ABSTRACT
Exosomes are nanovesicles released by all cells that can be found in the blood. A key point for their use as potential biomarkers in cancer is to differentiate tumour-derived exosomes from other circulating nanovesicles. Heat shock protein-70 (HSP70) has been shown to be abundantly expressed by cancer cells and to be associated with bad prognosis. We previously showed that exosomes derived from cancer cells carried HSP70 in the membrane while those from non-cancerous cells did not. In this work, we opened a prospective clinical pilot study including breast and lung cancer patients to determine whether it was possible to detect and quantify HSP70 exosomes in the blood of patients with solid cancers. We found that circulating exosomal HSP70 levels, but not soluble HSP70, reflected HSP70 content within the tumour biopsies. Circulating HSP70 exosomes increased in metastatic patients compared to non-metastatic patients or healthy volunteers. Further, we demonstrated that HSP70-exosome levels correlated with the disease status and, when compared with circulating tumour cells, were more sensitive tumour dissemination predictors. Finally, our case studies indicated that HSP70-exosome levels inversely correlated with response to the therapy and that, therefore, monitoring changes in circulating exosomal HSP70 might be useful to predict tumour response and clinical outcome.
KEYWORDS: Exosomes, HSP70, diagnosis, liquid biopsy, cancer
Introduction
A key issue to improve cancer patients’ outcome relies on earlier cancer diagnosis. Metastases, as opposed to primary tumours, are responsible for most cancer deaths and patients’ prognosis is closely linked to early cancer management. Today, cancer patient follow-up care relies mainly on imagery techniques that unfortunately are not sensitive enough to detect metastasis at an early stage. Thus, it is essential to develop new strategies for the early detection of recurrent or metastatic disease.
Although different circulating tumour markers have been characterised, only a few have demonstrated to be clinically useful for monitoring response to therapy and detecting early relapse. Therefore, there is an urgent need for development of biomarkers for the prediction of the effectiveness of the treatment or the chance of cancer recurrence. A major advance for metastasis diagnosis and monitoring has been the detection of circulating tumour cells (CTCs) in the blood. Current detection of CTCs from circulating blood is based on the epithelial cell adhesion molecule (EpCAM), frequently over-expressed in many cancers and seems to allow earlier detection of recurrence [1]. However, this approach suffers from important limitations, the main being that CTCs are rare events: only one single circulating tumour cell in a background of as many as 109 blood cells [2].
Exosomes constitute a heterogeneous population of small extracellular vesicles from 50 to 150 nm, present in all body fluids and involved in cell-cell communication [3]. Indeed, exosomes can be bioactive cargos of proteins, lipids and nucleotides that will be introduced into the receptor cells and thereby modify their physiology [4]. A broad range of cells secrete exosomes, including tumour cells, which have specific biological functions as they are able to promote cancer growth, metastasis formation and modulation of the immune system [5–8]. These properties have promoted exosomes as new potential biomarkers for the diagnosis and monitoring of cancer. However, since most cells can secrete exosomes that will be found circulating in the blood, a key issue is to differentiate those coming from cancer cells.
We and others have recently shown that whereas exosomes released by tumour cells express in their membrane the stress protein HSP70 (heat shock protein-70), exosomes released by normal cells do not [7,9–11]. We have called this sub-population of tumour-derived nanovesicles “HSP70-exosomes” [11]. Among the different HSPs, HSP70 is the most strongly and widely induced by different stresses. HSP70 is over-expressed in many cancer types, about 70% of solid tumours, and HSP70 expression level has been shown to be useful for the diagnosis, monitoring and response to treatment [12]. Taking into consideration these results and knowing that compelling recent literature supports that exosome-based diagnostics provide higher sensitivity and specificity over conventional biopsy or liquid biomarkers due to their stability in biofluids, we hypothesise that HSP70 exosomes might be used as biomarkers in the monitoring of cancer. To this end, we opened a prospective clinical pilot study called ExoDiag that aimed at quantifying HSP70 exosomes in the blood of cancer patients for the monitoring of malignant solid tumours.
