Allergy Clin Immunol 2014; 134:755–9.e1. [DOI] [PubMed] [Google Scholar]
- 11.Jappe U, Kreft B, Ludwig A, Przybilla B, Walker A, Becker W, et al. Allergy to red meat/offal is associated with IgE to a new mammalian cross-reactive carbohydrate determinant also found in cetuximab and cat IgA. Allergy 2010;65:164–5. [Google Scholar]
- 12.Landsteiner K, Miller CP. Serological studies on the blood of the primates, III: distribution of serological factors related to human isoagglutinogens in the blood of lower monkeys. J Exp Med 1925;42:863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galili U The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol 2005;83:674–86. [DOI] [PubMed] [Google Scholar]
- 14.Galili U Anti-Gal: an abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology 2013;140:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Platts-Mills TA, Li RC, Keshavarz B, Smith AR, Wilson JM. Diagnosis and management of patients with the alpha-Gal syndrome. J Allergy Clin Immunol Pract 2020;8:15–23.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Platts-Mills TA, Commins SP. Emerging antigens involved in allergic responses. Curr Opin Immunol 2013;25:769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jappe U, Minge S, Kreft B, Ludwig A, Przybilla B, Walker A, et al. Meat allergy associated with galactosyl-alpha-(1,3)-galactose (alpha-Gal)—closing diagnostic gaps by anti-alpha-Gal IgE immune profiling. Allergy 2018;73:93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson JM, Platts-Mills TAE. Meat allergy and allergens. Mol Immunol 2018; 100:107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer J, Lupberger E, Hebsaker J, Blumenstock G, Aichinger E, Yazdi AS, et al. Prevalence of type I sensitization to alpha-gal in forest service employees and hunters. Allergy 2017;72:1540–7. [DOI] [PubMed] [Google Scholar]
- 20.Maier S, Chung CH, Morse M, Platts-Mills T, Townes L, Mukhopadhyay P, et al. A retrospective analysis of cross-reacting cetuximab IgE antibody and its association with severe infusion reactions. Cancer Med 2015;4:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borrebaeck CK, Malmborg AC, Ohlin M. Does endogenous glycosylation prevent the use of mouse monoclonal antibodies as cancer therapeutics? Immunol Today 1993;14:477–9. [DOI] [PubMed] [Google Scholar]
- 22.Sheeley DM, Merrill BM, Taylor LC. Characterization of monoclonal antibody glycosylation: comparison of expression systems and identification of terminal alpha-linked galactose. Anal Biochem 1997;247:102–10. [DOI] [PubMed] [Google Scholar]
- 23.Lammerts van Bueren JJ, Rispens T, Verploegen S, van der Palen-Merkus T, Stapel S, Workman LJ, et al. Anti-galactose-alpha-1,3-galactose IgE from allergic patients does not bind alpha-galactosylated glycans on intact therapeutic antibody Fc domains. Nat Biotechnol 2011;29:574–6. [DOI] [PubMed] [Google Scholar]
- 24.Homann A, Schramm G, Jappe U. Glycans and glycan-specific IgE in clinical and molecular allergology: sensitization, diagnostics, and clinical symptoms. J Allergy Clin Immunol 2017;140:356–68. [DOI] [PubMed] [Google Scholar]
- 25.Amoah AS, Asuming-Brempong EK, Obeng BB, Versteeg SA, Larbi IA, Aryeetey Y, et al. Identification of dominant anti-glycan IgE responses in school children by glycan microarray. J Allergy Clin Immunol 2018;141:1130–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Apostolovic D, Tran TA, Sanchez-Vidaurre S, Cirkovic Velickovic T, Starkhammar M, Hamsten C, et al. Red meat allergic patients have a selective IgE response to the alpha-Gal glycan. Allergy 2015;70:1497–500. [DOI] [PubMed] [Google Scholar]
