Abstract
The prevalence of the causative agents of feline upper respiratory tract disease (URTD) has been previously documented in many regions worldwide, but has yet to be reported in eastern Canada. The objectives of this study were to determine the prevalence of feline herpesvirus-1 (FHV-1), feline calicivirus (FCV), Chlamydia felis (C. felis), and Bordetella bronchiseptica (B. bronchiseptica) in a population of shelter cats with clinical signs related to URTD on Prince Edward Island, Canada; to compare the prevalence of FHV-1 and FCV as detected by polymerase chain reaction (PCR) and virus isolation (VI) in this population; and lastly, to determine whether factors, such as co-infections, time of year, concurrent feline leukemia virus (FeLV)- or feline immunodeficiency virus (FIV)-positive status, or clinical signs, were associated with prevalence of particular pathogens. Conjunctival, nasal mucosal, and oropharyngeal swabs were collected from 82 cats with clinical signs consistent with URTD. Samples were pooled in transport medium and PCR was used to detect FHV-1, FCV, and C. felis and VI was also used to detect FHV-1 and FCV. A separate swab was submitted for aerobic bacterial culture to detect B. bronchiseptica. Feline herpesvirus-1 (FHV-1) was the most prevalent in this population, followed by C. felis, B. bronchiseptica, and FCV. Of the 4 cats that were positive for B. bronchiseptica, 3 were concurrently positive for FHV-1. All positive B. bronchiseptica cultures were resistant to cefovecin. The prevalence for FHV-1 was lowest in autumn (seasons P < 0.001) and was positively associated with the presence of nasal discharge (P = 0.018) and coughing (P = 0.043).
Résumé
La prévalence des agents causals de maladies du tractus respiratoire supérieur félin (URTD) a été préalablement documentée dans plusieurs régions du monde mais n’a pas encore été rapportée dans l’est du Canada. Les objectifs de la présente étude étaient de déterminer la prévalence d’herpès virus félin-1 (FHV-1), du calicivirus félin (FCV), de Chlamydia felis et de Bordetella bronchiseptica dans une population de chats de refuge de l’Île-du-Prince-Édouard, Canada avec des signes cliniques reliés au URTD; de comparer la prévalence de FHV-1 et FCV telle que détecter par réaction d’amplification en chaîne par la polymérase (PCR) et l’isolement viral (VI) dans ces populations; et finalement, déterminer si des facteurs, tels que les co-infections, la période de l’année, le statut concomitant positif pour le virus de la leucémie féline (FeLV) ou le virus de l’immunodéficience féline (FIV) ou les signes cliniques étaient associés avec la prévalence d’un agent pathogène en particulier. Des écouvillons de la conjonctive, de la muqueuse nasale et de l’oropharynx furent obtenus de 82 chats avec des signes cliniques compatibles avec URTD. Les échantillons étaient regroupés dans un milieu de transport et la PCR utilisée pour détecter FHV-1, FCV et C. felis et l’isolement viral fut également utilisé pour détecter FHV-1 et FCV. Un écouvillon séparé fut soumis pour culture bactérienne aérobie afin de détecter B. bronchiseptica. Le FHV-1 était le plus prévalent dans cette population, suivi par C. felis, B. bronchiseptica et FCV. Des quatre chats qui étaient positifs pour B. bronchiseptica, trois étaient positifs également pour FHV-1. Tous les isolats de B. bronchiseptica obtenus étaient résistants au céfovecin. La prévalence de FHV-1 était à son plus bas en automne (P < 0,001 pour les saisons) et était associée positivement avec la présence d’écoulement nasal (P = 0,018) et de la toux (P = 0,043).
(Traduit par Docteur Serge Messier)
Introduction
Feline upper respiratory tract disease (URTD) is a common disease complex of domestic cats worldwide that has a particularly great impact in animal shelters, where the disease propagates readily between stray and surrendered cats. Upper respiratory tract disease (URTD) is the primary illness reported both in cats housed in shelters and during the post-adoption period (1,2). Factors that contribute to the development of URTD include stress, over-crowding, poor ventilation, as well as lack of the necessary space, staffing, and tools for effective disease control programs (1,3–5). The result is that URTD remains among the leading causes of euthanasia of cats in traditional shelters, second only to overcrowding (5). This disease presents not only a heavy financial burden for animal shelters and new owners, but also significant management and disease control challenges, and can be associated with decreased adoptability as well as a high incidence of return of cats that become ill during the post-adoption period.
Clinical signs associated with URTD include sneezing, nasal discharge, ocular discharge, conjunctivitis, coughing, oral ulceration, anorexia, fever, and lethargy, either alone or in any combination. The primary causative agents associated with URTD include feline herpesvirus-1 (FHV-1), feline calicivirus (FCV), Chlamydia felis (C. felis), and more recently, Bordetella bronchiseptica (B. bronchiseptica) and Mycoplasma felis (M. felis). Identification of the causative pathogens can be challenging and is often not pursued due to financial limitations. Consequently, treatments are often non-specific and may be inappropriate or unnecessary based on the prevalent pathogens in a given shelter, resulting in wasted resources.
The pathogens involved in URTD are most commonly diagnosed using oropharyngeal, nasal, and conjunctival swabs. Feline herpesvirus-1 (FHV-1), FCV, and C. felis are typically identified by polymerase chain reaction (PCR), while FHV-1 and FCV can also be detected with virus isolation (VI) (2,6–10). More recently, multiplex PCR has been developed and may be of considerable value, although its availability is currently limited and it requires further investigation (11). Bordetella bronchiseptica is often diagnosed by a bacterial culture and PCR for B. bronchiseptica is also available, although one study found that it was 10 times less sensitive than bacterial culture (10).