Patients and methods
Study population and specimen collection
40 adult patients with either non-metastatic or metastatic solid tumours were included in the study (NCT02662621). Briefly, 20 women with breast cancer, 10 men and 10 women with non-small cell lung cancer were included (Table 1). Eligibility criteria for cancer patients are listed in Table 1. Additionally, 14 healthy volunteers with no previous cancer history, negative serology for HIV, HCV and HBC and aged 50–70 years old were also included. Age group of healthy volunteers is in agreement with the average age of occurrence of the different solid tumours studied. Upon patient written consent, serology to HIV, HCV and HBV was tested. Only patients with negative serology for these infectious agents were included. During the study, 10 ml of blood, collected in an EDTA tube were necessary for each exosomal HSP70 analysis and additional 10 ml of blood, collected with preservative cell save (Cell Search®) were required for each CTCs analysis. Initial sampling. Blood were collected prior to any treatment (radiotherapy, hormone therapy, surgery or chemotherapy). Follow-up. Sample collection schedule were dependent from the standard treatment line. (i) Follow-up D1 C(x)-Day1CureX: upon a surgical treatment, initial follow-up blood samples for HSP70 analysis were collected during the first visit after surgery; (ii) upon hormone therapy, blood samples were collected at each follow-up oncology visit, in average every 6 months; (iii) upon chemotherapy samples were collected every two cures. Disease progression. If disease progression was observed, blood was collected and the study was stopped.
Table 1.
Patient’s characteristics.
| Patient |
|
|---|---|
| N = 40 | |
| Age | |
| N | 40 |
| Mean (Std) | 62.5 (10.2) |
| Median [min–max] | 57.0 [36.0–83.0] |
| Sex | |
| Women | 26 (65.0%) |
| Men | 14 (35.0%) |
| OMS | |
| 0 | 18 (45.0%) |
| 1 | 17 (42.5%) |
| 2 | 2 (5.0%) |
| 3 | 2 (5.0%) |
| Missing | 1 (2.5%) |
| Localisation | |
| Breast | 17 (42.5%) |
| Lung | 20 (50.0%) |
| Missing | 3 (7.5%) |
| Breast hystologic type | |
| Lobular | 4 (23.5%) |
| Ductal | 13 (76.5%) |
| Lung histologic type | |
| Adenocarcinoma | 16 (80.0%) |
| Squamous cell carcinoma | 4 (20%) |
| Stagde M | |
| M0 | 20 (50.0%) |
| M1 | 14 (35.0%) |
| Mx | 2 (5.0%) |
| Missing |
4 (1.0%) |
| Controls |
|
| |
N = 14 |
| Age | |
| N | 14 |
| Mean (Std) | 55.0 (5.7) |
| Median [min–max] | 53.0 [50.0–69.0] |
| Sex | |
| Women | 7 (50.0%) |
| Men | 7 (50.0%) |
| OMS | |
| 0 | 14 (100.0%) |
Isolation, characterisation and quantification of exosomes
Exosomes were isolated from fresh plasma samples using an optimised protocol derived from Théry et al [13]. Briefly, plasma samples were differentially centrifuged 300 × g for 5 min at 4°C and then 17,000 × g for 10 min at 4°C. Next, supernatant obtained from the previous step were ultracentrifuged at 2,00,000 × g for 1 h at 4°C (Beckman Coulter, Optima XPN-100, Brea, California, the USA). The supernatants were carefully removed and the exosome pellets re-suspended in 100 µl of 1% RIPA lysis buffer or PBS and frozen at −80°C until further use.
Exosome presence was verified by transmission electron microscopy (TEM). The samples dissolved in PBS buffer were dropped into a carbon-coated copper grid and then were stained with 3% uranyl acetate. Images of the sample were captured using a Hitachi 7500 electron microscope (Hitachi high technologies, Tokyo, Japan).
