Abstract
Rationale: Aspirin-exacerbated respiratory disease is characterized by severe asthma, nonsteroidal antiinflammatory drug hypersensitivity, nasal polyposis, and leukotriene overproduction. Systemic corticosteroid therapy does not completely suppress lifelong aspirin hypersensitivity. Omalizumab efficacy against aspirin-exacerbated respiratory disease has not been investigated in a randomized manner.
Objectives: To evaluate omalizumab efficacy against aspirin hypersensitivity, leukotriene E4 overproduction, and symptoms during an oral aspirin challenge in patients with aspirin-exacerbated respiratory disease using a randomized design.
Methods: We performed a double-blind, randomized, crossover, placebo-controlled, single-center study at Sagamihara National Hospital between August 2015 and December 2016. Atopic patients (20–79 yr old) with aspirin-exacerbated respiratory disease diagnosed by systemic aspirin challenge were randomized (1:1) to a 3-month treatment with omalizumab or placebo, followed by a >18-week washout period (crossover design). The primary endpoint was the difference in area under logarithm level of urinary leukotriene E4 concentration versus time curve in the intent-to-treat population during an oral aspirin challenge.
Measurements and Main Results: Sixteen patients completed the study and were included in the analysis. The area under the logarithm level of urinary leukotriene E4 concentration versus time curve during an oral aspirin challenge was significantly lower in the omalizumab phase (median [interquartile range], 51.1 [44.5–59.8]) than in the placebo phase (80.8 [interquartile range, 65.4–87.8]) (P < 0.001). Ten of 16 patients (62.5%) developed oral aspirin tolerance up to cumulative doses of 930 mg in the omalizumab phase (P < 0.001).
Conclusions: Omalizumab treatment inhibited urinary leukotriene E4 overproduction and upper/lower respiratory tract symptoms during an oral aspirin challenge, resulting in aspirin tolerance in 62.5% of the patients with aspirin-exacerbated respiratory disease.
Keywords: aspirin-exacerbated respiratory disease, aspirin hypersensitivity, aspirin tolerance, leukotriene E4, omalizumab
At a Glance Commentary
Scientific Knowledge on the Subject
Aspirin-exacerbated respiratory disease (AERD) is characterized by asthma, hypersensitivity to aspirin and other nonsteroidal antiinflammatory drugs, chronic rhinosinusitis, mast cell activation, and overproduction of cysteinyl leukotrienes. The management of AERD involves guideline-based treatments for asthma and chronic rhinosinusitis, and avoidance of all medications that inhibit cyclooxygenase-1. Although systemic corticosteroid treatment was reported to partially attenuate upper and lower respiratory tract symptoms after aspirin challenge in AERD, all types of antiasthma medication have failed to completely suppress aspirin hypersensitivity, which continues throughout the life span of patients.
What This Study Adds to the Field
This study is the first double-blind, randomized, placebo-controlled, crossover study to evaluate the efficacy of omalizumab against the overproduction of leukotriene E4, aspirin hypersensitivity, and disease symptoms during an oral aspirin challenge in patients with AERD and at least one positive result in the specific IgE test for common environmental allergens. Omalizumab treatment suppressed overproduction of urinary leukotriene E4 and 11,15-dioxo-9α-hydroxy-2,3,4,5-tetranorprostan-1,20-dioic acid, which is a key feature of the pathogenesis of AERD, after an aspirin challenge. Furthermore, aspirin hypersensitivity disappeared in more than half of the patients after 3 months of omalizumab therapy, and serious eosinophilic airway inflammation and mast cell activation in AERD were effectively suppressed by omalizumab starting within 24 hours.
Aspirin-exacerbated respiratory disease (AERD) is characterized by severe asthma, hypersensitivity to aspirin and other nonsteroidal antiinflammatory drugs, chronic rhinosinusitis with nasal polyposis, and overproduction of cysteinyl leukotrienes (1–4). Although nonatopic AERD is common, a subset of patients develop elevated total serum IgE levels (5). Pathologically, AERD is associated with mast cell activation (6–8) and serious eosinophilic inflammation in the respiratory mucosa (9). The requirement for high-dose corticosteroids to control asthma and the frequent recurrence of chronic rhinosinusitis with nasal polyposis after surgery reflect the aggressive, persistent nature of the disease (1–3). Systemic corticosteroid therapy was shown to partially attenuate aspirin-induced airway symptoms in AERD, but did not completely suppress aspirin-hypersensitivity reactions to aspirin exposure (10).
In AERD, aspirin hypersensitivity is usually lifelong, and accidental exposure to nonsteroidal antiinflammatory drugs causes subsequent life-threatening asthma exacerbations. However, no treatment completely suppresses aspirin-hypersensitivity reactions, except for aspirin desensitization (2, 11). Therefore, it is important to identify medications that strongly suppress hypersensitivity reactions after exposure to nonsteroidal antiinflammatory drugs (2, 3, 12, 13).
Omalizumab, a humanized recombinant monoclonal anti-IgE antibody, binds selectively to the IgE constant region to block free IgE binding to IgE receptors (14–17). Previous studies demonstrated the effectiveness of omalizumab for severe allergic asthma (15, 17–21) and other allergic diseases (22). Furthermore, omalizumab reduced high-affinity IgE Fc receptor expression on mast cells (23, 24), basophils (25, 26), and dendritic cells (27), which may suppress their activation.
In a previous clinical observational study, we found that a 12-month treatment for AERD using omalizumab showed clinical effectiveness for upper and lower respiratory tract symptoms, and resulted in a marked reduction to near-normal levels of urinary leukotriene E4 (LTE4) and the prostaglandin D2 metabolite 9α,11β-prostaglandin F2, both of which when overproduced represent a steady-state feature of AERD (28). Other case studies reported that patients with AERD who were successfully treated with omalizumab developed aspirin tolerance (29). However, the efficacy of omalizumab against AERD, aspirin hypersensitivity, and overproduction of LTE4 after an aspirin challenge has not been investigated in a randomized manner. In addition, it is not clear at what time point after the initiation of omalizumab treatment clinical effectiveness is observed in patients with AERD. Based on these factors, we hypothesized that omalizumab would suppress the overproduction of LTE4 and clinical symptoms during an aspirin challenge in patients with AERD.
Our aim in this study was to evaluate the efficacy of omalizumab against the overproduction of LTE4 after an aspirin challenge, aspirin hypersensitivity, and severe upper and lower respiratory symptoms—all of which are characteristic features of AERD—using a randomized design (SOSA [Sagamihara Omalizumab Treatment Strategy for AERD]). We measured the levels of the urinary mediators LTE4 and 11,15-dioxo-9α-hydroxy-2,3,4,5-tetranorprostan-1,20-dioic acid (tetranor-PGDM), the dominant cyclooxygenase product of mast cells (30), during an oral aspirin challenge. Hypersensitivity symptoms during an oral aspirin challenge, inflammatory biomarkers, and asthma- and nasal-related symptoms were also evaluated in relation to omalizumab treatment.
Methods
Study Design and Participants
The study was a randomized, double-blind, single-center, placebo-controlled, crossover trial with two treatment phases of 3-month duration in patients with AERD (Figure 1A). An oral aspirin challenge was conducted at the end of each treatment phase. The Ethics Committee of the National Hospital Organization Sagamihara National Hospital (Sagamihara, Japan) approved the study protocol. Written informed consent was obtained from all patients. This study was registered with the University Hospital Medical Information Network (number UMIN000018777; https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000021562) in Japan.
Figure 1.
