Abstract
In signaled active avoidance (SigAA), rats learn to suppress Pavlovian freezing and emit actions to remove threats and prevent footshocks. SigAA is critical for understanding aversively motivated instrumental behavior and anxiety-related active coping. However, with standard protocols ∼25% of rats exhibit high freezing and poor avoidance. This has dampened enthusiasm for the paradigm and stalled progress. We demonstrate that reducing shock imminence with long-duration warning signals leads to greater freezing suppression and perfect avoidance in all subjects. This suggests that instrumental SigAA mechanisms evolved to cope with distant harm and protocols that promote inflexible Pavlovian reactions are poorly designed to study avoidance.
In the signaled active avoidance paradigm (SigAA), rats learn to suppress Pavlovian reactions (e.g., freezing) and emit instrumental actions (e.g., shuttling) to escape warning signals (WSs) and prevent painful unconditioned stimuli (USs, typically footshocks). Understanding the psychological and neural mechanisms of SigAA is critical for several reasons. It is the prototypical paradigm for studying aversively motivated instrumental actions (Rescorla and Solomon 1967; Cain et al. 2013). Maladaptive or excessive avoidance responses (ARs) contribute to every major anxiety disorder (APA 2013). Lastly, adaptive ARs reduce emotional reactions and give subjects control over environmental threats (Kamin et al. 1963; Cain and LeDoux 2007; Choi et al. 2010; Boeke et al. 2017), suggesting a potential role in proactive coping behaviors and resilience in humans (LeDoux and Gorman 2001; van der Kolk 2006; Collins et al. 2014; Moscarello and Hartley 2017).
Despite its importance as a fundamental learning mechanism with clinical relevance, SigAA research has lagged behind research on Pavlovian threats and appetitive instrumental behavior (Krypotos et al. 2015; LeDoux et al. 2017; Cain 2019). The phenomenon of “poor avoidance” has been one major obstacle to progress. Avoidance acquisition is typically slower than Pavlovian conditioning, but most animals learn to prevent >80% of scheduled shocks in two-way shuttlebox tasks. However, some animals exhibit high freezing and rarely emit ARs (Keehn 1967; Choi et al. 2010; Martinez et al. 2013; Galatzer-Levy et al. 2014). For some tasks, poor avoidance is the rule rather than the exception (Solomon and Brush 1954; Neffinger and Gibbon 1975). From a practical standpoint, avoidance studies are more costly and time-consuming because poor avoiders must be replaced. Pretraining loss-of-function studies are also ill-advised with SigAA, since there is no reliable way to predict which animals will acquire ARs. Finally, the poor avoidance phenomenon raises questions about whether instrumental AR learning is a major component of defense worthy of study (Bolles 1975; Fanselow 1997, 2018). Animals evolved defensive mechanisms to cope with predators, not shocks, and it is difficult to see how a trial-and-error learning mechanism that often fails could have evolved under predatory pressure.
One simple explanation is that researchers have used suboptimal protocols for studying avoidance in the laboratory. SigAA is typically evaluated in small chambers with short-duration WSs and high-density shock protocols. These conditions are ideal for modeling a state of high predatory imminence that triggers hard-wired, stereotyped fear-like reactions (e.g., freezing) that are incompatible with ARs (Weiss et al. 1968; Fanselow and Lester 1988). However, prey animals spend much more time in a state of low predatory imminence where encounters with predators are temporally distant or uncertain. Perhaps instrumental avoidance mechanisms evolved to deal with these anxiety-like states, where animals must balance conflicting needs (Gray and McNaughton 2000; Cain 2019; Diehl et al. 2019). Under these “preencounter” conditions, less rigid defensive behaviors may not interfere with AR learning.
