Abstract
While serum amyloid A (SAA) has been investigated as a potential marker for septic arthritis in horses, no study has reported on whether SAA can be used to detect eradication of joint infection. Therefore, the objective of this study was to investigate whether the eradication of joint infection in experimentally induced septic arthritis in horses can be detected using serum and synovial fluid SAA. A total of 17 horses were randomly assigned to 3 groups. A middle carpal joint of each horse was injected with saline (control group, n = 3), lipopolysaccharide (LPS) (nonseptic synovitis group, n = 6), or Escherichia coli (septic arthritis group, n = 8) on day 0. Starting on day 1, horses underwent treatment for septic arthritis. Sequential samples of serum and synovial fluid were collected, and quantification of SAA was carried out. Concentrations of serum and synovial fluid SAA were compared among groups and time points. A concurrent study was conducted and determined that infection was eradicated on day 4 in this experimental model of septic arthritis. Concentrations of serum and synovial fluid SAA rapidly increased after inoculation of E. coli and were highest on day 3 and day 4, respectively. Thereafter, both serum and synovial fluid SAA decreased with eradication of joint infection, although they remained significantly increased from baseline until day 9 and day 10, respectively. Serum and synovial fluid SAA did not increase in the control or nonseptic synovitis group. These findings suggest that serial measurements rather than a single measurement of SAA are required to determine eradication of infection from septic arthritis in horses.
Résumé
Bien que l’amyloïde sérique (SAA) fut étudiée comme marqueur potentiel pour l’arthrite septique chez les chevaux, aucune étude n’a rapporté si SAA peut être utilisée pour détecter l’élimination d’une infection articulaire. Ainsi, l’objectif de la présente étude était d’examiner si l’élimination d’une infection articulaire lors d’arthrite septique induite expérimentalement chez les chevaux peut être détectée en utilisant la SAA du sérum et du liquide synovial. Un total de 17 chevaux fut réparti de manière aléatoire en trois groupes. Une articulation carpienne médiale de chaque cheval fut injectée avec de la saline (groupe témoin, n = 3), du lipopolysaccharide (LPS) (groupe synovite non-septique, n = 6) ou Escherichia coli (groupe arthrite septique, n = 8) au jour 0. En débutant au jour 1, les chevaux furent soumis à un traitement pour arthrite septique. Des échantillons séquentiels de sérum et de liquide synovial furent prélevés et la quantification de SAA effectuée. Les concentrations de SAA dans le sérum et le liquide synovial furent comparées parmi les groupes et à différents temps. Une étude concomitante était menée et a déterminé que l’infection était éliminée au jour 4 dans ce modèle expérimental d’arthrite septique. Les concentrations de SAA dans le sérum et le liquide synovial ont rapidement augmenté après l’inoculation d’E. coli et étaient maximales au jour 3 et au jour 4, respectivement. Par la suite, les concentrations de SAA du sérum et du liquide synovial ont diminué avec l’élimination de l’infection articulaire, bien qu’elles soient demeurées augmentées significativement par rapport au seuil de base jusqu’au jour 9 et jour 10, respectivement. Les concentrations de SAA du sérum et du liquide synovial n’ont pas augmenté dans les groupes témoin et synovite non-septique. Ces résultats suggèrent que des mesures en série plutôt qu’une mesure unique de SAA sont requises pour déterminer l’élimination de l’infection lors d’arthrite septique chez les chevaux.
(Traduit par Docteur Serge Messier)
Introduction
Septic arthritis is a severe arthropathy, commonly resulting from contamination of a joint after intra-articular injection or direct trauma in adult horses and from hematogenous spread of bacteria in neonatal foals (1). Septic arthritis can be life-threatening, with reported short-term survival rates of 45% to 84% in foals (1–3) and 84% to 90% in adult horses (4–5).
While the definitive diagnosis of septic arthritis is made by detection of bacteria in synovial fluid, identification of bacteria on Gram stain is successful in only 24% of cases (1). Moreover, isolation of bacteria from synovial fluid is successful in only 32% to 52% of cases when enrichment media are not used (6,7) and in 74% of cases using enrichment media (1). As a result of these low detection rates, clinical signs and cytological examination of synovial fluid remain the primary modalities used when diagnosing septic arthritis (8). Synovial fluid cytology is suboptimal; however, for monitoring the success of treatment and detecting eradication of infection in septic arthritis. We previously demonstrated a persistent increase in total protein (TP) and percentage of neutrophils long after the eradication of joint infection in an experimental model of septic arthritis (9). In addition, commonly reported treatments for septic arthritis, such as arthroscopic lavage, through-and-through joint lavage, and intra-articular administration of antibiotics, have been shown to increase values for common synovial inflammatory markers in synovial fluid (10–12). As a result, due to uncertainty about a point of eradication of joint infection, long-term use of antimicrobials has frequently been reported in clinical cases of septic arthritis (1–3).