Exosomes were evaluated for their size and concentration by nanoparticle tracking analysis (NTA) using a NS300 Instrument (Malvern Instruments, Malvern, the UK). Briefly, exosome preparations were homogenised by vortexing followed by dilution of 1:500 in filtered phosphate saline buffer and analysed by NanoSight NS300. Each sample analysis was conducted for 60 s. Data were analysed by Nanosight NTA 3.2 Analytical Software (Malvern Instruments, Malvern, the UK) with the detection threshold optimised for each sample and screen gain at 10 to track as many particles as possible with minimal background. A blank 0.1 μm-filtered 1× PBS was also run as a negative control. At least three analyses were done for each individual sample.
Immunohistochemistry
Formalin-fixed paraffin-embedded tumour tissue samples were also obtained for correlation analysis of HSP70 (ADI-SPA-810, Enzo Life science) expression between tissue and circulating exosomes. All IHC procedures were performed using a Benchmark apparatus (Ventana).
Statistical analysis
Statistical analysis was performed using the Graphpad Prism 8 software. Data presented are from at least three independent experiments. Error bars shown in graphical data represent mean ± SEM. When data are presented as box plots, the bar indicates the median, the box shows the interquartile range (25–75%) and the whiskers extend to 1.5 the interquartile range. For normally distributed data, significance of mean differences was determined using two-tailed paired or unpaired student t-test or ANOVA. For data that were not normally distributed, non-parametric Wilcoxon tests were used for paired analysis. A Firth logistic regression was used to determine the association between HSP70-exosomes concentration and the presence of metastasis. Odds ratio was given with its 95% confidence interval. For information, the best cut-off maximising both sensitivity and specificity was determined using ROC curves and the Youden index. Tests were two sided and a p value less than 0.05 was considered significant. All analyses were performed using SAS version 9.4.
Results and discussion
The ExoDiag prospective clinical study includes 40 cancer patients suffering from a lung cancer (n = 20) or a breast cancer (n = 20). Inclusion of patients started in 2016 and ended in March 2019. Patients were followed for 1 year (Table 1). Three patients with breast cancer had to be removed from the study because no follow up was possible (pre-mature death). Samples were taken at diagnosis, after surgery and before and after each cure. In addition, baseline assays of the patients were compared with 14 healthy volunteers (with bilateral alpha of 5% and 40 patients, we will have a power of more than 95% to compare exosome HSP70 concentrations between cases and controls).
Exosomes were isolated from plasma samples and characterised by the nanovesicle size (50–150 nm), by the expression of the exosomal markers such as TSG101, CD9, CD63, by the absence of the endoplasmic reticulum marker Grp94, and by their typical cup-shaped appearance as observed by TEM (Supplementary Figure 1). When comparing HSP70 in lysates of circulating exosomes versus soluble in the plasma by ELISA, we found that whereas HSP70 within exosomes was detected in all patients, soluble HSP70 level was in most patients undetectable (Figure 1(a)). This could be explained by the fact that HSP70 is stabilised by the cholesterol-rich membrane of the exosome [2]. We used an already validated biolayer interferometry (BLI) protocol using a peptide aptamer that binds to the extracellular part of membrane-bound HSP70, to capture exosomes expressing HSP70 in their membrane (HSP70-exosomes) from total blood exosomes [11,14]. We found that the number of HSP70-exosomes in the plasma samples from patients was significant higher than in those from healthy volunteers, in which the level of HSP70 exosomes was barely detectable (Figure 1), thus confirming our previous reports [11]. This result is all the more interesting given that in our cohort the total number of exosomes did not differ between patients and healthy volunteers (Supplementary Figure 2). HSP70-exosomes levels were increased in metastatic patients compared to non-metastatic patients and healthy volunteers, as quantified by BLI (Figure 1(b)) and ELISA (Figure 1(c,d)). Therefore, as happens in cells where the amount of HSP70 in the membrane is in general proportional to the intracellular expression of HSP70 [15], exosomes expressing HSP70 in the membrane also contained high amounts of this stress protein inside the vesicle.
Figure 1.