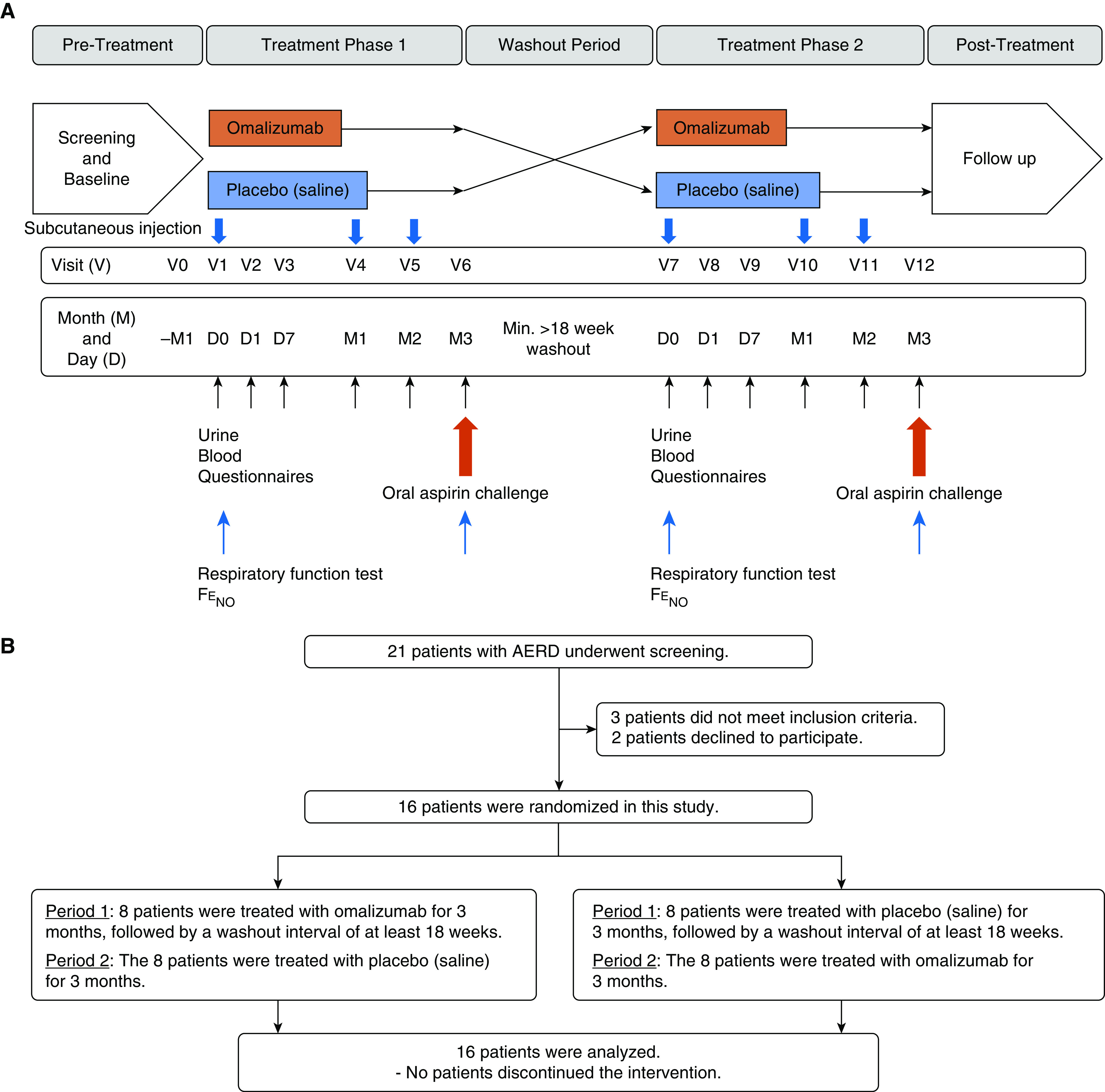
(A) Study design. (B) Flow diagram. AERD = aspirin-exacerbated respiratory disease; FeNO = fractional exhaled nitric oxide.
Patients 20–79 years of age with AERD were recruited from the National Hospital Organization Sagamihara National Hospital between August 24, 2015 and December 15, 2016. The patients were required to be clinically stable before undergoing an oral aspirin challenge (at least twice) safely. Asthma was diagnosed according to the American Thoracic Society criteria (31) by pulmonologists and allergists. In addition, AERD was diagnosed by a positive result for systemic aspirin challenge, based on a modified oral challenge protocol (32, 33) within the last 2 years before enrollment. Positive reactions were evaluated by a respiratory function test and/or clinical symptoms (nasal and/or extrapulmonary symptoms) (see Methods section in the online supplement). All patients had at least one positive result in the specific IgE test for common environmental allergens. Maintenance oral corticosteroids and other regular asthma treatments were unchanged for the duration of the study. Information regarding the inclusion and exclusion criteria for study patients, randomization, masking, and anti-IgE and placebo therapies are provided in the online supplement.
Study Outcomes
The primary endpoint was the difference in the area under the logarithm level of urinary biomarker concentration versus time curve (AUC[Before-24 h]) of LTE4 between the placebo and omalizumab phases during an oral aspirin challenge, because the AUC reflects the overall production of LTE4 in response to exposure to systemic aspirin. The secondary endpoints included the differences in urinary concentrations of LTE4 (34) and tetranor-PGDM (30) during the treatment phase (when there was no aspirin exposure), and the AUC(Before-24 h) of tetranor-PGDM during oral aspirin challenge, blood samples (peripheral blood eosinophil counts, periostin [35, 36], eosinophilic cationic protein, tryptase, platelet activation markers [37], cytokines, and chemokines), lung function (FEV1% predicted, FVC% predicted, forced expired flow between 25% and 75% of the volume expired, and fractional exhaled nitric oxide [FeNO]), Asthma Control Test (ACT) (38), Asthma Control Questionnaire-6 (ACQ-6) (39), Sino-Nasal Outcome Test-22 (SNOT-22) (40), asthma- and nasal-related symptom scores (visual analog scale [VAS]), and Global Evaluation of Treatment Effectiveness (GETE) (20). Adverse events and medication use and adherence were also assessed at every study visit.
Statistical Analyses
The baseline characteristics of patients were described as follows: number and percentage for categorical variables and the median (interquartile range [IQR]) for continuous variables. To compare the effects of treatments, the McNemar test for categorical variables and the Wilcoxon signed-rank test were used. A P value < 0.05 was considered statistically significant. Bonferroni corrections were applied to adjust for the impact of multiple comparisons. Our sample size estimate was based on a change in the logarithm level of urinary LTE4 concentration between before and after omalizumab treatment in our previous study (28). The mean change before and after omalizumab treatment in the log-transformed urinary LTE4 concentration (pg/mg of creatinine) was −0.70 with an SD of 0.525, and the number of participants was calculated to be nine patients at a significance level of 0.05 (two-sided) and power of 90%. To account for not being able to evaluate many endpoints during the long-term study period (>10 mo), and to perform at least two aspirin challenges in participants in stable condition in up to 40% of patients with AERD, we set an initial enrollment goal of 16 patients. Intent-to-treat data were analyzed unless otherwise indicated. All statistical analyses were performed using R version 3.2.4 (41).
The AUC[Before-24 h] of LTE4 and tetranor-PGDM was calculated using the trapezoidal rule. The AUC using the trapezoidal rule was defined as follows:
where ti (i = 1, 2, 3, 4, 5) is the observation time (Before, 3 h, 6 h, 9 h, and 24 h, respectively) and Ci is the logarithm level of LTE4 or tetranor-PGDM for each time point ti. To calculate the AUC, we assumed that Before equaled 0 h.
Results
Patient Recruitment and Characteristics
Screening visits were arranged for 21 patients; 16 were randomized to therapy and five were excluded before randomization (Figure 1B). The first patient was screened on August 24, 2015 and the last patient was screened on December 15, 2016. All patients completed the study as planned. The clinical characteristics of the patients are shown in Table 1. The study population consisted of 16 patients (10 female and six male) with a median age of 53 years (IQR, 44.0–60.0 yr), a median asthma onset age of 43.0 years (IQR, 27.0–56.3 yr), and a median serum IgE level of 169.0 IU/ml (IQR, 45.5–482.8 IU/ml). The median dose of monthly omalizumab was 300 mg (IQR, 150–600 mg). Three of the 16 patients (18.8%) received omalizumab every 2 weeks. The participants in this study demonstrated good adherence to the asthma medications.
Table 1.