Serum amyloid A is the major acute phase protein in horses, which is mainly synthesized by the liver non-specifically in response to inflammation and infection (13). Serum SAA peaks 36 to 48 h after an inflammatory or infectious insult and decreases quickly in response to treatment (14,15). Ludwig et al (16) demonstrated an increase in serum and synovial fluid SAA in an equine model of septic arthritis while SAA concentration remained within normal limits in an equine model of lipopolysaccharide (LPS)-induced synovitis. Unlike synovial fluid cytology, serum and synovial fluid SAA concentrations have been reported to be unaffected by the aforementioned treatment of septic arthritis (10–12). These features of SAA would make it an ideal biomarker not only for diagnosing septic arthritis, but also for monitoring its therapeutic success. To our knowledge, no study has investigated the use of SAA for determining eradication of infection during treatment of septic arthritis.
The objective of this study was to investigate temporal changes in concentrations of serum and synovial fluid SAA before and after eradication of infection in experimentally induced septic arthritis in horses and to determine if SAA could be used as a biomarker for eradication of infection. We hypothesized that serum and synovial fluid SAA concentrations would increase after septic arthritis was induced and decrease rapidly towards normal after infection was eradicated.
Materials and methods
Animals
A total of 17 adult horses, weighing 306 to 572 kg (median: 470 kg) and ranging from 2 to 20 y old (median: 7 y), were included in the study. All horses were healthy based on physical examinations and free of musculoskeletal disease in the carpi based on lameness examinations and 4 radiographic views (lateral-medial, dorsopalmar, dorsolateral-palmaromedial oblique, and dorsomedial-palmarolateral oblique) of the carpi. All procedures were approved by the University of Saskatchewan Animal Care and Use Committee and Animal Research Ethics Board (AUP #20140098).
Experimental design
To determine if SAA could be used as a biomarker for eradication of infection, we used 2 experimental models: a nonseptic synovitis model induced by LPS (9,16,17) and a septic arthritis model using experimental Escherichia coli (9,18). A randomly assigned middle carpal joint was injected with LPS or E. coli and the contralateral joint was used as internal control. Both joints were subjected to a standardized treatment protocol (Table I).
Table I.
Experimental design and treatment protocol.
| Control group (n = 3) | Nonseptic synovitis group (n = 6) | Septic arthritis group (n = 8) | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Injected joint | Non-injected joint | Injected joint | Non-injected joint | Injected joint | Non-injected joint | |
| Day 0 | Injected with saline | No injection | Injected with LPS | No injection | Injected with E. coli | No injection |
| Day 1 | Arthroscopic lavage + IA gentamicin | No treatment | Arthroscopic lavage + IA gentamicin | Arthroscopic lavage + IA gentamicin | Arthroscopic lavage + IA gentamicin | Arthroscopic lavage + IA gentamicin |
| Day 2 to 4 | IV RLP gentamicin | No treatment | IV RLP gentamicin | IV RLP gentamicin | IV RLP gentamicin | IV RLP gentamicin |
| Day 1 to 6 | All horses were administered IV penicillin, gentamicin, and phenylbutazone | |||||
| Day 7 to 21 | All horses were administered oral trimethoprim-sulfamethoxazole | |||||
LPS — lipopolysaccharide; IV — intravenous; IA — intra-articular; RLP — regional limb perfusion.
Horses were randomly assigned to 3 groups and a randomly selected middle carpal joint (injected joint) was then injected with either saline (control group, n = 3), LPS (nonseptic synovitis group, n = 6), or E. coli (septic arthritis group, n = 8), respectively. Contralateral joints (non-injected joints) were not injected and served as internal control. Synovial fluid was collected from the injected and non-injected joints immediately before the model induction on day 0, which served as a baseline value, and thereafter before initiation of treatment on day 1 and on days 4, 7, 10, 14, 21, and 28.
Blood samples were collected from a jugular vein each day from day 0 to day 10, and days 14, 21, and 28. Synovial fluid and blood samples were analyzed for SAA concentration. Cytology, bacterial culture, and polymerase chain reaction (PCR) for detection of E. coli were also carried out on synovial fluid samples and the results were reported in a previous study (9). All horses were treated with arthroscopic lavage of the middle carpal joints, systemic and local administration (intra-articular injection and intravenous regional limb perfusion) of antibiotics, and anti-inflammatory drugs. Both injected and non-injected joints received arthroscopic lavage and local administration of antimicrobials for the nonseptic synovitis and septic arthritis groups. In the control group, only the injected joints received the treatment in order to evaluate the effect of the treatment on concentrations of serum and synovial fluid SAA (Table I).
Induction of experimental models
Nonseptic synovitis and septic arthritis were induced as described in a previous report (9). Briefly, nonseptic synovitis was induced by 50 ng of LPS (E. coli O55:B5 strain; Sigma-Aldrich, St. Louis, Missouri, USA) diluted into 1 mL of sterile phosphate-buffered saline solution (PBS, pH 7.4), aliquoted, and stored at −20°C until used. Before induction of nonseptic synovitis, aliquots of LPS (50 ng/mL) were thawed for 30 min and stored on ice until injection.
Septic arthritis was induced in the assigned middle carpal joint using 1.0 × 108 colony-forming units (CFU) of E. coli. The strain used for inoculation was isolated from a case of naturally occurring septic arthritis in a horse referred to the Veterinary Medical Centre of University of Saskatchewan for treatment. The stock culture was divided into 50 μL aliquots of 1.0 × 1010 CFU/mL and stored at −80°C. Individual aliquots were used for preparation of each inoculum to avoid in-vitro passages, which could affect the virulence of the strain.