To solve the poor avoidance problem and optimize avoidance training, we designed two experiments to evaluate AR learning while systematically varying threat intensity. In the first, WS duration was varied to test how US imminence affects AR learning. In the second, WS–US contingency was varied to test how US certainty affects AR learning. In Pavlovian studies, reducing US imminence or certainty appears to promote anxiety over fear; freezing reactions are diminished and more flexible antipredator strategies increase (Rescorla 1968; Blanchard et al. 1989; Helmstetter and Fanselow 1993; Cain et al. 2005; Waddell et al. 2006; Mobbs et al. 2007; Kim and Jung 2018; Goode et al. 2019). Lesions that impair freezing rescue ARs in poor avoiders, suggesting that freezing reactions interfere with avoidance (Choi et al. 2010; Lazaro-Munoz et al. 2010; Moscarello and LeDoux 2013). Pavlovian reactions also impair avoidance performance in humans (Rigoli et al. 2012). Thus, we predicted that both methods of reducing threat intensity would decrease Pavlovian freezing and improve AR learning.
Experiments were conducted on adult male and female Sprague–Dawley rats (Hilltop Lab Animals,) weighing 300–350 g on arrival (N = 8/group; 4 females, 4 males—unless otherwise noted). Rats were pair-housed by sex, had ad lib access to food and water, and were tested during the light phase of a 12:12-h light:dark schedule. All procedures were approved by the NKI-IACUC.
All rats received 10 d of two-way SigAA training in standard shuttleboxes equipped with speakers, houselights, cameras, grid floors and infrared beams to detect shuttling (Coulbourn Instruments). Sessions included a 5-min acclimation followed by 15 trials where warning stimuli (80 dB white noise) preceded scrambled 0.5 sec footshocks (males: 1.0 mA, females: 0.7 mA). Males received stronger shocks because they have higher shock thresholds (Pare 1969), and because pilot studies in our laboratory showed similar AR learning rates with these parameters, but not with equal shock intensities. Session 1 always began with an inescapable Pavlovian trial, ensuring that all subsequent WS-shuttles occurred during threat of shock. For all subsequent trials, shuttling to the opposite chamber side terminated the WS, produced feedback (5 sec, 5 kHz, 80 dB tone), and canceled the upcoming shock (if scheduled). Shuttling was automatically recorded by Graphic State software (Coulbourn Instruments) and freezing was recorded to video files for off-line analysis. Intertrial intervals (ITIs) averaged 2-min unless otherwise stated. Avoidance percentage was calculated for individuals each session [(WS−shuttles/Trials)*100]. Avoidance latency reflects the time from WS onset to shuttle, with failures recorded as the full WS duration. Freezing was scored during all WSs for select sessions by two experienced raters blind to group (interrater reliability correlation >0.9). To facilitate comparisons of freezing suppression between studies, Session 10 freezing was also analyzed as a percentage of Session 1 freezing (calculated for individuals and then averaged).
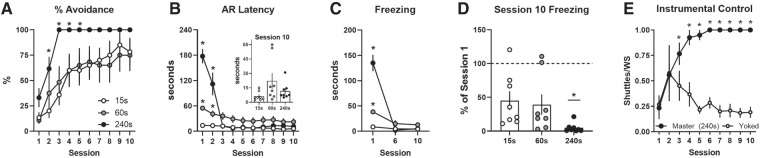
In Experiment 1, rats received avoidance training with 100% WS–US contingency (shock delivered on every failure trial), but the WS duration was varied (15, 60, or 240 sec; Fig. 1A,B). A two-way ANOVA (GraphPad Prism v8) indicated that AR% increased across training (Session: F(9,189) = 21.1, P < 0.01) and there was a significant effect of WS duration (Group: F(2,21) = 4.7, P = 0.02), however the pattern of change over time did not differ between groups (Group × Session: F(18,189) = 0.9). The effect of WS duration was driven mainly by the 240 sec group, where AR% was higher than the 15 sec group for Sessions 2–5 (Dunnett's tests). Remarkably, every rat in the 240 sec group showed perfect avoidance from Session 3 until the end of training (no failures). As expected for different WS durations, large differences in AR latency were observed across training (Group × Session: F(18,189) = 21.9, P < 0.01). These differences are not very informative early in training when failures are common and WS-duration determines AR latency. Interestingly, AR latencies were very similar by the end of training, even though rats in the 60 and 240 sec groups had much more time to emit ARs (Fig. 1B, inset). Large freezing differences were also apparent across training (Fig. 1C; Groups × Session: F(4,42) = 37.7, P < 0.01). This may be partly explained by the different WS durations. Rats in all groups froze for most of the WS early in training and freezing declined to similarly low levels as ARs were acquired. Dunnett's post tests revealed that rats in the 240 and 60 sec groups froze more than rats in the 15 sec group during Session 1 only (P < 0.01). Rats in the 240 sec group showed the strongest suppression of freezing by Session 10 (Fig. 1D; one-way ANOVA: F(2,21) = 3.4, P = 0.05; Dunnett's test versus 15 sec group: P < 0.05). This appears to reflect more than the programmed differences in WS duration; unlike the other groups, no rats in the 240 sec group maintained or increased freezing across training (as occurs in poor avoiders; Lazaro-Munoz et al. 2010).