Baseline Characteristics of All Patients at the Initial Study Visit
| Characteristic | N = 16 |
|---|---|
| Age, yr | 53.0 (44.0–60.0) |
| Sex, F, n (%) | 10 (62.5) |
| Asthma onset age, yr | 43.0 (27.0–56.3) |
| Body weight, kg | 56.4 (49.6–59.6) |
| Body mass index, kg/m2 | 21.4 (20.5–23.0) |
| Smoking status, never/past/current, n (%) | 8 (50.0)/6 (37.5)/2 (12.5) |
| Maintenance prednisolone, n (%) | 3 (18.8) |
| Oral prednisolone daily dose, mg/d, n = 3 | 2.5/2.5/7.0 |
| Inhaled corticosteroid, n (%) | 16 (100) |
| Inhaled corticosteroid (fluticasone equivalent) dose, μg/d | 655.0 (492.5–1,000.0) |
| Maintenance long-acting β-agonist, n (%) | 16 (100) |
| Maintenance leukotriene receptor antagonist, n (%) | 16 (100) |
| One or more exacerbations during 12 mo before enrollment, n (%) | 3 (18.8) |
| One or more hospitalizations during 12 mo before enrollment, n (%) | 0 (0) |
| Status of sinus surgery and polypectomy | |
| History of sinus surgery and polypectomy, n (%) | 7 (43.8) |
| Median number of lifetime nasal polyp surgeries | 0 (0–1.0) |
| Median time since last nasal polyp surgery, yr | 5.0 (3.5–10.5) |
| ACQ-6 | 0.8 (0.3–2.6) |
| ACT | 20.5 (14.5–24.5) |
| Peripheral eosinophil count per microliter | 370.0 (270.0–530.0) |
| Total serum IgE, IU/ml | 169.0 (45.5–482.8) |
| Omalizumab dose, mg/mo | 300 (150–600) |
| FEV1% predicted | 104.4 (92.7–112.0) |
| FEV1/FVC% | 75.2 (69.6–78.5) |
| FVC% predicted | 118.6 (111.2–128.0) |
| FEF25–75% (%) | 56.8 (43.5–70.7) |
| FeNO, ppb | 22.0 (17.1–31.0) |
Definition of abbreviations: ACQ-6 = Asthma Control Questionnaire-6; ACT = Asthma Control Test; FEF25–75 = forced expiratory flow, midexpiratory phase; FeNO = fractional exhaled nitric oxide.
Data are presented as medians and interquartile ranges unless otherwise indicated.
AUC(Before-24 h) and Levels of Urinary LTE4 and Tetranor-PGDM Concentrations during Oral Aspirin Challenge
The primary outcome and difference in the AUC(Before-24 h) of urinary LTE4 concentration between the placebo and omalizumab phases during an oral aspirin challenge are shown in Figure 2A and Table 2. There was a significant reduction in the AUC(Before-24 h) of urinary LTE4 concentrations in the omalizumab phase (51.1 [IQR, 44.5–59.8]) compared with the placebo phase (80.8 [IQR, 65.4–87.8]) (P < 0.001). In addition, there was a significant reduction in the AUC(Before-24 h) of urinary tetranor-PGDM concentrations, which was a secondary outcome, in the omalizumab phase (53.8 [51.3–56.8]) compared with the placebo phase (61.2 [57.9–64.0]) (P < 0.0001) (Figure 2B and Table 2).
Figure 2.
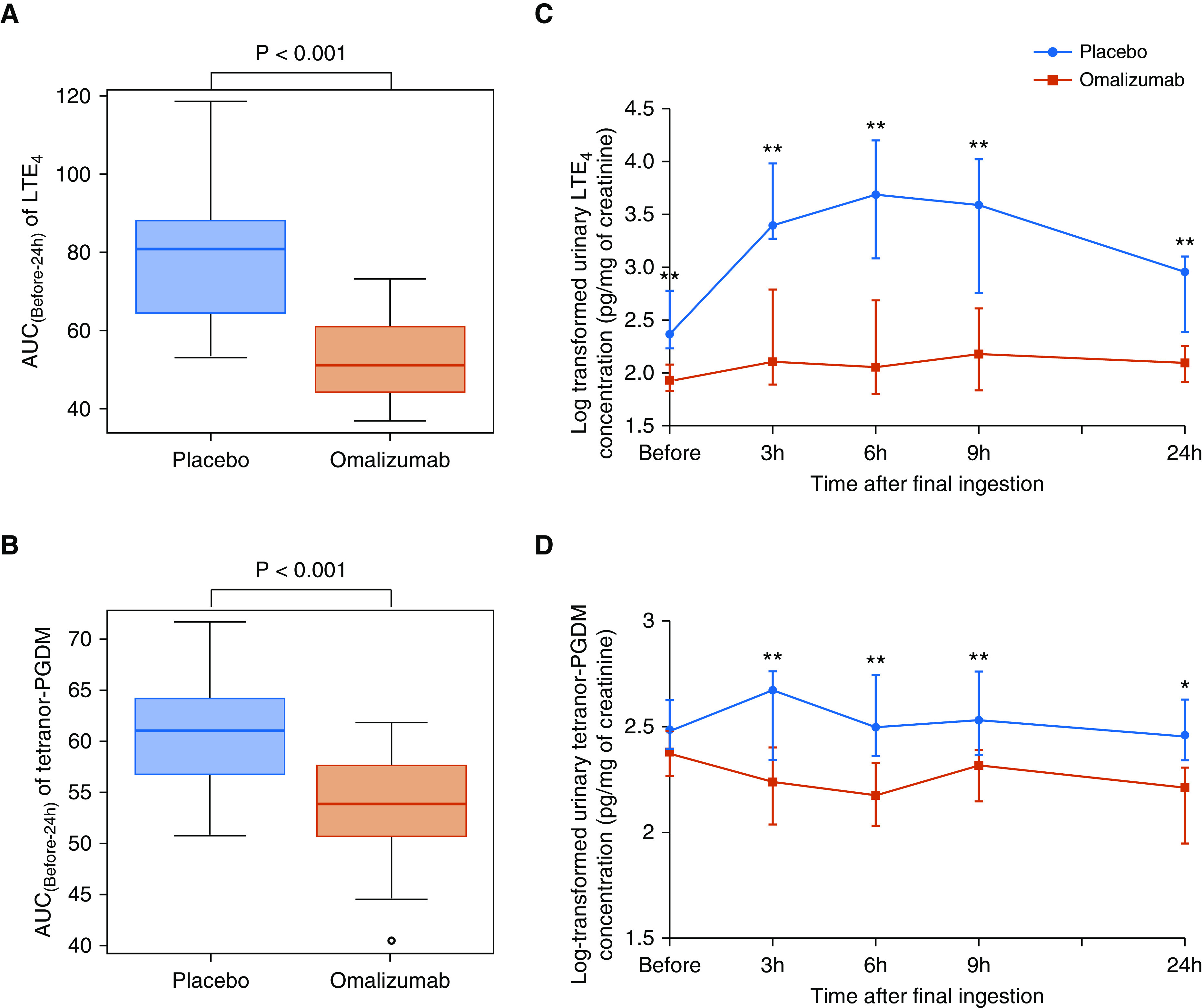
Difference in the area under the logarithm level of urinary biomarker concentration versus time curve (AUC(Before-24 h)) and levels of urinary LTE4 and tetranor-PGDM concentrations during oral aspirin challenge between the placebo and omalizumab phases. (A) AUC[Before-24 h] of LTE4. (B) AUC[Before-24 h] of tetranor-PGDM. (C and D) Time course of log-transformed levels of urinary LTE4 (C) and tetranor-PGDM (D) concentrations. Wilcoxon signed-rank test. *P < 0.05 and **P < 0.01. The log-transformed urinary LTE4 and tetranor-PGDM concentrations are expressed as medians and interquartile ranges. LTE4 = leukotriene E4; tetranor-PGDM = 11,15-dioxo-9α-hydroxy-2,3,4,5-tetranorprostan-1,20-dioic acid.
Table 2.