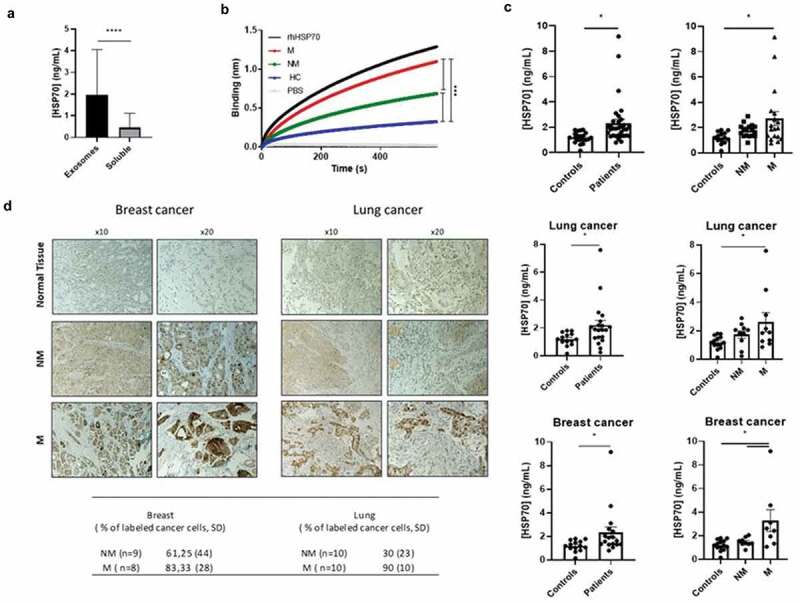
Exosomal HSP70 as a potential cancer biomarker. (a) Expression of circulating HSP70 within exosomes or soluble in paired samples at different time points (n = 68), Wilcoxon’s test, p < 0.0001. (b) Capture of tumour-derived exosomes by BLI using the peptide aptamer A8 from metastatic (M, n = 18), non-metastatic patients (NM, n = 19) and healthy volunteers (HC, n = 14), one way ANOVA, p < 0.0001. Recombinant human HSP70 (rhHSP70) and PBS are used as controls. (c) Expression of HSP70 within HSP70 exosomes either in all cancer patients (n = 37) or in lung cancer (n = 20) or breast cancer patients (n = 17) and healthy volunteers (n = 14), student t-test and ANOVA, p < 0.05, mean ± SEM. (d) Immunohistochemical HSP70 staining within tumour biopsy slides. A semi-quantitative evaluation was performed by counting positive cells in 100 cells, from three randomly selected fields. A representative immunohistochemistry picture of HSP70 expression is shown. NM = Non-metastatic, M = Metastatic.
As in plasma samples, the number of total exosomes in urine samples was measured by NTA and that of HSP70 exosomes by BLI (Supplementary Figure 3). In contrast to plasma samples, no differences were found in the number of HSP70 exosomes between metastatic and non-metastatic patients (Supplementary Figure 3). It should be mentioned that the cellular origin of the exosomes found in the blood and in the urines must be different.
Interestingly, when analysing HSP70 in tumour biopsies, we found that blood HSP70 exosomes matched HSP70 expression within the tumour (Figure 1(d)). This result suggests that circulating exosomes reproduce features within the tumour, particularly HSP70 status.
Next, we compared circulating HSP70 exosomes with CTCs quantified by Cell-Search (a current FDA-approved approach), as predictors of tumour progression. We performed receiver operating characteristic (ROC) curve analysis to discriminate metastatic from non-metastatic patients. As shown in Figure 2, HSP70 exosomes showed a better discrimination since the area under the curve (AUC) was higher when quantifying HSP70 exosomes in the blood than CTCs (AUC = 0.8968, CI95% = [0.7387–1], p < 0.0001 for HSP70 exosomes compared with 0.7857, CI95% = [0.5873–0.9842], p = 0.0048, for CTCs). Based on curve ROC analysis, we calculated a preliminary HSP70-exosome optimal cut off of 1.92 ng/ml (p = 0.0288, 95% Wald confidence limits < 0.001, 0.674). Due to the size of this pilot cohort (n = 40), the reliability of this HSP70-exosome cut-off value needs confirmation in larger cohorts. That HSP70 exosomes may be more reliable predictors of cancer dissemination than CTCs is not surprising since CTCs are rare events, less than 10 cells/ml of blood, whereas exosomes are found in large amounts in body fluids. To our knowledge, this is the first study comparing in a clinical cohort tumour-derived exosome with CTCs as biomarkers.