On day 0, horses were sedated with detomidine (Dormosedan; Pfizer Canada, Kirkland, Quebec), 0.015 mg/kg body weight (BW), intravenously (IV), and the selected joint was aseptically prepared. A 20-G, 3.8-cm needle attached to a 6-mL syringe was inserted into the middle carpal joint using a dorsolateral approach and a synovial fluid sample (4 mL) was collected. Upon collection of synovial fluid, the syringe was replaced with a 3-mL luer-lock syringe containing 1 mL of either saline, 50 ng LPS, or 1.0 × 108 CFU of E. coli and the solution was delivered into the middle carpal joint.
Experimental treatments
Experimental treatment is summarized in Table I. All horses underwent arthroscopic lavage 24 h post-induction (day 1). Sodium penicillin (Penicillin G, Sodium for injection, USP; Pharmaceutical Partners of Canada, Richmond Hill, Ontario), 22 000 IU/kg BW, IV, gentamicin sulfate (Gentocin; Intervet Canada, Kirkland, Quebec), 6.6 mg/kg BW, IV, and phenylbutazone (Phenylbutazone; Rafter 8 Products, Calgary, Alberta), 4.4 mg/kg BW, IV, were administered 30 min before surgery. Horses were premedicated with xylazine hydrochloride (Rompun; Bayer Health Care, Toronto, Ontario), 1 mg/kg BW, IV. Anesthesia was induced with ketamine hydrochloride (Vetalar, Bioniche, Bellevillle, Ontario), 2.2 mg/kg BW, IV, and diazepam (Diazepam, Sandoz Canada, Quebec), 0.1 mg/kg BW, IV, and maintained with isoflurane in oxygen. Horses were then positioned in dorsal recumbency with the front limbs suspended. The carpi (or carpus) were clipped, aseptically prepared, and draped for a routine carpal arthroscopic surgery. Arthroscopic surgery was carried out using the previously described approach to the middle carpal joint (19). After the initial articular examination, the middle carpal joint was copiously lavaged using 20 L of lactated Ringer’s solution. Skin incisions were closed using 2-0 monofilament polybutester in a simple interrupted suture pattern. In the nonseptic synovitis and septic arthritis groups, arthroscopic lavage was carried out in the non-injected joint first, followed by the injected joint to avoid contamination. In the control group, arthroscopic lavage was done only in the injected joint. When the surgical procedure was completed, the middle carpal joint was injected with 500 mg of gentamicin sulfate. A light sterile bandage was applied over the incisions before recovery. Horses were recovered unassisted in a padded recovery stall. Bandages were changed each time synovial fluid was collected and kept in place until suture removal on day 14. All horses were treated postoperatively with sodium penicillin (22 000 IU/kg BW, q6h, IV), gentamicin sulfate (6.6 mg/kg BW, q24h, IV), and phenylbutazone (2.2 mg/kg BW, q12h, IV) for 6 d, followed by trimethoprim-sulfamethoxazole (24 mg/kg BW, q12h orally) for an additional 14 d.
Intravenous regional limb perfusions were conducted on days 2, 3, and 4 (after synovial fluid collection). Horses were sedated with detomidine (0.01 mg/kg BW, IV) and butorphanol (0.01 mg/kg BW, IV). An 8-cm Esmarch tourniquet was applied 15 cm proximal to the accessory carpal bone with a gauze roll placed beneath the tourniquet over the cephalic vein. A 21-G butterfly infusion set (SURFLO Winged Infusion Set; Terumo Medical Products, Tokyo, Japan) was inserted into the cephalic vein distal to the tourniquet and 1 g (10 mL) of gentamicin was administered into the cephalic vein, followed by 50 mL sterile saline over 3 min. After the perfusate was administered, the butterfly infusion set was removed and a pressure bandage consisting of gauze and medical tape was placed over the injection site until the tourniquet was removed after 30 min.
SAA analysis
Synovial fluid samples were placed into an ethylenediaminetetraacetic acid (EDTA) tube and blood samples into a red-top serum tube. Blood samples were allowed to clot at ambient temperature. Both synovial fluid and serum samples were centrifuged at 1792 × g for 10 min at ambient temperature, following which the supernatant and serum were transferred into a microcentrifuge tube and stored at −80°C until analyzed for quantification of SAA concentration. Serum and synovial fluid SAA were quantified using a human SAA turbidimetric immunoassay (Eiken Chemical, Tokyo, Japan), previously validated for equine assays (20), on a chemistry analyzer (Cobas 6000 c501; F. Hoffmann-La Roche, Basel, Switzerland). Before quantification of SAA, 10 μL of hyaluronidase was added to 490 μL of synovial fluid to reduce viscosity. Automatic 1:10 dilution was carried out on the samples that were > 500 mg/L. The assay’s lower limit of quantification was 0.2 mg/L.
Clinical response to treatment
Physical examination (temperature, heart rate, and respiratory rate) and lameness evaluation were done immediately before and 4, 8, 12, 18, and 24 h after injection of LPS or E. coli and then at times of blood sample collection. Lameness was subjectively evaluated while horses were walking in a straight line and in a circle and was graded as sound, lame at the walk, or non-weight-bearing lame.