Difference in the Area under the Logarithm Level of Urinary Biomarker Concentration versus Time Curve during Oral Aspirin Challenge
| Placebo Phase (N = 16) | Omalizumab Phase (N = 16) | P Value | |
|---|---|---|---|
| LTE4 | 80.8 (65.4–87.8) | 51.1 (44.5–59.8) | <0.001 |
| Tetranor-PGDM | 61.2 (57.9–64.0) | 53.8 (51.3–56.8) | <0.001 |
Definition of abbreviations: LTE4 = leukotriene E4; tetranor-PGDM = 11,15-dioxo-9α-hydroxy-2,3,4,5-tetranorprostan-1,20-dioic acid.
Data are presented as medians and interquartile ranges. Significance testing was performed using the Wilcoxon signed-rank test.
Figures 2C and 2D show the time course of changes in the levels of urinary LTE4 and tetranor-PGDM concentrations during an oral aspirin challenge. The levels at each time point (3, 6, 9, and 24 h) after the oral aspirin challenge were significantly lower in the omalizumab phase than in the placebo phase.
Development of Aspirin Tolerance and Change in the Cumulative Provoking Aspirin Dose
In the placebo phase, all patients showed a positive reaction to the oral aspirin challenge. The median cumulative provocation dose of aspirin in the placebo phase was 150 mg (IQR, 90–210 mg). Conversely, in the omalizumab phase, 10 of 16 patients (62.5%) did not show a positive reaction, even after the highest cumulative dose (930 mg) was administered (P = 0.004; Table 3), indicating that these patients developed aspirin tolerance. Of the six patients with a positive reaction, the cumulative dose in four of the six patients increased from 90 mg to 210 mg. However, this was unchanged in the other two patients. Tables E1 and E2 and Figure E1 in the online supplement show the differences in VAS for asthma- and nasal-related symptoms and the maximal decline in FEV1 during oral aspirin challenge. VAS was scored significantly better at all time points in the omalizumab phase than in the placebo phase (see Table E1 and Figure E1). In addition, changes in FEV1 during the oral aspirin challenge were significantly smaller in the omalizumab phase than in the placebo phase (see Table E2). Moreover, there were significant correlations between the treatment-related improvement in FEV1 and that in VAS scores for wheezing and cough during the aspirin challenge (data not shown).
Table 3.
Change in the Cumulative Provoking Aspirin Dose during an Oral Aspirin Challenge
| Cumulative Provoking Aspirin Dose |
||||
|---|---|---|---|---|
| Number | Sex | Age (yr) | Placebo Phase | Omalizumab Phase |
| Developed aspirin tolerance | ||||
| 1 | M | 53 | 90 | 930* |
| 2 | F | 67 | 30 | 930* |
| 3 | M | 72 | 210 | 930* |
| 4 | M | 53 | 930 | 930* |
| 5 | F | 65 | 450 | 930* |
| 6 | F | 57 | 210 | 930* |
| 7 | F | 42 | 90 | 930* |
| 8 | M | 60 | 90 | 930* |
| 9 | F | 57 | 210 | 930* |
| 10 | M | 44 | 210 | 930* |
| Did not develop aspirin tolerance | ||||
| 11 | F | 21 | 90 | 210 |
| 12 | F | 60 | 210 | 210 |
| 13 | F | 49 | 90 | 210 |
| 14 | F | 52 | 210 | 210 |
| 15 | M | 44 | 90 | 210 |
| 16 | F | 40 | 90 | 210 |
Significance testing was performed using the McNemar test.
No symptoms were observed after the cumulative dose of aspirin (930 mg).
The baseline characteristics of patients who did or did not develop aspirin tolerance are presented in Tables E3 and E4. Patients who developed aspirin tolerance showed later asthma onset, as well as elevated peripheral blood platelet counts and serum levels of IL-22, compared with patients who did not develop aspirin tolerance. However, these associations were not observed after Bonferroni correction. Moreover, there was no association between the response to omalizumab treatment assessed by GETE and the development of aspirin tolerance (data not shown).
Time Course of Improved Symptoms
According to the GETE, seven of 16 patients (43.8%) reported a response to treatment within 7 days after starting treatment, and this increased to 11 of 16 patients (68.8%) at Month 3 (Figure 3A). Interestingly, three of 16 patients (18.8%) reported a rapid improvement of symptoms within 1 day of omalizumab treatment. In contrast, only two of 16 patients (12.5%) were responders in the placebo phase (Figure 3B).
Figure 3.
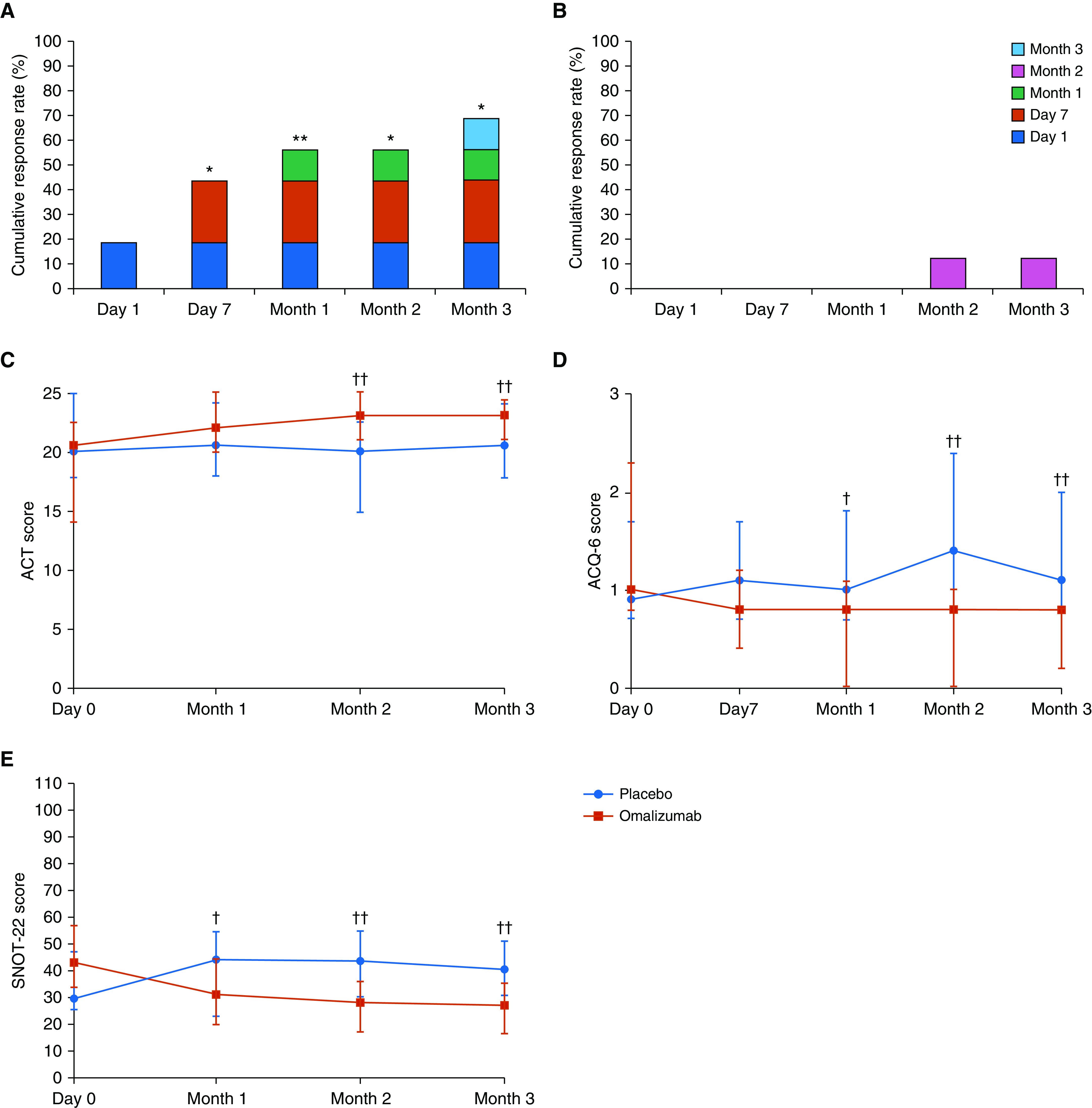
(A and B) Cumulative response rate according to the Global Evaluation of Treatment Effectiveness scale in the omalizumab (A) and placebo (B) phases. (C–E) Scores from the Asthma Control Test (ACT) (C), Asthma Control Questionnaire-6 (ACQ-6) (D), and Sino-Nasal Outcome Test-22 (SNOT-22) (E) in the omalizumab and placebo phases. McNemar test; *P < 0.05 and **P < 0.01 compared with the placebo phase. Global Evaluation of Treatment Effectiveness was used to classify responders and nonresponders. Wilcoxon signed-rank test; †P < 0.05 and ††P < 0.01 compared with the placebo phase. Circles (placebo phase) and squares (omalizumab phase) indicate median values, and bars indicate interquartile ranges.