Figure 1.
Reducing US imminence leads to perfect avoidance. (A) Mean percent avoidance by session. (B) Mean avoidance response (AR) latency by session. (inset) mean AR latency for individuals during Session 10. (C) Mean seconds freezing during warning signals for sessions 1, 6, and 10. (D) Mean Session 10 freezing expressed as a percentage of Session 1 freezing. (E) Mean number of shuttles per WS (separate experiment). Dots represent individuals. N = 8/group (4 females, 4 males except for Master (240 sec) group: 5 females, 3 males). Error bars = S.E.M. (*) P < 0.05 versus 15 sec-WS or Master groups.
One potential criticism of the long WS is that apparent ARs reflect locomotor activity not instrumental shuttling. To address this, we replicated AR training with the 240 sec WS (Master: 5 females, 3 males) and included Yoked controls. All rats in the 240 sec-WS group again attained perfect avoidance (Fig. 1E). Yoked controls shuttled far less frequently during the WSs than Master rats (<0.2 shuttles/trial on average by training end; Group × Session interaction (F(9,126) = 8.3, P < 0.01). This supports the notion that WS shuttles represent instrumental ARs even with long-duration WSs.
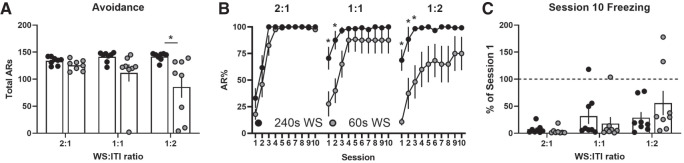
Experiment 1 was designed to test the effect of US imminence on avoidance learning. However, because WS duration was varied while the ITI was held constant, another explanation is possible. In Pavlovian studies, the conditioned stimulus (CS) to ITI ratio has a strong impact on performance of Pavlovian reactions (for review, see Balsam et al. 2010). Specifically, higher CS:ITI ratios weaken responding, perhaps because the long-duration signal loses informational value relative to the background context (Gibbon and Balsam 1989). Conversely, lower CS:ITI ratios (shorter CSs) predict the shock better than the context and elicit stronger Pavlovian reactions. Thus, it is possible that our long WS enhanced avoidance because it increased the WS:ITI ratio and weakened competing freezing reactions. To address this, we evaluated avoidance and freezing with WS:ITI ratios of 2:1, 1:1, and 1:2 using two different WS durations (60 and 240 sec). We trained four new groups of rats: 60 sec-WS:30 sec-ITI (2:1), 60 sec-WS:60 sec-ITI (1:1), 240 sec-WS:480 sec-ITI (1:2), and 240 sec-WS:240 sec-ITI (1:1). The remaining groups for the analysis came from Experiment 1: 60 sec-WS:120 sec-ITI (1:2) and 240 sec-WS:120 sec-ITI (2:1). Figure 2A depicts total (cumulative) ARs across training. A two-way ANOVA revealed a significant effect of WS-duration (F(1,42) = 12.9, P < 0.01) and a nonsignificant trend toward a WS-duration × Ratio interaction (F(2,42) = 2.6, P = 0.09). The main effect for Ratio was not significant (F(2,42) = 1.3). Thus, reducing the WS:ITI ratio failed to impair ARs and perfect avoidance was achieved for every rat trained with the 240 sec-WS. The same manipulation may reduce avoidance with a shorter WS; total ARs declined as the WS:ITI