Determination of eradication of infection
In conjunction with this study, another study was carried out to investigate the use of synovial fluid cytology to determine eradication of infection in experimentally induced septic arthritis in horses (9). In that study, joint infection was eradicated by day 4 during treatment of septic arthritis, which was confirmed using bacterial culture as well as PCR (9). Serum and synovial fluid samples were collected from all horses in this previous study (9) and used in the current study for investigating temporal changes in SAA concentrations in horses with experimentally induced nonseptic synovitis and septic arthritis.
Statistical analysis
All data were analyzed using a commercial statistics software program (Stata 15; StataCorp, College Station, Texas, USA). Values of SAA below the lower limit of quantification (0.2 mg/L) of the assay were interpreted as “0” for statistical analysis.
Mixed model analysis of variance (ANOVA) with restricted maximum likelihood was carried out to compare concentrations of serum and synovial fluid SAA over time within each group, as well as to compare concentrations of serum SAA and synovial fluid SAA in the injected joints among groups at each time point. For comparison of SAA concentrations over time within each group, post hoc pairwise comparisons were carried out to compare baseline value with each time point, as well as to compare each time point with a preceding time point. Group, time, and their interaction were included as fixed effects and individual joint was included as a random effect. Mixed model ANOVA with restricted maximum likelihood was also done to compare synovial fluid SAA concentration between the injected and non-injected joints at each time point. Injection, time, and their interaction were included as fixed effects and joint was included as a random effect. For all models, first-order autoregression model was selected for covariance of residuals based on likelihood ratio as well as Akaike information criterion and Bayesian information criterion. Post-hoc pairwise comparisons were done with Bonferroni adjustment and P < 0.05 was considered significant.
Results
Nonseptic synovitis model
After intra-articular injection of LPS, all horses (n = 6) had inflammatory synovial fluid parameters similar to those seen in clinical cases of septic arthritis (NCC > 30 × 103 cells/μL, TP > 40 g/L, and %N > 80%) (21), with negative bacterial culture (reported in our previous study) (9), which confirmed successful induction of nonseptic synovitis. Heart and respiratory rates remained within normal limits (heart rate < 44 bpm, respiratory rate < 28 bpm) in all horses, although 2 horses developed a mild transient fever (1 had 38.6°C 8 h after injection and the other had 38.7°C 12 h after injection). Horses with nonseptic synovitis developed varying degrees of transient lameness, peaking at 4 h after induction: sound (n = 2), lame at a walk (n = 2), and non-weight-bearing lame (n = 2). All horses were sound at a walk by 18 h after induction of nonseptic synovitis and lameness was not observed in any horse thereafter.
Septic arthritis model
After intra-articular injection of E. coli, successful induction of septic arthritis was confirmed in all horses (n = 8) by clinical signs, synovial fluid cytology parameters consistent with septic arthritis, and a positive bacterial culture (9). All horses developed marked fever (median: 39.2°C, range: 38.5°C to 40.5°C), tachycardia (median: 60 bpm, range: 48 to 64 bpm), tachypnea (median: 88 bpm, range: 44 to 100 bpm), marked effusion of the middle carpal joint, and non-weight-bearing lameness, peaking 12 h after inoculation of bacteria. After arthroscopic lavage at 24 h, all horses became sound at a walk and vital parameters stayed within normal limits for the remainder of the study.
Serum SAA concentration
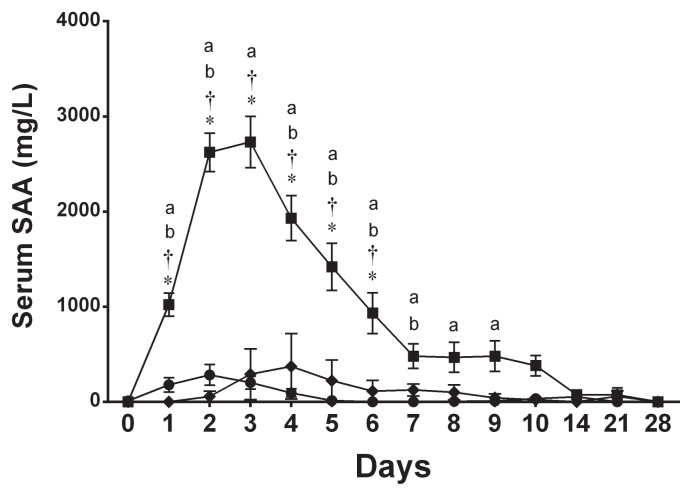
In the septic arthritis group, serum SAA concentration began increasing on day 1, peaked on day 3 (all P < 0.001), and remained significantly elevated until day 9 (P = 0.009) (Figure 1). When serum SAA concentration was compared to a preceding time point, it significantly increased from day 0 to day 1 and from day 1 to day 2 (both P < 0.001, Figure 1). Thereafter, a significant and gradual decrease in serum SAA concentration was observed each day from day 3 to day 7 (all P < 0.001; Figure 1). Serum SAA concentration in the septic arthritis group was significantly higher than in the control group and nonseptic synovitis group from day 1 to day 6 (all P < 0.001; Figure 1). There was no significant difference between the control and nonseptic synovitis groups at any time points. In the nonseptic synovitis group, while serum SAA response to intra-articular injection of LPS was variable on day 2 (median: 237 mg/L, range: 12 to 774 mg/L), there was no significant increase from baseline at any time points (Figure 1). Also, serum SAA concentration did not significantly increase from baseline at any time points in the control group (Figure 1). One horse showed a marked increase in serum SAA concentration on day 4 (1060 mg/L), however, while the other 2 horses had only a mild increase (0.6 mg/L and 61 mg/L), leading to a high standard error (Figure 1).