- 27.Stone CA Jr, Hemler JA, Commins SP, Schuyler AJ, Phillips EJ, Peebles RS Jr, et al. Anaphylaxis after zoster vaccine: implicating alpha-gal allergy as a possible mechanism. J Allergy Clin Immunol 2017;139:1710–3.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stone CA Jr, Commins SP, Choudhary S, Vethody C, Heavrin JL, Wingerter J, et al. Anaphylaxis after vaccination in a pediatric patient: further implicating alpha-gal allergy. J Allergy Clin Immunol Pract 2019;7:322–4.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinson ML, Waibel KH. Safe administration of a gelatin-containing vaccine in an adult with galactose-alpha-1,3-galactose allergy. Vaccine 2015;33:1231–2. [DOI] [PubMed] [Google Scholar]
- 30.Retterer MKC, Workman LJ, Bacon JR, Platts-Mills TAE. Specific IgE to gelatin as a cause of anaphylaxis to zoster vaccine. J Allergy Clin Immunol 2018;141: 1956–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischer J, Eberlein B, Hilger C, Eyer F, Eyerich S, Ollert M, et al. Alpha-gal is a possible target of IgE-mediated reactivity to antivenom. Allergy 2017;72:764–71. [DOI] [PubMed] [Google Scholar]
- 32.Rizer J, Brill K, Charlton N, King J. Acute hypersensitivity reaction to Crotalidae polyvalent immune Fab (CroFab) as initial presentation of galactose-alpha-1,3-galactose (alpha-gal) allergy. Clin Toxicol (Phila) 2017;55:668–9. [DOI] [PubMed] [Google Scholar]
- 33.Sell-Dottin K, Sola M, Caranasos T. Impact of newly emerging alpha-gal allergies on cardiac surgery: a case series. Clin Surg 2017;2. [Google Scholar]
- 34.Muglia C, Kar I, Gong M, Hermes-DeSantis ER, Monteleone C. Anaphylaxis to medications containing meat byproducts in an alpha-gal sensitized individual. J Allergy Clin Immunol Pract 2015;3:796–7. [DOI] [PubMed] [Google Scholar]
- 35.Swiontek K, Morisset M, Codreanu-Morel F, Fischer J, Mehlich J, Darsow U, et al. Drugs of porcine origin—a risk for patients with alpha-gal syndrome? J Allergy Clin Immunol Pract 2019;7:1687–90.e3. [DOI] [PubMed] [Google Scholar]
- 36.Vidal C, Mendez-Brea P, Lopez-Freire S, Gonzalez-Vidal T. Vaginal capsules: an unsuspected probable source of exposure to alpha-Gal. J Investig Allergol Clin Immunol 2016;26:388–9. [DOI] [PubMed] [Google Scholar]
- 37.Mozzicato SM, Tripathi A, Posthumus JB, Platts-Mills TAE, Commins SP. Porcine or bovine valve replacement in 3 patients with IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol Pract 2014;2:637–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawkins RB, Frischtak HL, Kron IL, Ghanta RK. Premature bioprosthetic aortic valve degeneration associated with allergy to galactose-alpha-1,3-galactose. J Card Surg 2016;31:446–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson JM, Schuyler AJ, Workman L, Gupta M, James HR, Posthumus J, et al. Investigation into the alpha-Gal syndrome: characteristics of 261 children and adults reporting red meat allergy. J Allergy Clin Immunol Pract 2019;7: 2348–58.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamsten C, Tran TA, Starkhammar M, Brauner A, Commins SP, Platts-Mills TA, et al. Red meat allergy in Sweden: association with tick sensitization and B-negative blood groups. J Allergy Clin Immunol 2013;132:1431–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Commins SP, James HR, Stevens W, Pochan SL, Land MH, King C, et al. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose-alpha-1,3-galactose. J Allergy Clin Immunol 2014;134:108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mabelane T, Basera W, Botha M, Thomas HF, Ramjith J, Levin ME. Predictive values of alpha-gal IgE levels and alpha-gal IgE: total IgE ratio and oral food challenge-proven meat allergy in a population with a high prevalence of reported red meat allergy. Pediatr Allergy Immunol 2018;29:841–9. [DOI] [PubMed] [Google Scholar]