Figure 2.
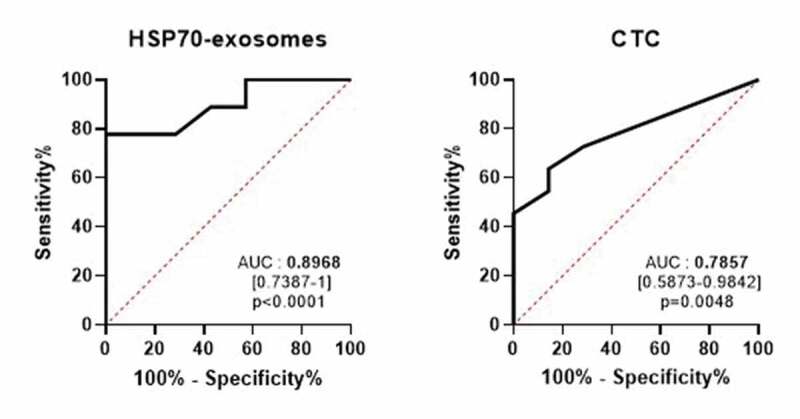
HSP70 exosomes compared to CTCs to discriminate metastatic from non-metastatic patients. ROC curve analysis for the detection level of HSP70 exosomes (left panel) or circulating tumour cells (CTCs, right panel), AUC = 0.8968, CI95% = [0.7387–1], p < 0.0001 compared to 0.7857, CI95% = [0.5873–0.9842], p = 0.0048.
To determine whether HSP70 exosomes could be used as predictors of response to the therapy, we compared HSP70-exosome levels at diagnosis and after treatment, at the same time as the first tumour evaluation by imagery. We found that patients in clinical response had a mean decrease of 77%, whereas patients in clinical progression had a mean increase of 92% (Figure 3(a)). For longer-term follow up, we demonstrated that variation in the level of HSP70 exosomes was associated with the disease status, as determined by scanner imagery. For instance, the level of exosomal HSP70 for patient 1, diagnosed with a metastatic HER2+ breast cancer, T4N1M1, varied according to treatments until the stabilisation after the 12th cure. Between the diagnosis and the second cure, the concentration of HSP70 decreased from 2.62 to 0.835 ng/ml corresponding to the start of the treatment (trastuzumab, pertuzumab and paclitaxel). At the fourth cure, we observed an increase from 0.835 to 3.931 ng/ml corresponding to the replacement of paclitaxel (taxol) by docetaxel (taxotere), due to excessive toxicity. At the sixth cure, we detected a decrease to 1.529 ng/ml that kept stable until the eighth cure. At the 10th cure, the level of HSP70 increased up to 2.874 ng/ml corresponding to a decrease in the dosage of taxotere. Finally, at the 12th cure, the concentration of HSP70 decreased to 0.938 ng/ml and then stabilised until the end of follow up. This stabilisation correlated with clinical stable disease, diagnosed by scanner imagery (Figure 3(b)). Interestingly, when compared HSP70 exosomes with that of the CA-15-3 breast cancer clinical marker, we observed that both reached a sustained low level when the disease stabilised.
Figure 3.