Figure 1.
Mean ± SE serum SAA concentration in the control group (◆), synovitis group (●), and septic arthritis group (■).
a Significant difference from baseline (day 0).
b Significant difference from a preceding time point.
* Significant difference from the control group.
† Significant difference from the synovitis group.
Synovial fluid SAA concentration
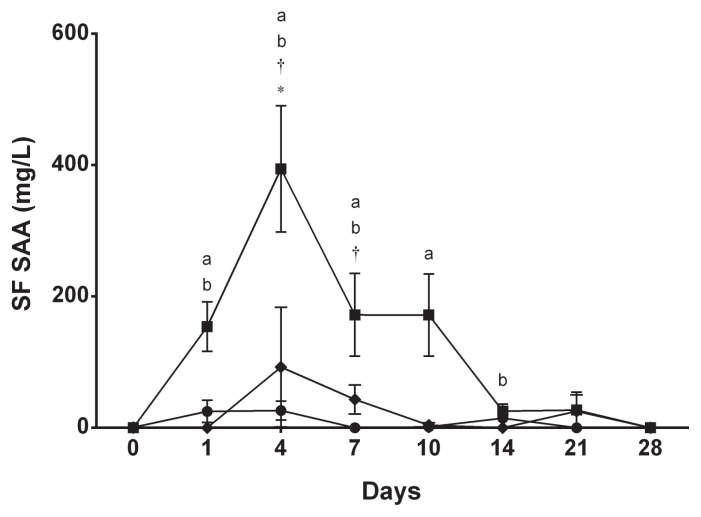
In the injected joint of horses in the septic arthritis group, synovial fluid SAA started increasing on day 1 (P = 0.03), reached its highest value on day 4 (P < 0.001), and remained elevated until day 10 (P = 0.007) (Figure 2). When compared between 2 consecutive time points, synovial fluid SAA concentration significantly increased from day 0 to day 1 (P = 0.03) and from day 1 to day 4 (P < 0.001) (Figure 2). Thereafter, synovial fluid SAA concentration decreased from day 4 to day 7 (P < 0.001), stayed at the same level until day 10 (P > 0.99), and decreased towards baseline value from day 10 to day 14 (P = 0.03) (Figure 2). Synovial fluid SAA concentration did not increase from baseline in the injected joint or the non-injected joint of the control and nonseptic synovitis groups (Figure 2). A marked increase in the injected joint was observed on day 4 (275 mg/L), however, in the same horse from the control group that showed a marked increase in serum SAA concentration on day 4. Concentrations of synovial fluid SAA in the injected joints were significantly higher in the septic arthritis group compared to the control group only on day 4 (P < 0.001) and compared to the nonseptic synovitis group from day 4 (P < 0.001) and day 7 (P = 0.04) (Figure 2).
Figure 2.
Mean ± SE synovial fluid (SF) SAA concentration in the injected joints in the control group (◆), synovitis group (●), and septic arthritis group (■).
a Significant difference from baseline (day 0).
b Significant difference from a preceding time point.
* Significant difference from the control group.
† Significant difference from the synovitis group.
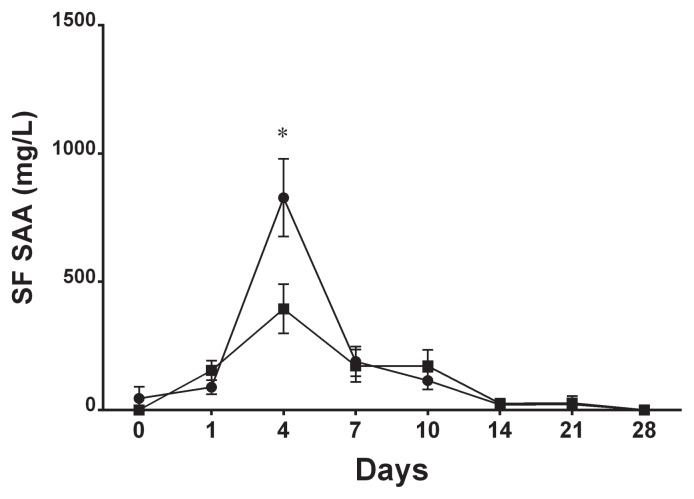
When the concentration of synovial fluid SAA was compared between the injected and non-injected joints within each group, there was no significant difference at any time point in the control and nonseptic synovitis groups. In the septic arthritis group, however, synovial fluid SAA concentration was approximately 2 times higher in the non-injected joint in all horses on day 4 (mean 828 mg/L versus 394 mg/L, P < 0.001; Figure 3).