The ACT, ACQ-6, SNOT-22, and VAS scored significantly better at most visits in the omalizumab phase compared with the placebo phase (see Figures 3 and E3, and Table E7). Statistically significant differences were first observed at Month 1 for ACQ-6 and SNOT-22, and at Month 2 for ACT. All differences remained statistically significant up to Month 3, although the differences in ACQ-6 and ACT were small, indicating they were not clinically important differences (42, 43). Regarding the VAS, statistically significant differences were first observed at Month 1 for asthma symptoms (dyspnea, wheezing, and cough) and at Month 2 for nasal symptoms (nasal congestion, anterior rhinorrhea, and anosmia). All remained statistically significant up to Month 3.
Urinary Concentrations of LTE4 and Tetranor-PGDM during the Treatment Phase
Significantly lower levels of urinary concentrations of LTE4 and tetranor-PGDM in the omalizumab phase compared with the placebo phase were observed as early as Day 1 (Figure 4 and Table 4), and the low levels of LTE4 were maintained until Month 3. Levels of tetranor-PGDM also tended to be lower in the omalizumab phase than in the placebo phase throughout the treatment period, although this did not reach statistical significance at Day 7, Month 2, or Month 3.
Figure 4.
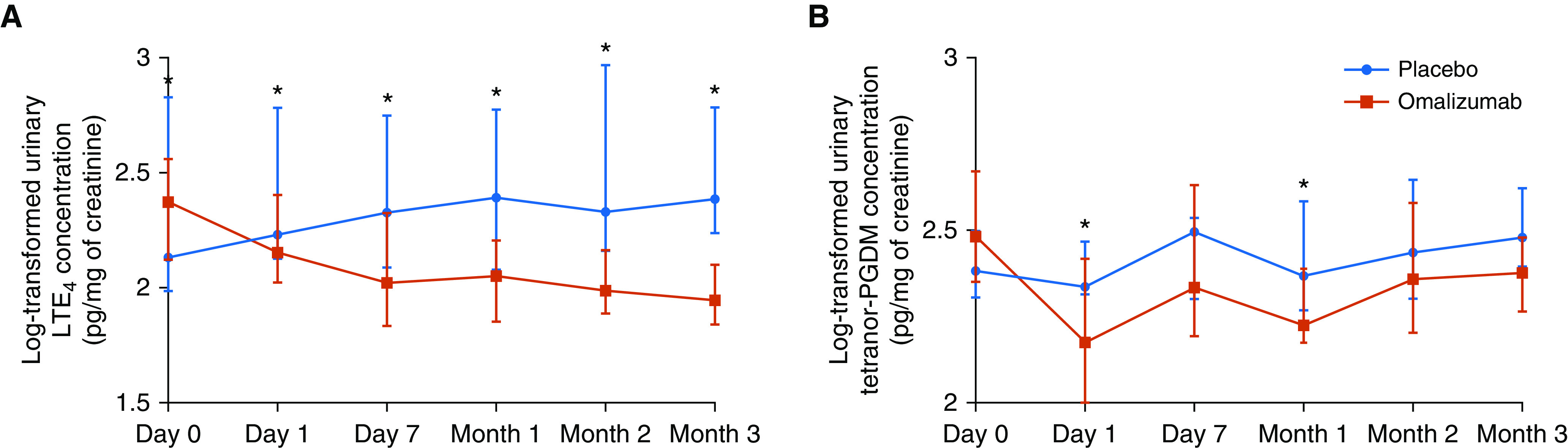
(A and B) Difference in the levels of urinary LTE4 (A) and tetranor-PGDM (B) concentrations between the placebo and omalizumab phases. Wilcoxon signed-rank test; *P < 0.05. Circles (placebo phase) and squares (omalizumab phase) indicate median values, and bars indicate interquartile ranges. LTE4 = leukotriene E4; tetranor-PGDM = 11,15-dioxo-9α-hydroxy-2,3,4,5-tetranorprostan-1,20-dioic acid.
Table 4.
Difference of Each Urine and Blood Biomarker between Placebo and Omalizumab Phases
| Variable | Placebo (N = 16) | Omalizumab (N = 16) | P Value | |
|---|---|---|---|---|
| Urine biomarkers, log-transformed |
||||
| LTE4, pg/mg of creatinine |
||||
| Day 0 |
2.1 (2.0–2.8) | 2.4 (2.1–2.6) | 0.404 | |
| Day 1 |
2.2 (2.1–2.8) | 2.1 (2.0–2.4) | 0.008 | |
| Day 7 |
2.3 (2.1–2.7) | 2.0 (1.8–2.3) | 0.002 | |
| Month 1 |
2.4 (2.1–2.8) | 2.0 (1.8–2.2) | 0.008 | |
| Month 2 |
2.3 (2.2–3.0) | 2.0 (1.9–2.2) | 0.002 | |
| Month 3 |
2.4 (2.2–2.8) | 1.9 (1.8–2.1) | <0.001 | |
| Tetranor-PGDM, pg/mg of creatinine |
||||
| Day 0 |
2.4 (2.3–2.5) | 2.5 (2.4–2.7) | 0.376 | |
| Day 1 |
2.3 (2.3–2.5) | 2.2 (2.0–2.4) | 0.021 | |
| Day 7 |
2.5 (2.3–2.5) | 2.3 (2.2–2.6) | 0.821 | |
| Month 1 |
2.4 (2.3–2.6) | 2.2 (2.2–2.4) | 0.016 | |
| Month 2 |
2.4 (2.3–2.6) | 2.4 (2.2–2.6) | 0.300 | |
| Month 3 |
2.5 (2.4–2.6) | 2.4 (2.3–2.5) | 0.051 | |
| Blood biomarkers |
||||
| Eosinophil count per microliter |
||||
| Day 0 |
380.0 (287.5–457.5) | 320.0 (257.5–482.5) | 0.497 | |
| Day 1 |
355.0 (302.5–502.5) | 395.0 (267.5–440.0) | 0.536 | |
| Day 7 |
385.0 (292.5–475.0) | 315.0 (187.5–430.0) | 0.028 | |
| Month 1 |
400.0 (295.0–607.5) | 275.0 (150.0–370.0) | 0.006 | |
| Month 2 |
385.0 (297.5–562.5) | 325.0 (172.5–372.5) | 0.007 | |
| Month 3 |
335.0 (250.0–442.5) | 220.0 (170.0–290.0) | 0.026 | |
| Periostin, ng/ml |
||||
| Day 0 |
113.5 (103.2–157.0) | 111.0 (98.8–133.0) | 0.934 | |
| Day 1 |
121.0 (97.8–146.0) | 111.5 (104.8–131.2) | 0.831 | |
| Day 7 |
126.0 (98.8–177.0) | 105.5 (91.5–128.5) | 0.004 | |
| Month 1 |
109.0 (98.0–160.2) | 107.5 (92.8–119.0) | 0.038 | |
| Month 2 |
133.5 (96.8–161.8) | 94.5 (85.8–116.2) | 0.001 | |
| Month 3 |
123.5 (100.5–137.0) | 96.0 (86.8–113.0) | 0.001 | |
| Eosinophil cationic protein, μg/L | |
|||
| Day 0 |
13.3 (9.6–17.9) | 11.3 (6.3–13.4) | 0.021 | |
| Day 1 |
11.7 (7.5–15.0) | 12.0 (5.0–14.9) | 0.940 | |
| Day 7 |
15.4 (7.8–20.9) | 11.0 (4.9–13.3) | 0.039 | |
| Month 1 |
12.8 (7.5–16.2) | 9.3 (6.1–14.1) | 0.376 | |
| Month 2 |
16.2 (11.6–18.2) | 9.6 (5.9–13.7) | 0.039 | |
| Month 3 |
8.1 (5.6–15.0) | 7.3 (5.4–10.4) | 0.083 | |
| Tryptase, μg/L |
||||
| Day 0 |
3.4 (2.7–5.1) | 3.1 (2.4–4.0) | 0.049 | |
| Day 1 |
3.1 (2.8–4.9) | 3.1 (2.3–4.2) | 0.696 | |
| Day 7 |
3.1 (2.6–4.6) | 2.9 (2.5–3.6) | 0.022 | |
| Month 1 |
3.1 (2.8–4.1) | 3.0 (2.3–3.6) | 0.009 | |
| Month 2 |
3.0 (2.9–5.2) | 2.8 (2.4–3.6) | 0.140 | |
| Month 3 | 3.1 (2.7–4.6) | 2.9 (2.6–3.5) | 0.003 | |
Definition of abbreviations: LTE4 = leukotriene E4; tetranor-PGDM = 11,15-dioxo-9α-hydroxy-2,3,4,5-tetranorprostan-1,20-dioic acid.