While prevalence of the pathogens implicated in URTD has been investigated worldwide, to our knowledge the prevalence of FHV-1, FCV, C. felis, and B. bronchiseptica in feline populations in eastern Canada, and more specifically, on Prince Edward Island, has not been described. The main objectives of this cross-sectional study were to determine the prevalence of FHV-1, FCV, C. felis, and B. bronchiseptica in a sample of shelter cats on Prince Edward Island showing clinical signs associated with URTD and to establish the antimicrobial susceptibility profile of any positive B. bronchiseptica cultures obtained. The secondary objectives were to compare the agreements in detection of FHV-1 and FCV using PCR and VI; describe concurrent infections; and identify potential risk factors, such as seasonal trends, feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) status, feline characteristics such as age, sex, weight, neuter status, and body condition score, and the presence of URTD-related clinical signs.
Materials and methods
This was a cross-sectional study that included cats housed at the Prince Edward Island Humane Society in eastern Canada from August 2013 to November 2014. The cats had various recorded background characteristics, such as being stray, feral, or surrendered. Cats included in this study had to have displayed one or more clinical signs consistent with URTD, as identified by Humane Society personnel, and could not have had any previous record of antibiotic therapy or currently be receiving antibiotic therapy. Cats that met the inclusion criteria were given subjective scores (0, 1, 2, or 3) by a single veterinarian evaluator based on the presence and severity of clinical signs, namely, ocular discharge, nasal discharge, sneezing, and coughing. Once identified by shelter staff as meeting the inclusion criteria, the veterinarian was notified and the cat was evaluated and samples collected in the next 1 to 3 d. For each cat, the date of evaluation, individual humane society number, body weight, body condition score, approximate age, sex, neuter status (if available), clinical sign(s), and the associated subjective score, were recorded.
Sample collection and testing
A total of 8 samples, including 7 swabs and a single venous blood sample, were collected from each cat, with samples taken from the conjunctiva (2 in total, 1 swab for each eye), nasal mucosa (2 in total, 1 swab for each naris), and oropharynx (3 swabs in total) to conduct the following: PCR for FHV-1, FCV, and C. felis; VI for FHV-1 and FCV; and aerobic bacterial culture for B. bronchiseptica.
Specifically, the conjunctival swabs were acquired 5 min after 1 drop of proparacaine hydrochloride ophthalmic solution (Alcaine; Alcon Laboratories, Fort Worth, Texas, USA) was instilled in each eye, by gently rolling a separate sterile cotton-tipped applicator along the lower conjunctiva for each of the left and right eye. Two nasal swabs were taken from each cat by rolling sterile pediatric cotton-tipped applicators along the nasal mucosa of each of the nares (left and right). Lastly, 3 oropharyngeal swabs were taken by rotating a sterile cotton-tipped applicator in the oropharynx of each cat.
The URTD samples for PCR and virus isolation were collected using the commercially available Starswab Multitrans Collection and Transport System (Starplex Scientific, Etobicoke, Ontario) utilizing standard flocked swabs and fine-tipped nasopharyngeal swabs. Each feline URTD sample consisted of 2 vials (component of the Starswab Multitrans Collection and Transport System) with each vial containing 3 swabs, which included nasal, conjunctival, and oropharyngeal samples. The sample for B. bronchiseptica isolation was collected from the oropharynx and placed in the commercially available Starswab II collection system (Starplex Scientific).
The samples for PCR and virus isolation were pooled based on the side (right or left) that the samples were collected from, with 1 swab from each sampling site placed in the pooled viral transport media. This resulted in 3 swabs each in 2 separate vials, as already described. Lastly, a peripherally collected blood sample was obtained from each cat to evaluate for FeLV antigen and FIV antibody, using the SNAP FeLV/FIV enzyme-linked immunosorbent assay (ELISA) test (IDEXX Laboratories, Markham, Ontario). All samples were collected from awake, non-sedated animals.
The study protocol was approved by the University of Prince Edward Island Animal Care Committee.
PCR and VI sample handling
The 2 vials, each containing 3 swabs in transport medium for each case, were submitted to the Regional Diagnostic Virology Services (RDVS) laboratory at Atlantic Veterinary College (AVC). The media in the 2 vials for each submission were pooled and aliquoted (4 cryotubes) before being stored in a −80°C freezer prior to processing. The samples were processed in batches, with the bulk of the samples being processed after the entire specimen collection for the project was complete.
Nucleic acid extraction of URTD swab samples for FHV-1, FCV, and C. felis PCR
The QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) was used to extract nucleic acid for FHV-1 PCR and C. felis PCR. Nucleic acid was extracted using 200 μL of URTD swab sample or cell culture supernatant following the manufacturer’s extraction protocol for eye, nasal, or pharyngeal swabs using spin and vacuum procedures.
The QIAamp RNA Mini Kit (Qiagen) was used to extract nucleic acid for FCV PCR. Nucleic acid was extracted using 140 μL of URTD swab sample or cell culture supernatant following manufacturer’s extraction protocol for purification of viral RNA using spin and vacuum procedures.
PCR amplification of FHV-1, FCV, and C. felis
FHV-1
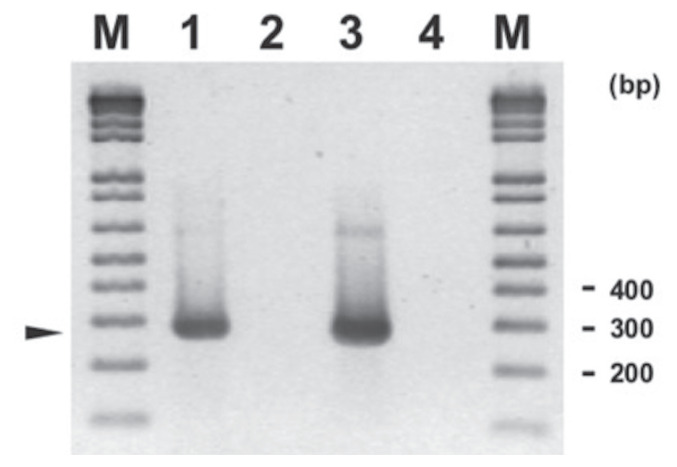
The primers and amplification conditions used for FHV-1 PCR were those developed by Sykes et al (12) that were adapted in the RDVS laboratory at AVC with minor modifications. The amplification uses a 50-μL reaction that contains 4 mM of magnesium chloride (MgCl2), 200 μM each of dNTP Blend (Applied Biosystems, Waltham, Massachusetts, USA), 0.4 M each of reverse and forward primers, 2.5 U AmpliTaq Gold DNA Polymerase (Applied Biosystems), an appropriate quantity of PCR buffer and RNAse free water, and 5 μL of template (extracted sample). The PCR conditions consisted of 5 min at 95°C, followed by 35 cycles of 94°C for 60 s, 56°C for 30 s, and 72°C for 30 s, and final extension step of 7 min, yielding an amplicon of 287 base pairs (bp). The electrophoresis gel for the PCR of FHV-1 is shown in Figure 1.