Figure 3.
Mean ± SE synovial fluid SAA concentration in the injected joints (■) and the non-injected joints (●) in the septic arthritis group.
* Significant difference between joints.
SAA as a biomarker for eradication of infection
After intra-articular inoculation of E. coli, serum SAA increased significantly within 24 h, peaked on day 3, and started to decline thereafter (Figure 1). This decline corresponds to the eradication of infection, which was confirmed on day 4 in our previous study (9). Similarly, synovial fluid SAA in the injected joint increased on day 1 and peaked on day 4 with subsequent decline after eradication of infection (Figure 2). Although both serum SAA and synovial fluid SAA in the injected joint started decreasing after joint infection was eradicated, they remained significantly elevated from the baseline value until day 9 and day 10, respectively. Accordingly, a single measurement of concentrations of serum and synovial SAA was suboptimal to detect eradication of infection in our experimental model of septic arthritis in horses.
Discussion
In this study, concentrations of serum and synovial fluid SAA increased significantly and rapidly after intra-articular inoculation of E. coli but did not change significantly from baseline after intraarticular injection of LPS or saline. This systemic and local expression pattern of SAA observed in our experimental models of nonseptic synovitis and septic arthritis is consistent with a previous study (16) and supports the clinical use of SAA as a diagnostic marker for septic arthritis. However, both serum and synovial fluid were still significantly increased from baseline on day 9 and day 10, respectively, even though they started to decline with eradication of joint infection, which was determined to be at day 4 in our experimental model (9). Therefore, a single measurement of serum and synovial fluid SAA appears to be a suboptimal marker for eradication of infection. A gradual but significant and continuous decrease in concentrations of serum and synovial SAA after infection was eradicated (from day 4 to 10), however, suggests that serial measurements of SAA may be beneficial for monitoring of a positive response to treatment of horses affected by septic arthritis.
An unexpected finding in our experimental model of septic arthritis was a significant increase in synovial fluid SAA observed in the non-injected joints of all horses. Concentrations of SAA in the non-injected joints showed a similar trend of increase and decrease as in the injected joints, but with the non-injected joints showing higher magnitude on day 4. The pathophysiology of this unexpected finding is not clear at this stage. It was not considered to be the consequence of blood contamination during arthrocentesis. Although minimal to mild blood contamination was observed in some synovial fluid samples, it did not significantly affect cytological parameters (9) and was therefore not taken into account for estimating SAA concentration. In addition, increase in synovial fluid SAA was consistently observed along with increase in serum SAA concentration regardless of the degree of blood contamination. An alternative explanation for the high SAA concentration in the non-injected joints is that our treatment protocol induced an increase in synovial fluid SAA. We applied exactly the same treatment to the injected joints of the horses in the control group and to the non-injected joints of horses in the nonseptic synovitis group and did not observe a significant increase in SAA as a result of the treatment.
Another theory is that the treatment protocol, together with systemic inflammation caused by intra-articular inoculation of E. coli, increased the permeability of the synovial membranes in the non-injected joints, which resulted in leakage of SAA from plasma in horses with experimentally induced septic arthritis. Serum SAA concentrations were consistently higher than synovial SAA concentrations in all horses in our study. This finding is consistent with previous studies in experimentally induced septic arthritis (16), as well as in naturally occurring cases of septic arthritis, in which a moderate correlation was observed between serum and synovial fluid SAA concentrations (22). In addition, elevated protein levels were observed in the non-injected joints in our previous study (9), which supports leakage of serum SAA into the joints. Further research is warranted to explain the increase in synovial fluid SAA expression in the non-injected joints after induction of septic arthritis.
Based on clinical signs, synovial fluid analysis, and bacterial culture, septic arthritis was successfully induced in our study. In a pilot study, the authors used a lower dose of E. coli (7.5 × 106 CFU) based on a previous study (18). The clinical signs in a horse in the pilot study (weight-bearing with normal vital parameters) were consistent with the previous study (18), but are dissimilar to those typically seen in clinical cases (non-weight-bearing lameness). Therefore, the higher dose (1.0 × 108 CFU) was selected for our study to better simulate the clinical signs of naturally occurring septic arthritis.
The SAA response in our septic arthritis group was greater than in a previous study, in which Staphylococcus aureus isolated from a bovine mastitis case was used to induce septic arthritis (16). This difference in SAA response is most likely due to inherent differences in experimental models, i.e., inoculation dose and bacterial virulence, as horses in our study exhibited more severe clinical signs, i.e., tachycardia, hyperthermia, and non-weight-bearing lameness. Moreover, our nonseptic synovitis group showed a higher concentration of serum SAA than an equine septic arthritis model induced with Staphylococcus aureus (mean: 180 mg/L versus 119 mg/L at 24 h) (16). This finding suggests that SAA reflects the degree of tissue damage rather than a specific disease process, as it has been described in a previous study (13). In naturally occurring cases of septic arthritis, horses present with varying degrees of injury. In addition, serum SAA would be elevated when there is concurrent systemic inflammation such as in foals with septicemia. As these factors are likely to affect SAA response and confound interpretation of SAA, care should be taken when interpreting SAA in cases of suspected septic arthritis.