- 43.Kennedy JL, Stallings AP, Platts-Mills TA, Oliveira WM, Workman L, James HR, et al. Galactose-alpha-1,3-galactose and delayed anaphylaxis, angioedema, and urticaria in children. Pediatrics 2013;131:e1545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levin M, Apostolovic D, Biedermann T, Commins SP, Iweala OI, Platts-Mills TAE, et al. Alpha-gal phenotypes—lessons from various patient populations. Ann Allergy Asthma Immunol 2019;122:598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Commins SP. Invited commentary: alpha-gal allergy: tip of the iceberg to a pivotal immune response. Curr Allergy Asthma Rep 2016;16:61. [DOI] [PubMed] [Google Scholar]
- 46.Wolbing F, Fischer J, Koberle M, Kaesler S, Biedermann T. About the role and underlying mechanisms of cofactors in anaphylaxis. Allergy 2013;68:1085–92. [DOI] [PubMed] [Google Scholar]
- 47.Wilson JM, Platts-Mills TAE. The oligosaccharide galactose-a-1,3-galactose and the α-Gal syndrome: insights from an epitope that is causal in IgE-mediated immediate and delayed anaphylaxis. EMJ Allergy Immunol 2018;3:89–98. [Google Scholar]
- 48.Morisset M, Richard C, Astier C, Jacquenet S, Croizier A, Beaudouin E, et al. Anaphylaxis to pork kidney is related to IgE antibodies specific for galactose-alpha-1,3-galactose. Allergy 2012;67:699–704. [DOI] [PubMed] [Google Scholar]
- 49.Steinke JW, Pochan SL, James HR, Platts-Mills TAE, Commins SP. Altered metabolic profile in patients with IgE to galactose-alpha-1,3-galactose following in vivo food challenge. J Allergy Clin Immunol 2016;138:1465–7.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mullins RJ, James H, Platts-Mills TA, Commins S. Relationship between red meat allergy and sensitization to gelatin and galactose-alpha-1,3-galactose. J Allergy Clin Immunol 2012;129:1334–42.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caponetto P, Fischer J, Biedermann T. Gelatin-containing sweets can elicit anaphylaxis in a patient with sensitization to galactose-alpha-1,3-galactose. J Allergy Clin Immunol Pract 2013;1:302–3. [DOI] [PubMed] [Google Scholar]
- 52.Roenneberg S, Bohner A, Brockow K, Arnold A, Darsow U, Eberlein B, et al. alpha-Gal—a new clue for anaphylaxis in mastocytosis. J Allergy Clin Immunol Pract 2016;4:531–2. [DOI] [PubMed] [Google Scholar]
- 53.Galili U, Basbaum CB, Shohet SB, Buehler J, Macher BA. Identification of erythrocyte Gal alpha 1–3Gal glycosphingolipids with a mouse monoclonal antibody, Gal-13. J Biol Chem 1987;262:4683–8. [PubMed] [Google Scholar]
- 54.Hendricks SP, He P, Stults CL, Macher BA. Regulation of the expression of Gal alpha 1–3Gal beta 1–4GlcNAc glycosphingolipids in kidney. J Biol Chem 1990; 265:17621–6. [PubMed] [Google Scholar]
- 55.Diswall M, Angstrom J, Karlsson H, Phelps CJ, Ayares D, Teneberg S, et al. Structural characterization of alpha1,3-galactosyltransferase knockout pig heart and kidney glycolipids and their reactivity with human and baboon antibodies. Xenotransplantation 2010;17:48–60. [DOI] [PubMed] [Google Scholar]
- 56.Michel CC, Nanjee MN, Olszewski WL, Miller NE. LDL and HDL transfer rates across peripheral microvascular endothelium agree with those predicted for passive ultrafiltration in humans. J Lipid Res 2015;56:122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Labbe SM, Grenier-Larouche T, Croteau E, Normand-Lauziere F, Frisch F, Ouellet R, et al. Organ-specific dietary fatty acid uptake in humans using positron emission tomography coupled to computed tomography. Am J Physiol Endocrinol Metab 2011;300:E445–53. [DOI] [PubMed] [Google Scholar]
- 58.Roman-Carrasco P, Lieder B, Somoza V, Ponce M, Szepfalusi Z, Martin D, et al. Only α-Gal bound to lipids, but not to proteins, is transported across enterocytes as an IgE reactive molecule that can induce effector cell activation. Allergy 2019; 74:1956–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson JM, Nguyen AT, Schuyler AJ, Commins SP, Taylor AM, Platts-Mills TAE, et al. IgE to the mammalian oligosaccharide galactose-alpha-1,3-galactose is associated with increased atheroma volume and plaques with unstable characteristics—brief report. Arterioscler Thromb Vasc Biol 2018;38:1665–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med 2007; 13:719–24. [DOI] [PubMed] [Google Scholar]