ratio dropped for the 60 sec WS. Further, in the 1:2 condition, rats in the 60 sec group avoided less than rats in the 240 sec group (planned Sidak's comparison). Figure 2B shows learning rates for the same six groups. Two-way ANOVAs revealed significant differences in the 1:1 (Group × session: F(9,126) = 4.0, P < 0.01) and 1:2 (Group: F(1,14) = 9.0, P < 0.01) experiments, but not in the 2:1 experiment (Group: F(1,14) = 3.6, P = 0.08, Group × Session: F(9,126) = 1.2, P = 0.31). The benefit of the long 240 sec-WS was especially apparent early in training with lower WS:ITI ratios (longer ITIs); planned Sidak's comparisons revealed better avoidance in the 240 sec group (P < 0.05) for sessions 1–2 (1:1 experiment) and sessions 1–3 (1:2 experiment). Suppression of freezing was more sensitive to the WS:ITI ratio (Fig. 2C). Session 10 freezing increased as the WS:ITI ratio dropped (Ratio: F(2,42) = 0.03, P = 0.03), but this effect was not modulated by WS-duration (WS-duration: F(1,42) = 0.09; WS-duration × Ratio: F(2,42) = 1.4). This suggests that the WS:ITI ratio is not a major determinant of AR acquisition with very long WSs. However, there are indications that reducing this ratio promotes freezing and impairs AR learning with shorter WSs. The reverse also appears true; using a 2:1 ratio led to very low Session 10 freezing and perfect avoidance for seven of eight rats trained with the 60 sec-WS. Exploring a wider range of WS:ITI ratios may help clarify these findings.
Figure 2.
Reducing the WS:ITI ratio fails to impair avoidance with a 240 sec warning signal. (A) Total avoidance responses emitted across 10 sessions of training. (B) Mean percent avoidance by session. (C) Mean Session 10 freezing expressed as a percentage of Session 1 freezing. N = 8/group (four females, four males). Bars represent separate groups. Bar height indicates group mean. Dots represent individuals. Error bars = S.E.M. (*) P < 0.05 for 240 sec versus 60 sec WS groups.
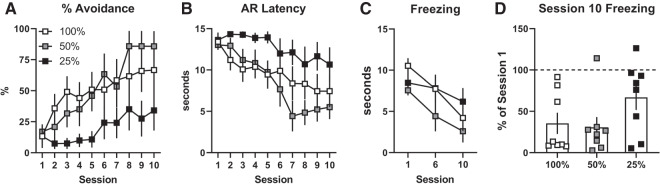
In Experiment 2, rats received SigAA training with a standard 15 sec WS except the likelihood of receiving a shock on failure trials was varied (100%, 50%, or 25%; Fig. 3A,B). A two-way ANOVA indicated group differences in acquisition rate for both AR% and AR latency (Group × Session interactions: F(18,189) = 1.8, P = 0.02; F(18,189) = 2.0, P = 0.01), however, reducing WS–US contingency did not improve learning. These differences appear to be driven by a deficit in the 25% group, where rats shuttled on average more slowly and less frequently. On average, freezing declined across avoidance training but there were no significant group differences. A two-way ANOVA revealed a significant effect for Session (F(2,42) = 21.6, P < 0.01), but not for Group (F(2,21) = 0.26) or the Group × Session interaction (F(4,42) = 1.7; Fig. 3C). Similarly, Session 10 freezing was lower on average than Session 1 but no group differences were observed (Fig. 3D; One-way ANOVA: F(2,21) = 2.1).