In our study, individual differences in SAA response were observed following intra-articular injection of LPS. This is in accordance with previous studies, in which variable SAA response was seen in cattle after intravenous administration of LPS (23) and in horses following intra-articular administration of LPS (24). In addition, 1 out of 3 horses in the control group showed a marked increase in serum and synovial fluid SAA on day 4. The horses in the control group received general anesthesia, arthroscopic lavage of the injected joint, and regional limb perfusions. There are conflicting results on the effect of general anesthesia on SAA concentration (11,25). These results are based on a small number of horses, however, and further studies are therefore warranted to evaluate the effect of general anesthesia on SAA concentration. Arthroscopy is considered minimally invasive and previous studies have shown little to no increase in SAA concentration after this procedure (11,26). To our knowledge, the effect of intravenous regional limb perfusion has not been reported. While intravenous regional limb perfusion has been shown to cause vascular and perivascular irritation (27), there was no notable swelling at the injection site for regional limb perfusion in the horse with markedly elevated SAA. In addition, none of the horses in the nonseptic synovitis group showed elevation of SAA after intravenous regional limb perfusion. We suspect this particular horse in the control group was transiently affected by subclinical inflammation that resulted in systemic elevation of SAA with leakage in the joint subjected to the treatment protocol on day 4.
A limitation of our study was that synovial fluid was not collected between day 1 and day 4 in order to minimize the disturbance in synovial fluid cytology parameters caused by repeated arthrocentesis. As a result, we were unable to follow concentration of synovial fluid SAA during this period. Nevertheless, our study adds essential information on changes in concentrations of serum and synovial fluid SAA during a successful treatment of experimentally induced septic arthritis.
In conclusion, the results of this study support the use of concentrations of serum and synovial fluid SAA as a diagnostic marker in horses with septic arthritis. However, our study shows that serum and synovial fluid SAA concentrations taken at a single time point may be suboptimal to determine eradication of joint infection. Instead, serial measurements of SAA are required in order to evaluate a positive response to treatment, when a decrease in serum and synovial fluid SAA concentrations will be seen once joint infection has been eradicated. Moreover, due to a large individual difference, the non-specific nature of SAA response to inflammation, and the increase seen in the non-injected joints, serum and synovial fluid SAA should be used in conjunction with other diagnostic modalities, such as clinical signs and synovial fluid cytology, when diagnosing and monitoring septic arthritis.
Acknowledgment
The authors thank the Western College of Veterinary Medicine Mark and Pat DuMont Equine Orthopedics Research Fund for funding this research.
References
- 1.Schneider RK, Bramlage LR, Moore RM, Mecklenburg LM, Kohn CW, Gabel AA. A retrospective study of 192 horses affected with septic arthritis/tenosynovitis. Equine Vet J. 1992;24:436–442. doi: 10.1111/j.2042-3306.1992.tb02873.x. [DOI] [PubMed] [Google Scholar]
- 2.Steel CM, Hunt AR, Adams PL, et al. Factors associated with prognosis for survival and athletic use in foals with septic arthritis: 93 cases (1987–1994) J Am Vet Med Assoc. 1999;215:973–977. [PubMed] [Google Scholar]
- 3.Smith LJ, Marr CM, Payne RJ, Stoneham SJ, Reid SW. What is the likelihood that Thoroughbred foals treated for septic arthritis will race? Equine Vet J. 2004;36:452–456. doi: 10.2746/0425164044868396. [DOI] [PubMed] [Google Scholar]
- 4.Walmsley EA, Anderson GA, Muurlink MA, Whitton RC. Retrospective investigation of prognostic indicators for adult horses with infection of a synovial structure. Aust Vet J. 2011;89:226–231. doi: 10.1111/j.1751-0813.2011.00720.x. [DOI] [PubMed] [Google Scholar]
- 5.Wright IM, Smith MR, Humphrey DJ, Eaton-Evans TC, Hillyer MH. Endoscopic surgery in the treatment of contaminated and infected synovial cavities. Equine Vet J. 2003;35:613–619. doi: 10.2746/042516403775467225. [DOI] [PubMed] [Google Scholar]
- 6.Madison JB, Sommer M, Spencer PA. Relations among synovial membrane histopathologic findings, synovial fluid cytologic findings, and bacterial culture results in horses with suspected infectious arthritis: 64 cases (1979–1987) J Am Vet Med Assoc. 1991;198:1655–1661. [PubMed] [Google Scholar]
- 7.Taylor AH, Mair TS, Smith LJ, Perkins JD. Bacterial culture of septic synovial structures of horses: Does a positive bacterial culture influence prognosis? Equine Vet J. 2010;42:213–218. doi: 10.2746/042516409X480403. [DOI] [PubMed] [Google Scholar]