Data are presented as medians and interquartile ranges. Significance testing was performed using the Wilcoxon signed-rank test.
Blood Biomarkers during the Treatment Phase
Day 7 was the first day statistically significant differences were observed for peripheral blood eosinophil counts and serum periostin. All remained statistically significant until Month 3 (see Figure E2 and Table 4). The levels of serum eosinophilic cationic protein and tryptase were significantly lower at several time points in the omalizumab phase than in the placebo phase. In contrast, there were no significant differences in the platelet surface markers of free plasma platelets (CD62P and CD63) and the levels of soluble P-selectin between the placebo and omalizumab phases (see Table E5). The concentration of serum B-cell activating factor, a tumor necrosis factor family/B-lymphocyte stimulator member, was significantly increased in the omalizumab phase compared with the placebo phase (see Table E6), although this was not observed after Bonferroni correction.
Respiratory Functions and FeNO during the Treatment Phase
All respiratory functions, except for FVC% predicted and FeNO, were significantly higher in the omalizumab phase than in the placebo phase (see Table E8). Regarding the forced oscillation technique, there were no significant differences in all parameters (respiratory system resistance and reactance) between the placebo and omalizumab phases (data not shown).
Safety and Adverse Events
Subcutaneous bleeding was reported as an adverse event in two patients (placebo phase, n = 0; omalizumab phase, n = 2). Two patients in the omalizumab phase reported injection-site erythema that was mostly mild. However, no moderate or severe adverse events related to omalizumab use, including generalized urticarial or anaphylaxis, were reported during the study period.
Discussion
To the best of our knowledge, this is the first randomized, placebo-controlled trial to evaluate the efficacy of omalizumab against overproduction of LTE4, aspirin hypersensitivity, and upper and lower respiratory tract symptoms—all of which are important pathogenic factors in AERD—during an oral aspirin challenge. Omalizumab suppressed LTE4 and tetranor-PGDM overproduction during a systemic aspirin challenge, suggesting that omalizumab has inhibitory effects on mast cell function during an aspirin challenge, and therefore potentially during clinical adverse responses to aspirin. In addition, omalizumab significantly suppressed the urinary concentrations of LTE4 and tetranor-PGDM during treatment periods (when there was no aspirin exposure), suggesting that it has inhibitory effects on ongoing mast cell activation. Although no medication has been reported to completely suppress hypersensitivity symptoms after aspirin exposure (3, 10), 10 of 16 patients (62.5%) in our study became tolerant to aspirin under omalizumab treatment. These study findings indicate that omalizumab might be a potential orphan drug for AERD.
With regard to general asthma, the response to omalizumab was characterized by a progressive onset, and at least 12 weeks of omalizumab treatment was required before we could determine whether a satisfactory response had been achieved (15, 44). In contrast, in previous studies, early onset (within 1 d to 4 wk) of omalizumab efficacy was reported for most patients with chronic idiopathic urticaria/chronic spontaneous urticaria (45, 46). The findings of our study also demonstrated early onset of omalizumab efficacy: 3 of 16 patients (18.8%) reported rapid symptomatic improvement within 1 day of omalizumab treatment, and an additional 4 patients reported an improvement within 7 days. When early responders (n = 7, response until Day 7) were compared with late responders (n = 4, response after Month 1), the early responders tended to have more severe symptoms at baseline (Day 0) and showed a rapid improvement of the symptoms until Day 7 after starting treatment (data not shown). Symptoms after oral aspirin challenge were also suppressed relatively early: 10 of 16 patients (62.5%) developed aspirin tolerance after only 3 months of omalizumab treatment.
In our study, urinary LTE4 levels were significantly lower in the omalizumab phase than in the placebo phase as early as 1 day after initiation of treatment. In addition, peripheral eosinophil counts and serum periostin levels were significantly suppressed within 7 days of omalizumab treatment. These findings suggest that omalizumab can begin to effectively suppress serious eosinophilic airway inflammation and mast cell activation in AERD (1, 7–9) within 24 hours, with the potential to improve aspirin hypersensitivity within 3 months. These findings were also supported by relatively rapid clinically significant improvements in GETE and each symptom score in the omalizumab phase compared with the placebo phase.
Previous studies suggested that omalizumab exerts its effects against general asthma by depleting free IgE and preventing the formation of an IgE/high-affinity IgE Fc receptor/mast cell axis (17, 23, 24). A duration of >10 weeks was reported to be required for the reduction of high-affinity IgE Fc receptors on mast cells (23). However, recent in vitro studies demonstrated that omalizumab dissociated prebound IgE from its high-affinity IgE Fc receptor on mast cells, and that the inhibition of mast cells by omalizumab started rapidly, within 24 hours (47, 48). Increased levels of prostaglandin D2 in urine were reported in stable patients with AERD compared with patients with general asthma (1, 8, 49, 50). The early suppression of LTE4 and tetranor-PGDM overproduction by omalizumab during the treatment periods (when there was no aspirin exposure) observed in our study may be explained by its suppressive efficacy against ongoing, exposure-independent mast cell activation, which is an important pathogenetic feature of AERD (1, 7–9, 50). However, the mechanism of ongoing mast cell activation in AERD remains to be determined.
GETE is a subjective and single-item questionnaire that is completed by patients to determine the perceived effectiveness of a treatment before and after omalizumab treatment on a five-point categorical scale. The response scale is not centered on 0, or no change, because its three levels indicate a positive improvement and only one level definitively reflects a worse outcome. Therefore, although GETE simply measures perceived treatment effectiveness, it is commonly used in clinical trials of omalizumab therapy (14, 20, 51). Of note, it was previously reported that GETE responses were similar between patients and investigators (20).
The management of AERD involves guideline-based treatments for asthma and chronic rhinosinusitis and avoiding all medications that inhibit cyclooxygenase-1 (2, 3, 12). However, patients with severe asthma require daily corticosteroid therapy and/or frequent steroid bursts to control their disease (1–3). Given the possible side effects of chronic corticosteroid therapy (e.g., osteoporosis, increased risk of infection, diabetes mellitus, weight gain, and adrenal suppression) (52), the ability to taper or stop corticosteroid treatment is a major clinical benefit of omalizumab. In this study, no moderate-to-severe adverse events (e.g., anaphylaxis) or other clinically relevant adverse events were observed during the omalizumab phase.