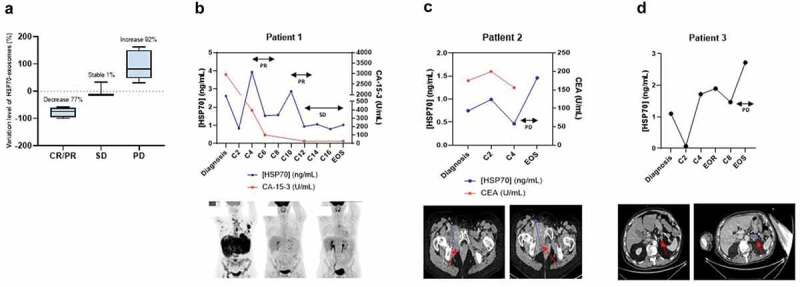
Exosome HSP70 levels correlates with the response to treatment. (a) Exosomal HSP70 variation ratio between diagnosis and the first tumour evaluation according to the clinical status. CR/PR = Complete/Partial response (n = 6), SD = Stable disease (n = 3), PD = Progression disease (n = 11), p = 0.0043. (b)–(d): Case study of the correlation between HSP70 levels in exosomes (blue lines) and their response to the different cures (C2, C4, etc.). CA-15-3 and CEA clinical tumour marker measurements, when available, are indicated by a red line. EOR = End of radiotherapy, EOS = End of study, SD = Stable Diseased PD = Progression disease, PR = Partial response. Panels below show the respective scan imagery. Metastasis is visualised by black spots (patient 1) and red arrows (patients 2 and 3).
Patient 2 was diagnosed with a metastatic NSCLC, T3N2M1. The levels of HSP70 and CEA (a gold standard lung cancer marker) followed the same variations during the different treatments until the disease clinical progression. Indeed, this patient had brain metastases as revealed by scanner imagery. Interestingly, this progression correlated with an important increase in exosomal HSP70 levels (from 0.466 to 1.461 ng/ml) (Figure 3(c)).
Patient 3 was also diagnosed with a metastatic NSCLC, T3N2M1. Exosomal HSP70 level varied depending on the treatment, especially between second and fourth cures where the concentration of HSP70 increased from 0.071 to 1.719 ng/ml, which corresponded to a change in platinum salts due to bad tolerance. Then, after eighth cure, exosomal HSP70 level progressively increased from 1.468 to 2.72 ng/ml, increase that associated with ganglionic cerebral and peritoneal cancer progression, as observed by imagery (Figure 3(d)). Altogether, these results are consistent with data in the literature, since several studies have reported that the expression of HSP70 within the tumour biopsies correlated with tumour volume [16,17]. We hypothesise that the treatments which lead to a reduction in the size of the tumour, consequently lead also to a decrease in the amount of HSP70 exosomes released. Conversely, non-responder patients might have a tumour growth that translates to a higher release of HSP70 exosomes. Although these case reports suggest a correlation between HSP70 exosomes and conventional tumour markers, the curve of HSP70 exosomes have several peaks compared to that of conventional tumoural markers (Figure 3(b,c)). Further studies are needed to determine whether these peaks reflect a higher sensitivity to detect changes in the physiological state of the tumour [18].
High expression of HSPs, particularly HSP70 and HSP90, has been shown to correlate with clinical parameters such as diagnosis, prognosis and/or response to therapy in a wide variety of cancers, both at the intracellular [15,19–25] and extracellular levels [2,11,16,24,26–30]. Our findings here provide for the first time in a prospective cohort an overview of HSP70 expression (circulating, both in exosomes and soluble, and within the tumour) and indicate that exosomal HSP70 hold promises as a potential predictor of tumour growth/spread for the monitoring of cancer patients bearing HSP70-expressing tumours. Further, towards a more personalised patient care, our follow-up studies point out the interest of measuring for each patient the difference between exosomal HSP70 level at baseline and the value after a cure. We intend to perform a multi-centre study that will include larger homogeneous cohorts to refine cut-off values and to confirm whether monitoring variations in circulating exosomal HSP70 levels could be used to detect responders to the therapy.
From a functional point of view, we previously showed that HSP70-exosomes restrain tumour immune surveillance by promoting myeloid-derived suppressor cells’ (MDSCs) functions [7,11]. Thus, the abundant HSP70 exosomes found in metastatic patients may lead to a pro-tumoural environment. Since HSP70-targeting therapies are being developed in cancer, quantifying HSP70 exosomes from a simple blood sample might be a strategy to select the patients likely to benefit from these innovative therapies [11,14,31,32].