- 8.Morton AJ. Diagnosis and treatment of septic arthritis. Vet Clin North Am Equine Pract. 2005;21:627–649. doi: 10.1016/j.cveq.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Koziy R, Yoshimura S, Dickinson R, et al. Use of standard diagnostic techniques to determine eradication of infection in experimental equine septic arthritis. Can J Vet Res. 2019;83:24–33. [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez-Teran AF, Rubio-Martinez LM, Villarino NF, Sanz MG. Effects of repeated intra-articular administration of amikacin on serum amyloid A, total protein and nucleated cell count in synovial fluid from healthy horses. Equine Vet J Suppl. 2012;43:12–16. doi: 10.1111/j.2042-3306.2012.00637.x. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Teran AF, Bracamonte JL, Hendrick S, et al. Effect of arthroscopic lavage on systemic and synovial fluid serum amyloid A in healthy horses. Vet Surg. 2016;45:223–230. doi: 10.1111/vsu.12439. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Teran AF, Bracamonte JL, Hendrick S, et al. Effect of repeated through-and-through joint lavage on serum amyloid A in synovial fluid from healthy horses. Vet J. 2016;210:30–33. doi: 10.1016/j.tvjl.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsen S, Andersen P. The acute phase protein serum amyloid A (SAA) as a marker of inflammation in horses. Equine Vet Educ. 2007;19:38–46. [Google Scholar]
- 14.Nunokawa Y, Fujinaga T, Taira T, et al. Evaluation of serum amyloid A protein as an acute-phase reactive protein in horses. J Vet Med Sci. 1993;55:1011–1016. doi: 10.1292/jvms.55.1011. [DOI] [PubMed] [Google Scholar]
- 15.Hultén C, Demmers S. Serum amyloid A (SAA) as an aid in the management of infectious disease in the foal: Comparison with total leucocyte count, neutrophil count and fibrinogen. Equine Vet J. 2002;34:693–698. doi: 10.2746/042516402776250360. [DOI] [PubMed] [Google Scholar]
- 16.Ludwig EK, Brandon Wiese R, Graham MR, et al. Serum and synovial fluid serum amyloid A response in equine models of synovitis and septic arthritis. Vet Surg. 2016;45:859–867. doi: 10.1111/vsu.12531. [DOI] [PubMed] [Google Scholar]
- 17.UCVM Class of 2016. Banse H, Cribb AE. Comparative efficacy of oral meloxicam and phenylbutazone in 2 experimental pain models in the horse. Can Vet J. 2017;58:157–167. [PMC free article] [PubMed] [Google Scholar]
- 18.Lloyd KC, Stover SM, Pascoe JR, Adams P. Synovial fluid pH, cytologic characteristics, and gentamicin concentration after intra-articular administration of the drug in an experimental model of infectious arthritis in horses. Am J Vet Res. 1990;51:1363–1369. [PubMed] [Google Scholar]
- 19.McIlwraith CW, Wright IM, Nixon AJ, Boening KJ. Diagnostic and Surgical Arthroscopy in the Horse. 3rd ed. Oxford, UK: Mosby-Elsevier; 2005. Diagnostic and surgical arthroscopy of the carpal joints; pp. 47–127. [Google Scholar]
- 20.Jacobsen S, Kjelgaard-Hansen M, Hagbard Petersen H, Jensen AL. Evaluation of a commercially available human serum amyloid A (SAA) turbidometric immunoassay for determination of equine SAA concentrations. Vet J. 2006;172:315–319. doi: 10.1016/j.tvjl.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Steel CM. Equine synovial fluid analysis. Vet Clin North Am Equine Pract. 2008;24:437–454. doi: 10.1016/j.cveq.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Robinson CS, Singer ER, Piviani M, Rubio-Martinez LM. Are serum amyloid A or D-lactate useful to diagnose synovial contamination or sepsis in horses? Vet Rec. 2017;181:425. doi: 10.1136/vr.104386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobsen S, Andersen PH, Toelboell T, Heegaard PM. Dose dependency and individual variability of the lipopolysaccharide-induced bovine acute phase protein response. J Dairy Sci. 2004;87:3330–3339. doi: 10.3168/jds.S0022-0302(04)73469-4. [DOI] [PubMed] [Google Scholar]
- 24.Jacobsen S, Niewold TA, Halling-Thomsen M, et al. Serum amyloid A isoforms in serum and synovial fluid in horses with lipopolysaccharide-induced arthritis. Vet Immunol Immunopathol. 2006;110:325–330. doi: 10.1016/j.vetimm.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Pepys MB, Baltz ML, Tennent GA, Kent J, Ousey J, Rossdale PD. Serum amyloid A protein (SAA) in horses: Objective measurement of the acute phase response. Equine Vet J. 1989;21:106–109. doi: 10.1111/j.2042-3306.1989.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 26.Jacobsen S, Nielsen JV, Kjelgaard-Hansen M, et al. Acute phase response to surgery of varying intensity in horses: A preliminary study. Vet Surg. 2009;38:762–769. doi: 10.1111/j.1532-950X.2009.00564.x. [DOI] [PubMed] [Google Scholar]
- 27.Levine DG, Epstein KL, Neelis DA, Ross MW. Effect of topical application of 1% diclofenac sodium liposomal cream on inflammation in healthy horses undergoing intravenous regional limb perfusion with amikacin sulfate. Am J Vet Res. 2009;70:1323–1325. doi: 10.2460/ajvr.70.11.1323. [DOI] [PubMed] [Google Scholar]