- 61.Kounis NG, Hahalis G. Serum IgE levels in coronary artery disease. Atherosclerosis 2016;251:498–500. [DOI] [PubMed] [Google Scholar]
- 62.Kounis NG. Coronary hypersensitivity disorder: the Kounis syndrome. Clin Ther 2013;35:563–71. [DOI] [PubMed] [Google Scholar]
- 63.Abdelghany M, Subedi R, Shah S, Kozman H. Kounis syndrome: a review article on epidemiology, diagnostic findings, management and complications of allergic acute coronary syndrome. Int J Cardiol 2017;232:1–4. [DOI] [PubMed] [Google Scholar]
- 64.Wilson JM, McNamara CA, Platts-Mills TAE. IgE, alpha-Gal and atherosclerosis. Aging (Albany NY) 2019;11:1900–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dahlgren FS, Paddock CD, Springer YP, Eisen RJ, Behravesh CB. Expanding range of Amblyomma americanum and simultaneous changes in the epidemiology of spotted fever group rickettsiosis in the United States. Am J Trop Med Hyg 2016;94:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gaines DN, Operario DJ, Stroup S, Stromdahl E, Wright C, Gaff H, et al. Ehrlichia and spotted fever group rickettsiae surveillance in Amblyomma americanum in Virginia through use of a novel six-plex real-time PCR assay. Vector Borne Zoonotic Dis 2014;14:307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Apperson CS, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, et al. Tick-borne diseases in North Carolina: is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis 2008;8:597–606. [DOI] [PubMed] [Google Scholar]
- 68.Trout Fryxell RT, Steelman CD, Szalanski AL, Billingsley PM, Williamson PC. Molecular detection of Rickettsia species within ticks (Acari: Ixodidae) collected from Arkansas United States. J Med Entomol 2015;52:500–8. [DOI] [PubMed] [Google Scholar]
- 69.Karpathy SE, Slater KS, Goldsmith CS, Nicholson WL, Paddock CD. Rickettsia amblyommatis sp. nov., a spotted fever group rickettsia associated with multiple species of Amblyomma ticks in North, Central and South America. Int J Syst Evol Microbiol 2016;66:5236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Monzon JD, Atkinson EG, Henn BM, Benach JL. Population and evolutionary genomics of Amblyomma americanum, an expanding arthropod disease vector. Genome Biol Evol 2016;8:1351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Springer YP, Eisen L, Beati L, James AM, Eisen RJ. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol 2014;51:342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamsten C, Starkhammar M, Tran TA, Johansson M, Bengtsson U, Ahlen G, et al. Identification of galactose-alpha-1,3-galactose in the gastrointestinal tract of the tick Ixodes ricinus: possible relationship with red meat allergy. Allergy 2013;68:549–52. [DOI] [PubMed] [Google Scholar]
- 73.Araujo RN, Franco PF, Rodrigues H, Santos LC, McKay CS, Sanhueza CA, et al. Amblyomma sculptum tick saliva: alpha-Gal identification, antibody response and possible association with red meat allergy in Brazil. Int J Parasitol 2016;46: 213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim M, Straesser MD, Keshavarz B, Workman L, McGowan EC, Platts-Mills TAE, et al. IgE to galactose-alpha-1, 3-galactose wanes over time in patients who avoid tick bites. J Allergy Clin Immunol Pract 2020;8:364–7.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Nunen SA. Tick-induced allergies: mammalian meat allergy and tick anaphylaxis. Med J Aust 2018;208:316–21. [DOI] [PubMed] [Google Scholar]
- 76.Gonzalez-Quintela A, Dam Laursen AS, Vidal C, Skaaby T, Gude F, Linneberg A. IgE antibodies to alpha-gal in the general adult population: relationship with tick bites, atopy, and cat ownership. Clin Exp Allergy 2014;44:1061–8. [DOI] [PubMed] [Google Scholar]