The main advantage of the HSP70 exosomes is that these tumour-specific nanovesicles are found in large amounts in the peripheral blood, providing a minimally invasive method for serial assessment of predictive and prognostic markers during multi-stage cancer progression. Further progress is needed to bring the exosomes to clinical practice, especially concerning the isolation technique. We are currently pursuing this issue through the development of a nanovesicle isolation technic based on a microfluidic system-on-chip approach.
Supplementary Material
Acknowledgments
We would like to thank all members of the early phase unit from the Cancer Center Georges-François Leclerc.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article
Competing interest
The authors declare that they have no competing interests.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Gold B, Cankovic M, Furtado LV, et al. Do circulating tumor cells, exosomes, and circulating tumor nucleic acids have clinical utility? A report of the association for molecular pathology. J Mol Diagn. 2015. May;17(3):209–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cordonnier M, Chanteloup G, Isambert N, et al. Exosomes in cancer theranostic: diamonds in the rough. Cell Adhes Migr. 2017. February 6;1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Théry C, Ostrowski M, Segura E.. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009. August;9(8):581–593. [DOI] [PubMed] [Google Scholar]
- [4].Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007. June;9(6):654–659. [DOI] [PubMed] [Google Scholar]
- [5].Peinado H, Alečković M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012. June;18(6):883–891. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hoshino A, Costa-Silva B, Shen T-L, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015. November 19;527(7578):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chalmin F, Ladoire S, Mignot G, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010. February;120(2):457–471. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li L, Cao B, Liang X, et al. Microenvironmental oxygen pressure orchestrates an anti- and pro-tumoral γδ T cell equilibrium via tumor-derived exosomes. Oncogene. 2018. December 13. [DOI] [PubMed] [Google Scholar]
- [9].Multhoff G, Botzler C, Wiesnet M, et al. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995. April 10;61(2):272–279. [DOI] [PubMed] [Google Scholar]
- [10].Multhoff G. Heat shock protein 70 (Hsp70): membrane location, export and immunological relevance. Methods San Diego Calif. 2007. November;43(3):229–237. [DOI] [PubMed] [Google Scholar]
- [11].Gobbo J, Marcion G, Cordonnier M, et al. Restoring anticancer immune response by targeting tumor-derived exosomes with a HSP70 peptide aptamer. J Natl Cancer Inst. 2016. March;108(3):djv330. [DOI] [PubMed] [Google Scholar]
- [12].Murphy ME. The HSP70 family and cancer. Carcinogenesis. 2013. June;34(6):1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Théry C, Amigorena S, Raposo G, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. In: Current Protocols in Cell Biology [Internet]. John Wiley & Sons, Inc.; 2001. Available from: http://onlinelibrary.wiley.com.gate2.inist.fr/doi/10.1002/0471143030.cb0322s30/abstract [DOI] [PubMed]
- [14].Rérole A-L, Gobbo J, Thonel AD, et al. Peptides and aptamers targeting HSP70: a novel approach for anticancer chemotherapy. Cancer Res. 2011. January 15;71(2):484–495. [DOI] [PubMed] [Google Scholar]
- [15].Seigneuric R, Mjahed H, Gobbo J, et al. Heat shock proteins as danger signals for cancer detection. Front Oncol [Internet] 2011. November 10;1 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3355996/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gunther S, Ostheimer C, Stangl S, et al. Correlation of HSP70 serum levels with gross tumor volume and composition of lymphocyte subpopulations in patients with squamous cell and adeno non-small cell lung cancer. Front Immunol. 2015;6:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gehrmann MK, Kimm MA, Stangl S, et al. Imaging of HSP70-positive tumors with cmHsp70.1 antibody-conjugated gold nanoparticles. Int J Nanomedicine. 2015. September 8;10:5687–5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kulasingam V, Prassas I, Diamandis EP. Towards personalized tumor markers. Npj Precis Oncol. 2017. May 25;1(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee HW, Lee EH, Kim S-H, et al. Heat shock protein 70 (HSP70) expression is associated with poor prognosis in intestinal type gastric cancer. Virchows Arch Int J Pathol. 2013. October;463(4):489–495. [DOI] [PubMed] [Google Scholar]