Figure 1.
Agarose gel electrophoresis of feline herpesvirus-1 (FHV-1) amplification products (around 287 bp, arrowhead) on feline upper respiratory tract samples using primers and conditions described in article (with modification). Legend: M — 1 kb Plus DNA ladder (Invitrogen); Lane 1 — FHV-1 positive control; Lane 2 — FHV-1 negative control; Lane 3 — FHV-1 positive sample; and Lane 4 — FHV-1 negative sample. A sample represents pool of fluid from the following swabs: nasal, ocular, and oropharyngeal.
FCV
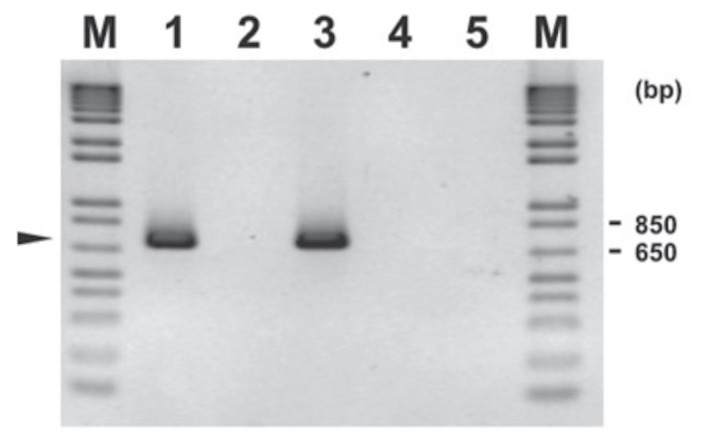
The primers and amplification conditions used for FCV were those developed by Sykes et al (13) that were adapted in the RDVS laboratory at AVC with minor modifications. First, 5 μL was added to the PCR tube that contained 45 μL MasterMix using Roche Titan One Tube RT-PCR System (Sigma, St. Louis, Missouri, USA). The PCR is a 50-μL reaction containing a final concentration of 0.4 μM each of forward and reverse primers, 200 μM each of dNTP Blend, 2.5 U of enzyme mix, 2.5 mM of MgCl2, an appropriate quantity of PCR buffer and RNAse free water, and 5 μL of template. After initial heating at 42°C for 60 min, the amplification was done for 40 cycles of 95°C for 60 s, 56°C for 60 s, 72°C for 30 s, followed by final extension of 7 min. The amplicon was 670 to 680 bp. The electrophoresis gel for the PCR of FVC is shown in Figure 2.
Figure 2.
Agarose gel electrophoresis of feline calicivirus (FCV) amplification products (around 673 bp, arrowhead) on feline upper respiratory tract samples using primers and conditions described in article (with modification). Legend: M-1 kb Plus DNA ladder (Invitrogen); Lane 1 — FCV positive control; Lane 2 — FCV negative control; Lane 3 — FCV positive sample tested by virus isolation (VI) and identified by polymerase chain reaction (PCR); and Lane 4 — FCV negative sample tested by VI and checked by PCR; and Lane 5 — FCV negative sample tested directly by PCR. A sample (Lane 3 to 5) represents pool of fluid from the following swabs: nasal, ocular and oropharyngeal. Virus isolation consists of growing the virus in cell culture using Crandell feline kidney (CRFK) cell line. For VI testing (Lane 3 and Lane 4), the specific cell culture fluid of inoculated sample was tested for FCV PCR.
Chlamydia felis (former name feline Chlamydia psittaci and Chlamydophila felis)
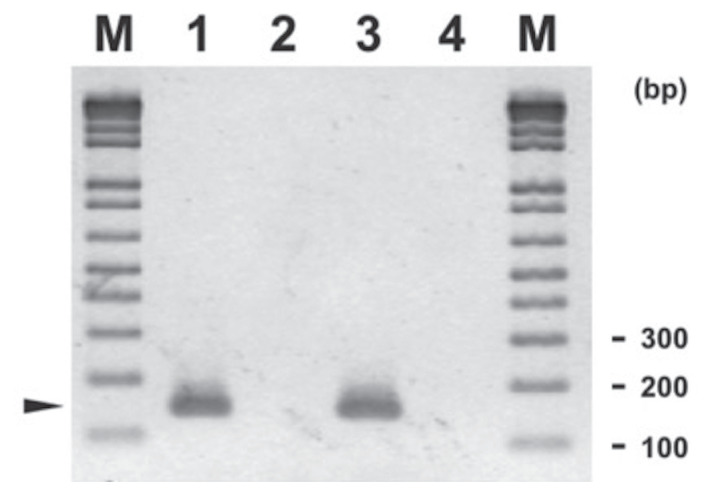
The C. felis PCR used the protocol of Collin (14) that was adapted in the RDVS laboratory at AVC with minor modifications. The PCR consists of 50-μL reaction of 5 μL template, 2.5 μM MgCl2, 200 μM each of dNTP Blend, 0.4 μM each of forward and reverse primers, 2.5 U of AmpliTaq Gold DNA Polymerase (Applied Biosystems), and an appropriate amount of PCR buffer and RNAse free water. The amplification was done with an initial denaturation at 94°C for 5 min, followed by 40 cycles of 94°C for 30 s, 59°C for 30 s, and 72°C for 30 s, and final extension of 72°C for 7 min. The amplification yielded a 147 bp amplicon. The electrophoresis gel for Chlamydia PCR is shown in Figure 3.