- 77.Diuk-Wasser MA, Gatewood AG, Cortinas MR, Yaremych-Hamer S, Tsao J, Kitron U, et al. Spatiotemporal patterns of host-seeking Ixodes scapularis nymphs (Acari: Ixodidae) in the United States. J Med Entomol 2006;43:166–76. [DOI] [PubMed] [Google Scholar]
- 78.Springer YP, Jarnevich CS, Barnett DT, Monaghan AJ, Eisen RJ. Modeling the present and future geographic distribution of the lone star tick, Amblyomma americanum (Ixodida: Ixodidae), in the Continental United States. Am J Trop Med Hyg 2015;93:875–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Raghavan RK, Peterson AT, Cobos ME, Ganta R, Foley D. Current and future distribution of the lone star tick, Amblyomma americanum (L.) (Acari: Ixodidae) in North America. PLoS One 2019;14:e0209082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stoltz LP, Cristiano LM, Dowling APG, Wilson JM, Platts-Mills TAE, Traister RS. Could chiggers be contributing to the prevalence of galactose-alpha-1,3-galactose sensitization and mammalian meat allergy? J Allergy Clin Immunol Pract 2019;7:664–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burns EC, Melancon DG. Effect of imported fire ant (Hymenoptera: Formicidae) invasion on lone star tick (Acarina: Ixodidae) populations. J Med Entomol 1977; 14:247–9. [DOI] [PubMed] [Google Scholar]
- 82.Paddock CD, Yabsley MJ. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum-associated zoonoses in the United States. Curr Top Microbiol Immunol 2007;315:289–324. [DOI] [PubMed] [Google Scholar]
- 83.Chinuki Y, Ishiwata K, Yamaji K, Takahashi H, Morita E. Haemaphysalis longicornis tick bites are a possible cause of red meat allergy in Japan. Allergy 2016; 71:421–5. [DOI] [PubMed] [Google Scholar]
- 84.Hashizume H, Fujiyama T, Umayahara T, Kageyama R, Walls AF, Satoh T. Repeated Amblyomma testudinarium tick bites are associated with increased galactose-alpha-1,3-galactose carbohydrate IgE antibody levels: a retrospective cohort study in a single institution. J Am Acad Dermatol 2018;78: 1135–41.e3. [DOI] [PubMed] [Google Scholar]
- 85.Karim S, Ribeiro JM. An insight into the Sialome of the lone star tick, Amblyomma americanum, with a glimpse on its time dependent gene expression. PLoS One 2015;10:e0131292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cabezas-Cruz A, Valdes JJ. Are ticks venomous animals? Front Zool 2014;11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nuttall PA. Wonders of tick saliva. Ticks Tick Borne Dis 2019;10:470–81. [DOI] [PubMed] [Google Scholar]
- 88.Cabezas-Cruz A, Espinosa PJ, Alberdi P, Simo L, Valdes JJ, Mateos-Hernandez L, et al. Tick galactosyltransferases are involved in alpha-Gal synthesis and play a role during Anaplasma phagocytophilum infection and Ixodes scapularis tick vector development. Sci Rep 2018;8:14224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Apostolovic D, Mihailovic J, Commins SP, Wijnveld M, Kazimirova M, Starkhammar M, et al. Allergenomics of the tick Ixodes ricinus reveal important alpha-Gal-carrying IgE-binding proteins in red meat allergy. Allergy 2020;75: 217–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crispell G, Commins SP, Archer-Hartman SA, Choudhary S, Dharmarajan G, Azadi P, et al. Discovery of alpha-gal-containing antigens in North American tick species believed to induce red meat allergy. Front Immunol 2019;10:1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park Y, Kim D, Boorgula GD, De Schutter K, Smagghe G, Simo L, et al. Alpha-gal and cross-reactive carbohydrate determinants in the N-Glycans of salivary glands in the lone star tick., 8. Basel: Amblyomma americanum. Vaccines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lack G Epidemiologic risks for food allergy. J Allergy Clin Immunol 2008;121: 1331–6. [DOI] [PubMed] [Google Scholar]