This study has some limitations. First, it included a limited sample size of 16 patients, which was estimated before the start of the study, from a single center. Second, because this study was performed in Japanese patients with AERD, results from populations with other ethnic and genetic backgrounds were not available. Third, a selection bias toward patients with relatively mild AERD may have occurred. This is because patients with severe AERD who were preferentially enrolled in our previous observational study (28) could not be enrolled in this randomized study because of previous omalizumab treatment (item 5 of the inclusion criteria). In addition, patients with severe AERD and uncontrolled asthmatic symptoms were not enrolled in this study because at least two safely performed oral aspirin challenges were required to evaluate the primary and secondary endpoints. Fourth, the possibility of prolonged carry-over effects could not be completely excluded because this was a randomized, crossover study. However, we extended the washout period to >18 weeks to minimize this effect, and with regard to the levels of urinary LTE4 and tetranor-PGDM, there was no significant difference in the levels in the omalizumab preceding group at visit 1 (just before omalizumab treatment) and those at visit 7 (just before placebo treatment) (data not shown). This suggests that there were no prolonged carry-over effects in urinary biomarker concentrations. Fifth, no placebo for omalizumab was available from the manufacturer in this investigator-initiated trial. However, this is a minor limitation because the study endpoints were objective outcomes with minimal sensitivity to subjective bias. Finally, although both prostaglandin D2 and cysteinyl leukotrienes have been reported to promote the chemotaxis of group 2 innate lymphoid cells (53, 54), we did not evaluate the efficacy of omalizumab against group 2 innate lymphoid cells in this study. Investigators should consider these limitations when designing future studies.
In conclusion, this randomized study showed the efficacy of omalizumab against the overproduction of LTE4, mast cell activation, and the results of aspirin challenge in patients with AERD. Omalizumab treatment led to the development of aspirin tolerance and markedly reduced urinary LTE4 concentrations during an oral aspirin challenge. These findings indicate that omalizumab has efficacy against the key pathogenic features of AERD and might be an important therapeutic candidate for AERD.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Mr. Ryosuke Kage, Ms. Katsue Sumi, Ms. Chiharu Shibuya, Ms. Itsuko Ito, and Dr. Haruhisa Mita for quantifying urinary LTE4 and tetranor-PGDM; Junya Ono BSc. (Shino-Test Co.) for measuring serum periostin levels; and Ms. Yuka Takahashi, Ms. Natsuko Hasebe, Ms. Emi Ohtsuka, Ms. Moe Egawa, Ms. Nozomi Hayakawa, and Ms. Masako Ikeno for their assistance in this study. Statistical analysis support was provided by StaGen Co. Ltd.
Footnotes
Supported by the Practical Research Project for Allergic Diseases and Immunology, Japan Agency for Medical Research and Development (grant number 19ek0410040h0003).
Author Contributions: All authors contributed to this research study. M.T. developed the study concept. H.H. and M.T. developed the study design. H.H., Y.F., C.M., K.K., K. Watai, Y.K., Y.N., Y. Hamada, Y.T., K.S., and T.T. were responsible for patient recruitment and data acquisition. H.H., C.M., and K.K. were involved in measuring the urinary levels of leukotriene E4, 11,15-dioxo-9a-hydroxy-2,3,4,5-tetranorprostan-1,20-dioic acid, and the levels of platelet activation markers. K.I. was involved in measuring serum periostin levels. StaGen Co. Ltd. conducted all statistical analyses. H.H. and Y.F. wrote the manuscript. K.I., K. Wakahara, N.H., Y. Hasegawa, and M.T. contributed to the critical revision of the manuscript. All authors additionally assisted in study design and data interpretation, and provided comments on the final article.
Data sharing statement: The data collected for this study and related documents will not be made available to others.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201906-1215OC on March 6, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Laidlaw TM, Boyce JA. Aspirin-exacerbated respiratory disease: new prime suspects. N Engl J Med. 2016;374:484–488. doi: 10.1056/NEJMcibr1514013. [DOI] [PubMed] [Google Scholar]
- 2.White AA, Stevenson DD. Aspirin-exacerbated respiratory disease. N Engl J Med. 2018;379:1060–1070. doi: 10.1056/NEJMra1712125. [DOI] [PubMed] [Google Scholar]
- 3.Kowalski ML, Agache I, Bavbek S, Bakirtas A, Blanca M, Bochenek G, et al. Diagnosis and management of NSAID-Exacerbated Respiratory Disease (N-ERD)-a EAACI position paper. Allergy. 2019;74:28–39. doi: 10.1111/all.13599. [DOI] [PubMed] [Google Scholar]
- 4.Christie PE, Tagari P, Ford-Hutchinson AW, Charlesson S, Chee P, Arm JP, et al. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991;143:1025–1029. doi: 10.1164/ajrccm/143.5_Pt_1.1025. [DOI] [PubMed] [Google Scholar]
- 5.Johns CB, Laidlaw TM. Elevated total serum IgE in nonatopic patients with aspirin-exacerbated respiratory disease. Am J Rhinol Allergy. 2014;28:287–289. doi: 10.2500/ajra.2014.28.4054. [DOI] [PubMed] [Google Scholar]
- 6.Fischer AR, Rosenberg MA, Lilly CM, Callery JC, Rubin P, Cohn J, et al. Direct evidence for a role of the mast cell in the nasal response to aspirin in aspirin-sensitive asthma. J Allergy Clin Immunol. 1994;94:1046–1056. doi: 10.1016/0091-6749(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 7.Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D2: a dominant mediator of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2015;135:245–252. doi: 10.1016/j.jaci.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyce JA. Aspirin sensitivity: lessons in the regulation (and dysregulation) of mast cell function. J Allergy Clin Immunol. 2019;144:875–881. doi: 10.1016/j.jaci.2019.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasser SM, Pfister R, Christie PE, Sousa AR, Barker J, Schmitz-Schumann M, et al. Inflammatory cell populations in bronchial biopsies from aspirin-sensitive asthmatic subjects. Am J Respir Crit Care Med. 1996;153:90–96. doi: 10.1164/ajrccm.153.1.8542168. [DOI] [PubMed] [Google Scholar]
- 10.Nizankowska E, Szczeklik A. Glucocorticosteroids attenuate aspirin-precipitated adverse reactions in aspirin-intolerant patients with asthma. Ann Allergy. 1989;63:159–162. [PubMed] [Google Scholar]
- 11.Cahill KN, Cui J, Kothari P, Murphy K, Raby BA, Singer J, et al. Unique effect of aspirin therapy on biomarkers in aspirin-exacerbated respiratory disease: a prospective trial. Am J Respir Crit Care Med. 2019;200:704–711. doi: 10.1164/rccm.201809-1755OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laidlaw TM, Cahill KN. Current knowledge and management of hypersensitivity to aspirin and NSAIDs. J Allergy Clin Immunol Pract. 2017;5:537–545. doi: 10.1016/j.jaip.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: a meta-analysis of the literature. J Allergy Clin Immunol. 2015;135:676–681.e1. doi: 10.1016/j.jaci.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 15.Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med. 2006;354:2689–2695. doi: 10.1056/NEJMct055184. [DOI] [PubMed] [Google Scholar]
- 16.Schulman ES. Development of a monoclonal anti-immunoglobulin E antibody (omalizumab) for the treatment of allergic respiratory disorders. Am J Respir Crit Care Med. 2001;164:S6–S11. doi: 10.1164/ajrccm.164.supplement_1.2103025. [DOI] [PubMed] [Google Scholar]
- 17.McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199:433–445. doi: 10.1164/rccm.201810-1944CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bousquet J, Cabrera P, Berkman N, Buhl R, Holgate S, Wenzel S, et al. The effect of treatment with omalizumab, an anti-IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthma. Allergy. 2005;60:302–308. doi: 10.1111/j.1398-9995.2004.00770.x. [DOI] [PubMed] [Google Scholar]