Figure 3.
Agarose gel electrophoresis of Chlamydia felis amplification products (around 147 bp, arrowhead) on feline upper respiratory tract samples using primers and conditions described in article (with modification). Legend: M — 1 kb Plus DNA ladder (Invitrogen); Lane 1 — C. felis positive control; Lane 2 — C. felis negative control; Lane 3 — C. felis positive sample; and Lane 4 — C. felis negative sample. A sample represents pool of fluid from the following swabs: nasal, ocular, and oropharyngeal.
Analysis of PCR products
The PCR products were analyzed by Bio-Rad Electrophoresis System (Bio-Rad Laboratories, Hercules, California, USA) in 1.5% agarose gel at 80 V (8 channels) to 100 V (18 channels) for 50 min in TBE (10.8 g Tris base, 5.5 g boric acid, and 0.75 g of EDTA, disodium salt in 1 L of water) electrophoresis buffer. The products were visualized by staining using SYBR Safe (Invitrogen, Carlsbad, California, USA) in TBE buffer. The molecular weight standard uses a 1 kb Plus DNA Ladder (Invitrogen).
General PCR procedure
All the PCR tests included appropriate positive and negative extraction and amplification controls. For FCV, the positive control was reference isolate from ATCC (782-VR), while for FHV and C. felis, the positive controls were reference isolates from AVC (FHV15972 and C. felis 12470). The procedures were conducted in the Molecular Diagnostic Suite and RDVS Laboratory using the Standard Operating Procedure for molecular diagnostic testing. All PCR amplifications were done in GeneAmp 9700 Thermocycler (Applied Biosystems).
Virus isolation for FHV-1 and FCV
Virus isolation using cell culture was carried out on all samples. One to 3-day-old Crandell feline kidney, CRFK (ATCC CCL-94) cells grown in 12.5 cm2 Falcon tissue culture flasks (VWR, Wayne, Pennsylvania, USA) were used for virus isolation.
Each URTD swab sample was diluted 1:50 using Minimum Essential Medium or MEM (Sigma), centrifuged and supernatant was filtered in a 45-μM filter before 350 μL of filtrate was inoculated unto the CRFK monolayer. It was incubated at 37°C for 30 min before 3.0 mL of MEM with 3% Fetal Bovine Serum (Sigma) was added. The inoculated cell cultures were incubated further for 10 d at 37°C and were monitored daily for presence of cytopathogenic effect (CPE). FHV-1 PCR and FCV PCR were carried out on all samples that showed CPE using similar extraction and amplification protocol as described for URTD swab samples.
Bacterial culture
Bacterial culture of oropharyngeal swab samples was done at the AVC Diagnostic Services Bacteriology Laboratory using standard microbiological procedures (15,16). Briefly, samples were plated on Columbia agar with 5% sheep blood and MacConkey agar and incubated aerobically at 35°C. Cultures were examined at 24- and 48-hour incubation for typical B. bronchiseptica colonies. Suspect B. bronchiseptica colonies were identified by matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) with BioTyper identification software (Microflex LT and MALDI BioTyper RTC 3.0 Software; Bruker Daltonics, Billerica, Massachusetts, USA) and by API 20 NE test strips (BioMérieux, St. Laurent, Quebec).
Antimicrobial susceptibility testing of B. bronchiseptica isolates was done using the disk diffusion assay, following the CLSI standard for other non-fermentative Gram-negative bacilli (17). In brief, a standardized bacterial suspension was inoculated onto Mueller-Hinton agar plates and paper disks impregnated with antimicrobial were placed on the surface of the agar. After incubation, the diameter of the zones of inhibition around the disk was measured and interpreted as susceptible or resistant. Interpretative criteria for non-fermentative Gram-negative bacilli isolated from humans and animals were applied, as no testing standards have been developed for B. bronchiseptica isolated from cats (17,18).
Statistical analyses
Summary statistics included prevalence values for those pathogens associated with feline URTD; binomial exact estimates were used to determine the 95% confidence intervals (CI). McNemar’s test was used to assess the level of agreement between PCR and VI methods for both FHV-1 and FCV.
Risk factor analyses were restricted to only those pathogens with at least 20% prevalence to ensure adequate sample sizes (minimum of 8 positive cats). Logistical regression models were used with the pathogen as the outcome of interest and the following risk factors were evaluated: season (summer, autumn, winter, and spring), weight (kg), neuter status (neutered versus intact), age (immature, adult, or geriatric), and body condition score (5-point scale with 1/2 point increments). Additionally, the following clinical signs were included as potential risk factors (dichotomous predictors; present versus absent): nasal discharge, ocular discharge, oral ulcers, sneezing, and coughing. As part of the model-building process, all unconditional associations with P < 0.2 were considered for the multivariable model and the final logistical regression model was evaluated with the Hosmer-Lemeshow goodness-of-fit test (19); Bonferroni adjustments were made for all pairwise comparisons.
Preliminary sample size calculations revealed that the inclusion of 96 cats in this study would achieve a precision of 10% prevalence, 95% of the time, with an 80% power to detect a real effect. All statistical analyses were done in Stata v15 (StataCorp, College Station, Texas, USA), and significance was set at P < 0.05.
Results
In total, samples were obtained from 82 cats with one or more clinical signs consistent with URTD. Conventional PCR and VI were carried out on samples pooled from conjunctival, nasal, and oropharyngeal swabs and bacterial culture and sensitivity was done on oropharyngeal swabs, collected from all of the 82 cats.
The apparent prevalence of FHV-1, FCV, and C. felis using PCR was 29.3% (95% CI = 20.3, 40.2%), 0% (95% CI = 0, 4.4%; using the binomial exact estimate), and 7.3% (95% CI = 3.2, 15.6%), respectively. In comparison, the apparent prevalence of FHV-1 and FCV using VI was 21.9% (95% CI = 14.2, 32.4%) and 4.9% (95% CI = 1.8, 12.5%), respectively. Lastly, the apparent prevalence of B. bronchiseptica in our study population by aerobic bacterial culture was 4.9% (95% CI = 1.8, 12.5%). This is shown in Table I.