Figure 3.
Reducing WS–US contingency does not improve avoidance. (A) Mean percent avoidance by session. (B) Mean avoidance response (AR) latency by session. (C) Mean seconds freezing during warning signals for Sessions 1, 6, and 10. (D) Mean Session 10 freezing expressed as a percentage of Session 1 freezing. Squares represent individuals. N = 8/group (4 females, 4 males). Error bars = S.E.M. (*) P < 0.05 versus 100% WS–US contingency group.
Last, all experiments included both female and male subjects. Avoidance learning with the 240s WS was nearly identical between the sexes as measured by AR% (Session: F(9,126) = 41.0, P < 0.01, Sex: F(1,14) = 2.3, Session × Sex: F(9,126) = 1.0) and AR latency (Session: F(9,126) = 39.3, P < 0.01, Sex: F(1,14) = 3.6, Session × Sex: F(9,126) = 0.6). Freezing across training was also very similar (Session: F(2,28) = 81.5, P < 0.01, Sex: F(1,14) = 0.29, Session × Sex: F(2,28) = 0.08). Sex differences were difficult to evaluate in the other conditions due to poor avoiders, which appear to occur randomly (equally likely in both sexes).
Our major finding is that reducing US imminence by extending WS duration greatly facilitates SigAA learning. In four separate groups trained with the long-duration WS, every rat learned and performed the task perfectly (no subsequent failures), sometimes in fewer than 30 trials. The benefits of the long-duration WS also resisted manipulations of the WS:ITI ratio that promote competing freezing reactions. Several observations also argue against the concern that shuttling during long-duration WSs reflects exploration rather than instrumental ARs. First, exploration was severely depressed early in training where rats froze for more than 60% of the WS. Second, once the response was acquired, ARs were emitted with short latencies (usually <15 sec). Third, yoked controls shuttled during the WS at a far lower rate than master rats.
What might explain the enhanced efficiency of SigAA with long-duration WSs? Though there are some reports of improved SigAA learning with slightly longer or more complex WSs (Solomon and Brush 1954; Levis and Stampfl 1972; Archer et al. 1984; Coll-Andreu et al. 1993; Satorra-Marin et al. 2001; Terburg et al. 2018), this has not been systematically studied. There are far more studies of US imminence using Pavlovian paradigms. These suggest that threats activate different components of the survival circuit depending on proximity to harm (modeled by CS–US delay; Walker and Davis 1997; Davis 1998; Sullivan et al. 2004; Waddell et al. 2006; Mobbs et al. 2007, 2009; Goode et al. 2020). Short-duration CSs recruit amygdala and periaqueductal gray to emit short-latency, inflexible, hard-wired responses that function to prevent threat escalation (e.g., freezing, a postencounter response) or escape harm (e.g., flight, a circa-strike response). Long-duration CSs recruit bed nucleus of the stria terminalis and prefrontal cortex to flexibly reorganize behavior (e.g., thigmotaxis, altered meal-patterns), presumably to prevent threat escalation and prepare the organism to defend against distant or uncertain harm. Importantly, high US-imminence restricts behavior to species-specific defense reactions whereas low US-imminence balances defense with other behaviors like exploration and reward procurement (Bolles 1970; Blanchard and Blanchard 1989; Gray and McNaughton 2000; Mobbs et al. 2015; Mobbs and Kim 2015; Fanselow 2018). Thus, long-duration WSs likely trigger less intense defensive strategies and allow for active responses like shuttling. This is consistent with observed patterns of freezing; though 240 sec-WS rats froze significantly early in training (∼62%), they had more time to emit the AR and experience the instrumental contingency than rats in the other groups. Freezing appeared to be more easily suppressed in this condition too. Thus, optimal trial-and-error SigAA mechanisms may have evolved under low threat conditions, where errors (failures to emit the AR) lead to more intense threats and not necessarily harm.