- 19.Holgate ST, Djukanović R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005;35:408–416. doi: 10.1111/j.1365-2222.2005.02191.x. [DOI] [PubMed] [Google Scholar]
- 20.Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60:309–316. doi: 10.1111/j.1398-9995.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- 21.Abraham I, Alhossan A, Lee CS, Kutbi H, MacDonald K. ‘Real-life’ effectiveness studies of omalizumab in adult patients with severe allergic asthma: systematic review. Allergy. 2016;71:593–610. doi: 10.1111/all.12815. [DOI] [PubMed] [Google Scholar]
- 22.Holgate ST. New strategies with anti-IgE in allergic diseases. World Allergy Organ J. 2014;7:17. doi: 10.1186/1939-4551-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell Fc epsilon RI expression and function. J Allergy Clin Immunol. 2004;114:527–530. doi: 10.1016/j.jaci.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 24.Chang TW, Chen C, Lin CJ, Metz M, Church MK, Maurer M. The potential pharmacologic mechanisms of omalizumab in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2015;135:337–342. doi: 10.1016/j.jaci.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 25.MacGlashan DW, Jr, Bochner BS, Adelman DC, Jardieu PM, Togias A, McKenzie-White J, et al. Down-regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997;158:1438–1445. [PubMed] [Google Scholar]
- 26.Lin H, Boesel KM, Griffith DT, Prussin C, Foster B, Romero FA, et al. Omalizumab rapidly decreases nasal allergic response and FcepsilonRI on basophils. J Allergy Clin Immunol. 2004;113:297–302. doi: 10.1016/j.jaci.2003.11.044. [DOI] [PubMed] [Google Scholar]
- 27.Prussin C, Griffith DT, Boesel KM, Lin H, Foster B, Casale TB. Omalizumab treatment downregulates dendritic cell FcepsilonRI expression. J Allergy Clin Immunol. 2003;112:1147–1154. doi: 10.1016/j.jaci.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi H, Mitsui C, Nakatani E, Fukutomi Y, Kajiwara K, Watai K, et al. Omalizumab reduces cysteinyl leukotriene and 9α,11β-prostaglandin F2 overproduction in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016;137:1585–1587.e4. doi: 10.1016/j.jaci.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 29.Phillips-Angles E, Barranco P, Lluch-Bernal M, Dominguez-Ortega J, López-Carrasco V, Quirce S. Aspirin tolerance in patients with nonsteroidal anti-inflammatory drug-exacerbated respiratory disease following treatment with omalizumab. J Allergy Clin Immunol Pract. 2017;5:842–845. doi: 10.1016/j.jaip.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Higashi N, Mita H, Yamaguchi H, Fukutomi Y, Akiyama K, Taniguchi M. Urinary tetranor-PGDM concentrations in aspirin-intolerant asthma and anaphylaxis. J Allergy Clin Immunol. 2012;129:557–559, 559.e1–e2. doi: 10.1016/j.jaci.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Guidelines for the evaluation of impairment/disability in patients with asthma: American Thoracic Society. Medical Section of the American Lung Association. Am Rev Respir Dis. 1993;147:1056–1061. doi: 10.1164/ajrccm/147.4.1056. [DOI] [PubMed] [Google Scholar]
- 32.Stevenson DD. Diagnosis, prevention, and treatment of adverse reactions to aspirin and nonsteroidal anti-inflammatory drugs. J Allergy Clin Immunol. 1984;74:617–622. doi: 10.1016/0091-6749(84)90115-5. [DOI] [PubMed] [Google Scholar]
- 33.Stevenson DD. Aspirin sensitivity and desensitization for asthma and sinusitis. Curr Allergy Asthma Rep. 2009;9:155–163. doi: 10.1007/s11882-009-0023-4. [DOI] [PubMed] [Google Scholar]
- 34.Mita H, Oosaki R, Mizushima Y, Kobayashi M, Akiyama K. Efficient method for the quantitation of urinary leukotriene E4: extraction using an Empore C18 disk cartridge. J Chromatogr B Biomed Sci Appl. 1997;692:461–466. doi: 10.1016/s0378-4347(97)00006-6. [DOI] [PubMed] [Google Scholar]
- 35.Kanemitsu Y, Matsumoto H, Izuhara K, Tohda Y, Kita H, Horiguchi T, et al. Increased periostin associates with greater airflow limitation in patients receiving inhaled corticosteroids. J Allergy Clin Immunol. 2013;132:305–312.e3. doi: 10.1016/j.jaci.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 36.Izuhara K, Conway SJ, Moore BB, Matsumoto H, Holweg CT, Matthews JG, et al. Roles of periostin in respiratory disorders. Am J Respir Crit Care Med. 2016;193:949–956. doi: 10.1164/rccm.201510-2032PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitsui C, Kajiwara K, Hayashi H, Ito J, Mita H, Ono E, et al. Platelet activation markers overexpressed specifically in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016;137:400–411. doi: 10.1016/j.jaci.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 38.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 40.Kennedy JL, Hubbard MA, Huyett P, Patrie JT, Borish L, Payne SC. Sino-nasal outcome test (SNOT-22): a predictor of postsurgical improvement in patients with chronic sinusitis. Ann Allergy Asthma Immunol. 2013;111:246–251.e2. doi: 10.1016/j.anai.2013.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.R Core Team R: a language and environment for statistical computing R Foundation for Statistical Computing; Vienna, Austria: 2016https://www.R-project.org/. [Google Scholar]
- 42.Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99:553–558. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the asthma control test. J Allergy Clin Immunol. 2009;124:719–723.e1. doi: 10.1016/j.jaci.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 44.Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125:1378–1386. doi: 10.1378/chest.125.4.1378. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan A, Ferrer M, Bernstein JA, Antonova E, Trzaskoma B, Raimundo K, et al. Timing and duration of omalizumab response in patients with chronic idiopathic/spontaneous urticaria. J Allergy Clin Immunol. 2016;137:474–481. doi: 10.1016/j.jaci.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 46.Kaplan AP, Giménez-Arnau AM, Saini SS. Mechanisms of action that contribute to efficacy of omalizumab in chronic spontaneous urticaria. Allergy. 2017;72:519–533. doi: 10.1111/all.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eggel A, Baravalle G, Hobi G, Kim B, Buschor P, Forrer P, et al. Accelerated dissociation of IgE-FcεRI complexes by disruptive inhibitors actively desensitizes allergic effector cells. J Allergy Clin Immunol. 2014;133:1709–1719.e8. doi: 10.1016/j.jaci.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serrano-Candelas E, Martinez-Aranguren R, Valero A, Bartra J, Gastaminza G, Goikoetxea MJ, et al. Comparable actions of omalizumab on mast cells and basophils. Clin Exp Allergy. 2016;46:92–102. doi: 10.1111/cea.12668. [DOI] [PubMed] [Google Scholar]
- 49.Buchheit KM, Cahill KN, Katz HR, Murphy KC, Feng C, Lee-Sarwar K, et al. Thymic stromal lymphopoietin controls prostaglandin D2 generation in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016;137:1566–1576.e5. doi: 10.1016/j.jaci.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taniguchi M, Mitsui C, Hayashi H, Ono E, Kajiwara K, Mita H, et al. Aspirin-exacerbated respiratory disease (AERD): current understanding of AERD. Allergol Int. 2019;68:289–295. doi: 10.1016/j.alit.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Braunstahl GJ, Chen CW, Maykut R, Georgiou P, Peachey G, Bruce J. The eXpeRience registry: the ‘real-world’ effectiveness of omalizumab in allergic asthma. Respir Med. 2013;107:1141–1151. doi: 10.1016/j.rmed.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 52.Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol. 2018;141:110–116, e7. doi: 10.1016/j.jaci.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 53.Eastman JJ, Cavagnero KJ, Deconde AS, Kim AS, Karta MR, Broide DH, et al. Group 2 innate lymphoid cells are recruited to the nasal mucosa in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2017;140:101–108, e3. doi: 10.1016/j.jaci.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White AA, Doherty TA. Role of group 2 innate lymphocytes in aspirin-exacerbated respiratory disease pathogenesis. Am J Rhinol Allergy. 2018;32:7–11. doi: 10.2500/ajra.2018.32.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