Table I.
Number of positives (apparent prevalence, with 95% confidence interval using the binomial exact estimate) of etiologic agents from samples collected from 82 cats at Prince Edward Island Humane Society from August 2013 to November 2014.
| Etiologic agent | PCR | VI | Aerobic culture |
|---|---|---|---|
| FHV-1 | 24 (29.3%, 19.7% to 0.4%) | 18 (21.9%, 13.6% to 32.5%) | N/A |
| FCV | 0 (0.0%, 0% to 4.4%) | 4 (4.9%, 1.3% to 12.0%) | N/A |
| C. felis | 6 (7.3%, 2.7% to 15.2%) | N/A | N/A |
| B. bronchiseptica | N/A | N/A | 4 (4.9%, 1.3% to 12.0%) |
PCR — polymerase chain reaction; VI — virus isolation; FHV-1 — feline herpesvirus; FCV — feline calicivirus; N/A — not applicable.
The McNemar’s test for agreement between PCR and VI results demonstrated significant differences (asymmetry) between these 2 methods, with the PCR method identifying more cats with FHV-1 than the virus isolation method (P = 0.014), while the virus isolation method identified more samples with FCV than the PCR method (P = 0.0455). In fact, there were no positive samples for FCV using PCR methods.
Multiple infections in a single cat were uncommon, occurring in only 3 of the 82 cats (3.7%). All the co-infections that did occur, however, were with FHV-1 and B. bronchiseptica, with a prevalence of 3.7%. Interestingly, only a single cat was positive for B. bronchiseptica alone and not also positive for FHV-1.
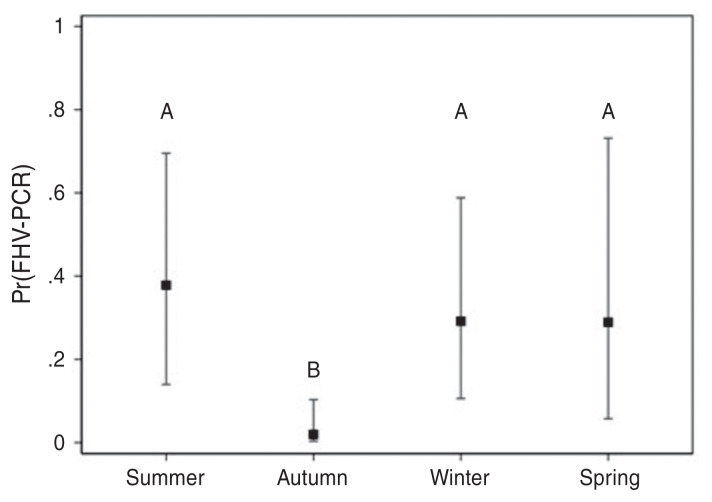
Risk factor analyses were only carried out for FHV, since it was the only pathogen related to feline URTD with prevalence greater than 20%. Both time of year (season) and clinical signs were associated with changes in prevalence of FHV-1 in this population (Figure 4). For season, the fewest number of detections occurred in autumn compared to spring, summer, and winter (Bonferroni-adjusted comparisons, P < 0.05).
Figure 4.
Estimated apparent prevalence of feline herpesvirus-1 (FHV-1) at PEI Humane Society across seasons from a logistical regression model using 82 cats with clinical signs related to upper respiratory tract disease from August 2013 to November 2014. Error bars represent the 95% confidence intervals and letters are assigned to significantly different Bonferroni-adjusted groups (P < 0.05).
With respect to clinical signs, a cough was significantly associated with the presence of FHV-1 (P = 0.033), with the odds of coughing cats having FHV-1 being 30 times greater than for non-coughing cats. Similarly, the presence of nasal discharge increased the odds of FHV-1 infection (P = 0.017), with cats with nasal discharge being 5.1 times more likely to have FHV-1 than cats without nasal discharge.
Lastly, all cultured B. bronchiseptica isolates were susceptible to several antibiotics, including amoxicillin-clavulanic acid, doxycycline, gentamicin, and marbofloxacin. All of these cultured isolates were resistant to cefovecin, ampicillin, penicillin, and cephalexin.
Discussion
This is the first epidemiological study of feline URTD in eastern Canada, specifically in Prince Edward Island. This study was conducted with the primary objective of determining the prevalence of FHV-1, FCV, C. felis, and B. bronchiseptica in a population of shelter cats with clinical signs related to URTD.
Feline herpesvirus-1 (FHV-1) is a double-stranded, enveloped DNA virus that is an important cause of rhinotracheitis in cats. It is not zoonotic and is not known to cause disease in non-felid species (6,20). The virus replicates in the upper respiratory tract, ocular epithelium, and neurons (20). After infection, lytic proliferation occurs with the virus following sensory axons to reach neuron cell bodies. It is shed through nasal, oral, and ocular secretions and, while it can remain in the environment for several days, direct exposure is thought to be the most important route of infection (20). Cats generally recover from acute infections within 2 to 3 wk, but approximately 80% of cats with FHV-1 remain infected for the rest of their lives and can experience intermittent viral reactivation (6,21), often during times of stress or immunosuppression. Some cats will also develop chronic ocular disease, including corneal ulcers and stromal keratitis (6).
Feline calicivirus (FCV) is a single-stranded, non-enveloped RNA virus that is not zoonotic and is widespread in feline populations worldwide (6). Cats with FCV will shed continuously, rather than the intermittent shedding that is seen in cats with FHV-1. As with FHV-1, however, cats may remain latently infected for life (7). The virus is shed in bodily secretions and can be very stable in the environment, with the ability to persist for a month (22). Naive and immunosuppressed cats often become ill, with kittens being the most severely affected (6). The disease is characterized by oral ulcerations, sneezing, nasal discharge, and less commonly, viral pneumonia and lameness (6). Occasionally, a mutated form of FCV can occur that is highly virulent and can cause much more severe, systemic disease (22,23). Clinical manifestations of virulent systemic-FCV (VS-FCV) are the result of profound vasculitis and can include: peripheral edema; ulceration of the skin and mucosal surfaces; hair loss; and necrosis of the ears, toes, and claws (22,23). Mortality from VS-FCV is high and outbreaks tend to be sporadic (22–24). Death often occurs from severe vasculitis, hepatocellular necrosis, disseminated intravascular coagulation, or other systemic complications (6,23,24).