Interestingly, our follow-up experiment suggests another possible way to improve SigAA efficiency: increase the WS:ITI ratio. Pavlovian studies show that increasing the CS:ITI ratio impairs Pavlovian reactions (Stein et al. 1958; Delamater and Holland 2008; Balsam et al. 2010). This is likely a result of the CS losing informational value relative to the background context (Gibbon and Balsam 1989), making the CS a weaker threat. A similar pattern emerges in freezing suppression during SigAA training; increasing the WS:ITI ratio produces weaker Session 10 freezing and near-perfect avoidance with the shorter 60 sec-WS. If replicated, this protocol could ensure good avoidance in all subjects with significantly shorter session durations.
Though our hypothesis about lowering threat intensity to improve avoidance was supported by the US-imminence experiment, it was not supported by reducing WS–US contingency. Rats receiving shocks on only 25% of failure trials did not perform better than rats in the 100% contingency condition. We see two possible explanations for this. First, SigAA learning depends, at least in part, on omission of expected US presentations (Hunter 1935; Kamin 1956; Bolles et al. 1966; Cain 2019). So even if 25% WS–US contingency reduces certainty and competing freezing reactions, this may have been offset by degradation of an important reinforcement signal. Second, 10 sessions of SigAA training may have been too few to observe the benefit of reduced WS–US contingency. Additional work is needed to clarify these points. We found only two other studies that varied shock delivery on failure trials (Boren and Sidman 1957; Neffinger and Gibbon 1975). Though these were conducted quite differently (contingency manipulated after standard training, poor avoiders eliminated, no-ITI protocols, bar-press avoidance etc.), both confirm that reducing the likelihood of shock on failure trials leads to an avoidance decrement.
In summary, we describe two simple procedural methods to improve SigAA learning and eliminate poor avoidance: increase the WS duration and/or the WS:ITI ratio. This removes a major obstacle to SigAA research that has dampened enthusiasm for the paradigm over decades. Experiments requiring pretraining manipulations can be used with confidence when controls reliably learn and perform ARs. The explanation for enhanced avoidance with low-intensity threats also aligns with functional behavior systems theories of defensive behavior and Pavlovian studies of US-imminence (Fanselow and Lester 1988; Waddell et al. 2006; Mobbs and Kim 2015). However, it is inconsistent with two-factor “fear” theories of avoidance that assume avoidance positively correlates with threat intensity (Mowrer and Lamoreaux 1946; Miller 1948; Levis 1989). This work may also help explain how strong avoidance responses are acquired in human anxiety even when harm is not imminent.
Acknowledgments
This work was supported by the National Institute of Mental Health of the National Institutes of Health under award numbers [R01MH114931] to C. Cain and [R21MH116242] to R. Sears. Additional funding for this project was provided by The William S. McIntyre Foundation to R. Sears. The authors thank Peter Balsam and Michael Fanselow for helpful discussions about the data.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.051557.120.
Freely available online through the Learning & Memory Open Access option.