- 93.Tordesillas L, Goswami R, Benede S, Grishina G, Dunkin D, Jarvinen KM, et al. Skin exposure promotes a Th2-dependent sensitization to peanut allergens. J Clin Invest 2014;124:4965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Woodfolk JA, Commins SP, Schuyler AJ, Erwin EA, Platts-Mills TA. Allergens, sources, particles, and molecules: why do we make IgE responses? Allergol Int 2015;64:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beck LA, Leung DY. Allergen sensitization through the skin induces systemic allergic responses. J Allergy Clin Immunol 2000;106:S258–63. [DOI] [PubMed] [Google Scholar]
- 96.Brown SJ, Knapp FW. Amblyomma americanum: sequential histological analysis of adult feeding sites on guinea pigs. Exp Parasitol 1980;49:303–18. [DOI] [PubMed] [Google Scholar]
- 97.Harrison OJ, Linehan JL, Shih HY, Bouladoux N, Han SJ, Smelkinson M, et al. Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science 2019;363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chandrasekhar JL, Cox KM, Loo WM, Qiao H, Tung KS, Erickson LD. Cutaneous exposure to clinically relevant lone star ticks promotes IgE production and hypersensitivity through CD4(+) T cell- and MyD88-dependent pathways in mice. J Immunol 2019;203:813–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cabezas-Cruz A, de la Fuente J, Fischer J, Hebsaker J, Lupberger E, Blumenstock G, et al. Prevalence of type I sensitization to alpha-gal in forest service employees and hunters: is the blood type an overlooked risk factor in epidemiological studies of the alpha-Gal syndrome? Allergy 2017;72:2044–7. [DOI] [PubMed] [Google Scholar]
- 100.Venturini M, Lobera T, Sebastian A, Portillo A, Oteo JA. IgE to α-Gal in foresters and forest workers from La Rioja, North of Spain. J Investig Allergol Clin Immunol 2018;28:106–12. [DOI] [PubMed] [Google Scholar]
- 101.Commins SP, Karim S. Development of a novel murine model of alpha-gal meat allergy. J Allergy Clin Immunol 2017;139:AB193. [Google Scholar]
- 102.Choudhary S, Iweala OI, Addison CT, Commins SP. Tick salivary extract induces alpha-gal allergy in alpha-gal deficient mice. J Allergy Clin Immunol 2019;143: AB252. [Google Scholar]
- 103.Bowman AS, Gengler CL, Surdick MR, Zhu K, Essenberg RC, Sauer JR, et al. A novel phospholipase A2 activity in saliva of the lone star tick, Amblyomma americanum (L.). Exp Parasitol 1997;87:121–32. [DOI] [PubMed] [Google Scholar]
- 104.Cabezas-Cruz A, Mateos-Hernandez L, Chmelar J, Villar M, de la Fuente J. Salivary prostaglandin E2: role in tick-induced allergy to red meat. Trends Parasitol 2017;33:495–8. [DOI] [PubMed] [Google Scholar]
- 105.Trager W Acquired immunity to ticks. J Parasitol Urbana 1939;25:57–78. [Google Scholar]
- 106.Wada T, Ishiwata K, Koseki H, Ishikura T, Ugajin T, Ohnuma N, et al. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest 2010;120:2867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Karasuyama H, Tabakawa Y, Ohta T, Wada T, Yoshikawa S. Crucial role for basophils in acquired protective immunity to tick infestation. Front Physiol 2018;9:1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schmidle P, Reidenbach K, Kugler C, Eberlein B, Biedermann T, Darsow U. Recall urticaria—a new clinical sign in the diagnosis of alpha-gal syndrome. J Allergy Clin Immunol Pract 2019;7:685–6. [DOI] [PubMed] [Google Scholar]
- 109.Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med 1984;160:1519–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hamadeh RM, Galili U, Zhou P, Griffiss JM. Anti-alpha-galactosyl immunoglobulin A (IgA), IgG, and IgM in human secretions. Clin Diagn Lab Immunol 1995; 2:125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hamanova M, Chmelikova M, Nentwich I, Thon V, Lokaj J. Anti-Gal IgM, IgA and IgG natural antibodies in childhood. Immunol Lett 2015;164:40–3. [DOI] [PubMed] [Google Scholar]