Chlamydia felis is an obligate, intracellular, Gram-negative bacterium that does not survive for any length of time outside the host (7,25). The clinical signs caused by this bacterium consist primarily of conjunctivitis and possibly mild respiratory signs (6,25). The conjunctivitis can be marked with hyperemia, ocular discharge, blepharospasm, and chemosis (6,25). The bacterium is shed in ocular secretions and requires close contact between animals for transmission (7,9). It is rare to find C. felis in healthy cats, compared to FCV and FHV-1, which are commonly identified in animals without clinical signs (6,8).
Bordetella bronchiseptica is a Gram-negative, coccobacillus bacterium that can infect many species, although it rarely causes disease in humans (7,26). It is shed in oral and nasal secretions, and it is thought that disease transmission occurs through direct contact with infected animals, as well as indirectly via contaminated surfaces (6,26). B. bronchiseptica colonizes the epithelium of the respiratory tract, where it may or may not cause clinical disease and can be associated with increased coughing (7,26). While it is able to induce respiratory disease, it is thought that this is more severe when infection occurs concurrently with other pathogens (6,26).
The reported prevalence of FHV-1 is extremely variable, ranging from 20% to 50% (2,5,6,10,20,27). Prevalence of FCV is similarly variable, from 10% to 50% (5,6,10,22,27). Chlamydia felis has been reported to have a prevalence of less than 10% (2,5,6,10,25,27,28). Bordetella bronchiseptica, which was recently implicated as having a significant role in URTD, has a reported prevalence of less than 15% (2,5,6,10,26,27), although the seroprevalence in some studies has been found to be much higher (10).
In our study, FHV-1 was the most prevalent organism detected both by PCR (29.3%) and VI (21.9%), followed by C. felis by PCR (7.3%) and B. bronchiseptica (4.9%) by aerobic bacterial culture. Feline calicivirus (FCV) was the pathogen with the lowest prevalence when using PCR for detection (0.0%), although it was equal in prevalence to B. bronchiseptica when using VI (4.9%).
While the prevalence of FHV-1, C. felis, and B. bronchiseptica in this population was similar to that previously reported elsewhere, the prevalence of FCV (0.0% by PCR and 4.9% by VI) was lower than what has been described in previous studies of shelter populations (10% to 50%) (5,10,22,27,28). This may be due to a true low prevalence of this pathogen in this region and is consistent with the fact that oral ulcerations were not identified in any of the cats. Another possible explanation is that sample collection occurred before or after viral shedding. This is considered unlikely, however, as FCV is typically shed continuously during infection, predominantly via oral, ocular, and nasal secretions (6), and all of these sites were included in our sampling. Additionally, viremia occurs only 3 to 4 d after infection before the onset of clinical signs, with most cats continuing to shed for weeks after clinical recovery and in some cases, for life (6).
Asymmetry was identified between PCR and virus isolation for both FHV-1 and FCV. In the case of FHV-1, PCR identified more cats, with a prevalence of 29.3% compared to 21.9% with virus isolation. This finding can be explained by the fact that PCR can detect whole virus, as well as fragments of viral DNA, while virus isolation detects only whole, viable virus. While this can result in PCR seeming to be more sensitive than virus isolation, virus isolation may better correspond to active infection. Conversely, virus isolation identified more cats with FCV in this study, with a prevalence of 4.9%, while PCR identified no cats with FCV. A possible explanation for this finding is that the PCR that was used to identify FCV was insensitive, thus highlighting a benefit of virus isolation when evaluating patients for FCV, as virus isolation in this case was more sensitive.
Bordetella bronchiseptica is considered a primary pathogen in cats, as clinical infection has been experimentally induced in pathogenfree cats. It is often thought of as an opportunistic infection, however, with various factors predisposing cats to clinical disease, including concurrent infection with FHV-1 and/or FCV, as well as environmental factors such as overcrowding (26). This appeared to be a consistent finding in our population, with all but 1 cat that cultured positive for B. bronchiseptica and also being identified as positive for FHV-1. This further supports that, while B. bronchiseptica has the potential to be a primary pathogen, it is more likely to contribute to URTD as a secondary or opportunistic infection in the clinical setting.
Only a single cat (1.2%) in our study was positive for FIV, with none (0%) being positive for FeLV. In 2 recent studies evaluating prevalence of both FIV and FeLV in shelter populations in Australia and the United Kingdom, the reported prevalence of FIV was considerably higher, ranging from 6.0% to 15.0% and 3.0% to 11.4%, respectively (29,30). Meanwhile the prevalence of FeLV was similar to that found in our study, i.e., 1.0% to 4.0% and 0.0% to 3.0%, respectively (29,30). Possible explanations for the lower prevalence of FIV in our study include several factors such as the age and sex of the population, with FIV being more common in older male cats (29–31), as well as the fact that some animals may have been surrendered or owned strays rather than feral cats.
The low prevalence of bacterial pathogens in this population provides evidence that empirical use of antibiotics in cats displaying signs of feline URTD is not definitively indicated. In some shelter populations, any cat with URTD is automatically placed on antimicrobial therapy, such as doxycycline. While doxycycline is often an appropriate antibiotic choice for C. felis and B. bronchiseptica, daily and twice daily dosing can be challenging in a busy shelter environment. While more attractive options, such as the long-acting, injectable, third-generation cephalosporin, cefovecin (Convenia), may seem ideal for a shelter environment, this antibiotic has previously been shown to be less efficacious than amoxicillin-clavulanic acid or doxycycline (3). This was consistent with our study, in which we found all B. bronchiseptica isolates recovered were susceptible to doxycycline, amoxicillin-clavulanic acid, and marbofloxacin, but resistant to cefovecin, cephalexin, and clindamycin. These 3 drugs are inherently ineffective against B. bronchiseptica (32). Thus, while we cannot support the use of antimicrobial therapy in all cats displaying clinical signs of URTD, if antibiotics are to be used, then doxycycline or amoxicillin-clavulanic acid remain reasonable first-line choices.