- [20].Stangl S, Tontcheva N, Sievert W, et al. Heat shock protein 70 and tumor-infiltrating NK cells as prognostic indicators for patients with squamous cell carcinoma of the head and neck after radiochemotherapy: A multicentre retrospective study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Int J Cancer. 2017. December 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mano R, Zilber S, Di Natale RG, et al. Heat shock proteins 60 and 70 are associated with long-term outcome of T1-stage high-grade urothelial tumors of the bladder treated with intravesical Bacillus Calmette-Guérin immunotherapy. Urol Oncol. 2018;36(12):531.e9–531.e17. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gehrmann M, Specht HM, Bayer C, et al. Hsp70–a biomarker for tumor detection and monitoring of outcome of radiation therapy in patients with squamous cell carcinoma of the head and neck. Radiat Oncol Lond Engl. 2014. June 9;9(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tavassol F, Starke OF, Kokemüller H, et al. Prognostic significance of heat shock protein 70 (HSP70) in patients with oral cancer. Head Neck Oncol. 2011. February 23;3(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liu W, Li J, Zhang P, et al. A novel pan-cancer biomarker plasma heat shock protein 90alpha and its diagnosis determinants in clinic. Cancer Sci. 2019. September;110(9):2941–2959. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ono K, Eguchi T, Sogawa C, et al. HSP-enriched properties of extracellular vesicles involve survival of metastatic oral cancer cells. J Cell Biochem. 2018;119(9):7350–7362. . [DOI] [PubMed] [Google Scholar]
- [26].Ostheimer C, Gunther S, Bache M, et al. Dynamics of heat shock protein 70 serum levels as a predictor of clinical response in non-small-cell lung cancer and correlation with the hypoxia-related marker osteopontin. Front Immunol. 2017;8:1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gráf L, Barabás L, Madaras B, et al. High serum Hsp70 level predicts poor survival in colorectal cancer: results obtained in an independent validation cohort. Cancer Biomark Sect Dis Markers. 2018;23(4):539–547. [DOI] [PubMed] [Google Scholar]
- [28].Tang T, Yang C, Brown HE, et al. Circulating heat shock protein 70 is a novel biomarker for early diagnosis of lung cancer. Dis Markers. 2018;2018:6184162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Saini J, Sharma PK. Clinical, prognostic and therapeutic significance of heat shock proteins in cancer. Curr Drug Targets. 2018;19(13):1478–1490. [DOI] [PubMed] [Google Scholar]
- [30].Breuninger S, Erl J. Quantitative analysis of liposomal heat shock protein 70 (Hsp70) in the blood of tumor patients using a novel LipHsp70 ELISA. J Clin Cell Immunol [Internet] 2014. [cited 2020 March17];5(5). Available from: https://www.omicsonline.org/open-access/quantitative-analysis-of-liposomal-heat-shock-protein-hsp-in-the-blood-of-tumor-patients-2155-9899-5-264.php?aid=38483 [Google Scholar]
- [31].Shevtsov M, Multhoff G. Heat shock protein–peptide and HSP-based immunotherapies for the treatment of cancer. Front Immunol [Internet] 2016. April 29 [cited 2017 May18];7 Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4850156/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Boudesco C, Cause S, Jego G, et al. Hsp70: a cancer target inside and outside the cell. Methods Mol Biol Clifton NJ. 2018;1709:371–396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Théry C, Amigorena S, Raposo G, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. In: Current Protocols in Cell Biology [Internet]. John Wiley & Sons, Inc.; 2001. Available from: http://onlinelibrary.wiley.com.gate2.inist.fr/doi/10.1002/0471143030.cb0322s30/abstract [DOI] [PubMed]