- 112.Aguilar R, Ubillos I, Vidal M, Balanza N, Crespo N, Jimenez A, et al. Antibody responses to alpha-Gal in African children vary with age and site and are associated with malaria protection. Sci Rep 2018;8:9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ohdan H, Swenson KG, Kruger Gray HS, Yang YG, Xu Y, Thall AD, et al. Mac-1-negative B-1b phenotype of natural antibody-producing cells, including those responding to Gal alpha 1,3Gal epitopes in alpha 1,3-galactosyltransferase-deficient mice. J Immunol 2000;165:5518–29. [DOI] [PubMed] [Google Scholar]
- 114.Parker W, Yu PB, Holzknecht ZE, Lundberg K, Buckley RH, Platt JL. Specificity and function of “natural” antibodies in immunodeficient subjects: clues to B cell lineage and development. J Clin Immunol 1997;17:311–21. [DOI] [PubMed] [Google Scholar]
- 115.Wilson JM, Schuyler AJ, Schroeder N, Platts-Mills TA. Galactose-alpha-1,3-galactose: atypical food allergen or model IgE hypersensitivity? Curr Allergy Asthma Rep 2017;17:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yilmaz B, Portugal S, Tran TM, Gozzelino R, Ramos S, Gomes J, et al. Gut microbiota elicits a protective immune response against malaria transmission. Cell 2014;159:1277–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cretin N, Bracy J, Hanson K, Iacomini J. The role of T cell help in the production of antibodies specific for Gal alpha 1–3Gal. J Immunol 2002;168:1479–83. [DOI] [PubMed] [Google Scholar]
- 118.Rispens T, Derksen NI, Commins SP, Platts-Mills TA, Aalberse RC. IgE production to alpha-gal is accompanied by elevated levels of specific IgG1 antibodies and low amounts of IgE to blood group B. PLoS One 2013;8:e55566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kollmann D, Nagl B, Ebner C, Emminger W, Wohrl S, Kitzmuller C, et al. The quantity and quality of alpha-gal-specific antibodies differ in individuals with and without delayed red meat allergy. Allergy 2017;72:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Apostolovic D, Rodrigues R, Thomas P, Starkhammar M, Hamsten C, van Hage M. Immunoprofile of alpha-Gal- and B-antigen-specific responses differentiates red meat-allergic patients from healthy individuals. Allergy 2018;73: 1525–31. [DOI] [PubMed] [Google Scholar]
- 121.McGowan EC, Platts-Mills TAE, Wilson JM. Food allergy, eosinophilic esophagitis and the enigma of IgG4. Ann Allergy Asthma Immunol 2019; 122:563–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ristivojevic MK, Grundstrom J, Tran TAT, Apostolovic D, Radoi V, Starkhammar M, et al. alpha-Gal on the protein surface affects uptake and degradation in immature monocyte derived dendritic cells. Sci Rep 2018;8:12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Iweala O, Brennan PJ, Commins SP. Serum IgE specific for alpha-Gal sugar moiety can bind glycolipid. J Allergy Clin Immunol 2017;139:AB88. [Google Scholar]
- 124.Liu S, Kandeva T, Tchervenkov J. CD1d-mediated interaction between activated T cells and B cells is essential to B-cell proliferation and anti-alpha-Gal antibody production. Transplant Proc 2009;41:398–402. [DOI] [PubMed] [Google Scholar]
- 125.Iweala OI, Savage PB, Commins SP. A role for CD1d-restricted invariant natural killer T cells and glycolipids in alpha-Gal allergy. J Allergy Clin Immunol 2018; 141:AB288. [Google Scholar]
- 126.Brestoff JR, Tesfazghi MT, Zaydman MA, Jackups R Jr, Kim BS, Scott MG, et al. The B antigen protects against the development of red meat allergy. J Allergy Clin Immunol Pract 2018;6:1790–1.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Galili U, Buehler J, Shohet SB, Macher BA. The human natural anti-Gal IgG, III: the subtlety of immune tolerance in man as demonstrated by crossreactivity between natural anti-Gal and anti-B antibodies. J Exp Med 1987;165: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hilger C, Fischer J, Wolbing F, Biedermann T. Role and mechanism of galactose- alpha-1,3-galactose in the elicitation of delayed anaphylactic reactions to red meat. Curr Allergy Asthma Rep 2019;19:3. [DOI] [PMC free article] [PubMed] [Google Scholar]