The most perplexing finding in this study was that there were 47 cats (57.3%) with clinical signs consistent with URTD in which none of the previously mentioned pathogens was identified. There are several possible explanations for this, specifically, potential issues with the diagnostic sensitivity, i.e., intermittent shedding of pathogens, inadequate sample collection or handling, or the presence of a pathogen(s) other than those we tested for, or that perhaps the clinical signs typically associated with URTD are not specific to the disease. In particular, both Mycoplasma spp. and influenza virus can cause clinical signs of URTD and conjunctivitis in cats (6). Mycoplasma spp. are Gram-negative pleomorphic bacteria that are considered commensal organisms of the feline upper respiratory tract and are known to be difficult to culture and identify. There is evidence, however, that some species may play a primary or secondary role in respiratory disease and conjunctivitis (6,33–35). Similarly, while influenza virus is considered rare in cats, with no well-adapted strain established in feline populations, there have been reports of natural infection in cats with H5N1 and H1N1 (6). Infected cats can have a range of clinical signs that can include increased respiratory effort, nasal discharge, and/or conjunctivitis (6).
This was the first study in eastern Canada, and more specifically, on Prince Edward Island, to evaluate the prevalence of several infectious agents implicated in feline URTD in a shelter environment. Given the number of cats with documented clinical signs of URTD in which an infectious agent was not identified, future studies may benefit from evaluating samples for Mycoplasma spp. and influenza virus, as their role as primary pathogens remains unclear. Additionally, more novel diagnostic testing methods, such as multiplex PCR assays, may provide a more cost-effective means of testing for and identifying several possible pathogens in cats with URTD from a single sample. Further study is needed, however, in order to assess the sensitivity and specificity of this method.
Acknowledgments
The authors are grateful to the Atlantic Veterinary College Companion Animal Trust Fund for financial support and the PEI Humane Society for assistance in sample collection and use of their facilities. Thanks also to Jan Giles, Lorraine Lund, Matthew Saab, and Maria Vasquez of the Atlantic Veterinary College Diagnostic Services Bacteriology Laboratory, and Robyn MacPhee, Pamela Maloney, and Tina Polstra of the Regional Diagnostic Virology Services laboratory for their assistance with sample processing and testing.
References
- 1.Gourkow N, Lawson JH, Hamon SC, Phillips JC. Descriptive epidemiology of upper respiratory disease and associated risk factors in cats in an animal shelter in coastal western Canada. Can Vet J. 2013;54:132–138. [PMC free article] [PubMed] [Google Scholar]
- 2.Veir JK, Ruch-Gallie R, Spindel ME, Lappin MR. Prevalence of selected infectious organisms and comparison of two anatomic sampling sites in shelter cats with upper respiratory tract disease. J Feline Med Surg. 2008;10:551–557. doi: 10.1016/j.jfms.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litster AL, Wu CC, Constable PD. Comparison of the efficacy of amoxicillin-clavulanic acid, cefovecin, and doxycycline in the treatment of upper respiratory tract disease in cats housed in an animal shelter. J Am Vet Med Assoc. 2012;241:218–226. doi: 10.2460/javma.241.2.218. [DOI] [PubMed] [Google Scholar]
- 4.Burns RE, Wagner DC, Leutenegger CM, Pesavento PA. Histologic and molecular correlation in shelter cats with acute upper respiratory infection. J Clin Microbiol. 2011;49:2454–2460. doi: 10.1128/JCM.00187-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bannasch MJ, Foley JE. Epidemiologic evaluation of multiple respiratory pathogens in cats in animal shelters. J Feline Med Surg. 2005;7:109–119. doi: 10.1016/j.jfms.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohn LA. Feline respiratory disease complex. Vet Clin North Am Small Anim Pract. 2011;41:1273–1289. doi: 10.1016/j.cvsm.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Binns SH, Dawson S, Speakman AJ, et al. A study of feline upper respiratory tract disease with reference to prevalence and risk factors for infection with feline calicivirus and feline herpesvirus. J Feline Med Surg. 2000;2:123–133. doi: 10.1053/jfms.2000.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holst BS, Hanås S, Berndtsson LT, et al. Infectious causes for feline upper respiratory tract disease — A case-control study. J Feline Med Surg. 2010;12:783–789. doi: 10.1016/j.jfms.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sykes JE, Anderson GA, Studdert VP, Browning GF. Prevalence of feline Chlamydia psittaci and feline herpesvirus 1 in cats with upper respiratory tract disease. J Vet Intern Med. 1999;13:153–162. doi: 10.1892/0891-6640(1999)013<0153:pofpaf>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Helps CR, Lait P, Damhuis A, et al. Factors associated with upper respiratory tract disease caused by feline herpesvirus, feline calicivirus, Chlamydophila felis and Bordetella bronchiseptica in cats: Experience from 218 European catteries. Vet Rec. 2005;156:669–673. doi: 10.1136/vr.156.21.669. [DOI] [PubMed] [Google Scholar]
- 11.Sykes JE, Allen JL, Studdert VP, Browning GF. Detection of feline calicivirus, feline herpesvirus 1 and Chlamydia psittaci mucosal swabs by multiplex RT-PCR/PCR. Vet Microbiol. 2001;81:95–108. doi: 10.1016/s0378-1135(01)00340-6. [DOI] [PubMed] [Google Scholar]
- 12.Sykes JE, Browning GF, Anderson G, Studdert VP, Smith HV. Differential sensitivity of culture and the polymerase chain reaction for detection of feline herpesvirus 1 in vaccinated and unvaccinated cats. Arch Virol. 1997;142:65–74. doi: 10.1007/s007050050059. [DOI] [PubMed] [Google Scholar]
- 13.Sykes JE, Studdert VP, Browning GF. Detection and strain differentiation of feline calicivirus in conjunctival swabs by RT-PCR of the hypervariable region of the capsid protein gene. Arch Virol. 1998;143:1321–1334. doi: 10.1007/s007050050378. [DOI] [PubMed] [Google Scholar]
- 14.Collin JK. Chlamydia psittaci PCR assay for avian, bovine, feline and ovine. In: Lauerman LH, editor. Nucleic Acid Amplification Assays for Diagnosis of Animal Diseases. Madison, Wisconsin: American Association of Veterinary Laboratory Diagnosticians; 1998. p. 10. [Google Scholar]
- 15.Baron EJ, Thomson RB., Jr . Specimen collection, transport, and processing: Bacteriology. In: Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW, editors. Manual of Clinical Microbiology. 10th ed. Washington DC: ASM Press; 2011. pp. 228–271. [Google Scholar]
- 16.Songer J, Post K. Veterinary Microbiology. 1st ed. St. Louis, Missouri: Elsevier Saunders; 2005. pp. 10–20. [Google Scholar]
- 17.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard. 4th ed. Wayne, Pennsylvania: CLSI; 2013. [Google Scholar]
- 18.Kilgore PE, Coenye T. Bordetella and related genera. In: Carroll KC, Pfaller MA, Landry ML, et al., editors. Manual of Clinical Microbiology. 12th ed. Washington DC: ASM Press; 2019. pp. 858–870. [Google Scholar]
- 19.Dohoo IR, Martin SW, Stryhn H, editors. Veterinary Epidemiologic Research. 2nd ed. Charlottetown, Prince Edward Island: VER Inc; 2009. p. 413. [Google Scholar]
- 20.Thiry E, Addie D, Belák S, et al. Feline herpesvirus infection. ABCD guidelines on prevention and management. J Feline Med Surg. 2009;11:547–555. doi: 10.1016/j.jfms.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drazenovich TL, Fascetti AJ, Westermeyer HD, et al. Effects of dietary lysine supplementation on upper respiratory and ocular disease and detection of infectious organisms in cats within an animal shelter. Am J Vet Res. 2009;70:1391–1400. doi: 10.2460/ajvr.70.11.1391. [DOI] [PubMed] [Google Scholar]
- 22.Radford AD, Addie D, Belák S, et al. Feline calicivirus infection. ABCD guidelines on prevention and management. J Feline Med Surg. 2009;11:556–564. doi: 10.1016/j.jfms.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurley KE, Pesavento PA, Pedersen NC, Poland AM, Wilson E, Foley JE. An outbreak of virulent systemic feline calicivirus disease. J Am Vet Med Assoc. 2004;224:241–249. doi: 10.2460/javma.2004.224.241. [DOI] [PubMed] [Google Scholar]
- 24.Hurley KF, Sykes JE. Update on feline calicivirus: New trends. Vet Clin North Am Small Anim Pract. 2003;33:759–772. doi: 10.1016/s0195-5616(03)00025-1. [DOI] [PubMed] [Google Scholar]
- 25.Gruffydd-Jones T, Addie D, Belák S, et al. Chlamydophila felis infection. ABCD guidelines on prevention and management. J Feline Med Surg. 2009;11:605–609. doi: 10.1016/j.jfms.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egberink H, Addie D, Belák S, et al. Bordetella bronchiseptica infection in cats. ABCD guidelines on prevention and management. J Feline Med Surg. 2009;11:610–614. doi: 10.1016/j.jfms.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Martino B, Di Francesco CE, Meridiani I, Marsilio F. Etiological investigation of multiple respiratory infections in cats. New Microbiol. 2007;30:455–461. [PubMed] [Google Scholar]
- 28.Kang BT, Park HM. Prevalence of feline herpesvirus 1, feline calicivirus and Chlamydophila felis in clinically normal cats at a Korean animal shelter. J Vet Sci. 2008;9:207–209. doi: 10.4142/jvs.2008.9.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westman ME, Paul A, Malik R, et al. Seroprevalence of feline immunodeficiency virus and feline leukaemia virus in Australia: Risk factors for infection and geographical influences (2011–2013) JFMS Open Rep. 2016;2:1–11. doi: 10.1177/2055116916646388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stavisky J, Dean RS, Molloy MH. Prevalence of and risk factors for FIV and FeLV infection in two shelters in the United Kingdom (2011–2012) Vet Rec. 2017;181:451. doi: 10.1136/vr.103857. [DOI] [PubMed] [Google Scholar]
- 31.Hosie MJ, Addie D, Belák S, et al. Feline immunodeficiency: ABCD guidelines on prevention and management. J Feline Med Surg. 2009;11:575–584. doi: 10.1016/j.jfms.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lappin MR, Blondeau J, Boothe D, et al. Antimicrobial use guidelines for treatment of respiratory tract disease in dogs and cats: Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases. J Vet Intern Med. 2017;31:279–294. doi: 10.1111/jvim.14627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chandler JC, Lappin MR. Mycoplasmal respiratory infections in small animals: 17 cases (1988–1999) J Am Anim Hosp Assoc. 2002;38:111–119. doi: 10.5326/0380111. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez M, Manzanilla EG, Lioret A, Leon M, Thibault JC. Prevalence of feline herpesvirus-1, feline calicivirus, Chlamydophila felis and Mycoplasma felis DNA and associated risk factors in cats in Spain with upper respiratory tract disease, conjunctivitis and/or gingivostomatitis. J Feline Med Surg. 2017;19:461–469. doi: 10.1177/1098612X16634387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Low HC, Powell CC, Veir JK, Hawley JR, Lappin MR. Prevalence of feline herpesvirus 1, Chlamydophila felis, and Mycoplasma spp. DNA in conjunctival cells collected from cats with and without conjunctivitis. Am J Vet Res. 2007;68:643–648. doi: 10.2460/ajvr.68.6.643. [DOI] [PubMed] [Google Scholar]