References
- APA. 2013. Diagnostic and statistical manual of mental disorders. American Psychiatric Publishing, Arlington, VA. [Google Scholar]
- Archer T, Ogren S, Johansson G. 1984. Stimulus conditions affecting the rate of acquisition in a computer-operated version of the two-way active avoidance procedure. Scand J Psychol 25: 89–95. 10.1111/j.1467-9450.1984.tb01003.x [DOI] [Google Scholar]
- Balsam PD, Drew MR, Gallistel CR. 2010. Time and associative learning. Comp Cogn Behav Rev 5: 1–22. 10.3819/ccbr.2010.50001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. 1989. Antipredator defensive behaviors in a visible burrow system. J Comp Psychol 103: 70–82. 10.1037/0735-7036.103.1.70 [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Hori K. 1989. Ethoexperimental approaches to the study of defensive behavior. In Ethoexperimental approaches to the study of behavior (ed. Blanchard RJ, Brain PF, Blanchard DC, Parmigiani S), pp. 114–136. Kluwer Academic Publishers, Dordrecht. [Google Scholar]
- Boeke EA, Moscarello JM, LeDoux JE, Phelps EA, Hartley CA. 2017. Active avoidance: neural mechanisms and attenuation of Pavlovian conditioned responding. J Neurosci 37: 4808–4818. 10.1523/JNEUROSCI.3261-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles RC. 1970. Species-specific defense reactions and avoidance learning. Psychol Rev 77: 32–48. 10.1037/h0028589 [DOI] [Google Scholar]
- Bolles RC. 1975. Theory of motivation. Harper & Row, New York. [Google Scholar]
- Bolles RC, Stokes LW, Younger MS. 1966. Does CS termination reinforce avoidance behavior? J Comp Physiol Psychol 62: 201–207. 10.1037/h0023678 [DOI] [PubMed] [Google Scholar]
- Boren JJ, Sidman M. 1957. Maintenance of avoidance behaviour with intermittent shocks. Can J Psychol 11: 185–192. 10.1037/h0083704 [DOI] [PubMed] [Google Scholar]
- Cain CK. 2019. Avoidance problems reconsidered. Curr Opin Behav Sci 26: 9–17. 10.1016/j.cobeha.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CK, Godsil BP, Jami S, Barad M. 2005. The L-type calcium channel blocker nifedipine impairs extinction, but not reduced contingency effects, in mice. Learn Mem 12: 277–284. 10.1101/lm.88805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CK, LeDoux JE. 2007. Escape from fear: a detailed behavioral analysis of two atypical responses reinforced by CS termination. J Exp Psychol Anim Behav Process 33: 451–463. 10.1037/0097-7403.33.4.451 [DOI] [PubMed] [Google Scholar]
- Cain CK, Sullivan GM, LeDoux JE. 2013. The neurobiology of fear and anxiety: contributions of animal models to current understanding. In Neurobiology of mental illness (ed. Charney DS, Buxbaum JD, Sklar P, Nestler EJ), pp. 549–566. Oxford University Press, New York. [Google Scholar]
- Choi JS, Cain CK, LeDoux JE. 2010. The role of amygdala nuclei in the expression of auditory signaled two-way active avoidance in rats. Learn Mem 17: 139–147. 10.1101/lm.1676610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll-Andreu M, Marti-Nicolovius M, Portell-Cortes I, Morgado-Bernal I. 1993. Facilitation of shuttle-box avoidance by the platform method: effects of conditioned stimulus duration. Physiol Behav 53: 349–352. 10.1016/0031-9384(93)90216-3 [DOI] [PubMed] [Google Scholar]
- Collins KA, Mendelsohn A, Cain CK, Schiller D. 2014. Taking action in the face of threat: neural synchronization predicts adaptive coping. J Neurosci 34: 14733–14738. 10.1523/JNEUROSCI.2152-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. 1998. Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry 44: 1239–1247. 10.1016/S0006-3223(98)00288-1 [DOI] [PubMed] [Google Scholar]
- Delamater AR, Holland PC. 2008. The influence of CS-US interval on several different indices of learning in appetitive conditioning. J Exp Psychol Anim Behav Process 34: 202–222. 10.1037/0097-7403.34.2.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl MM, Bravo-Rivera C, Quirk GJ. 2019. The study of active avoidance: a platform for discussion. Neurosci Biobehav Rev 107: 229–237. 10.1016/j.neubiorev.2019.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. 1997. Species-specific defense reactions: retrospect and prospect. In Learning, motivation, and cognition: the functional behaviorism of Robert C Bolles (ed. Bouton ME, Fanselow MS), pp. 321–341. American Psychological Association, Washington, D.C. [Google Scholar]
- Fanselow MS. 2018. The role of learning in threat imminence and defensive behaviors. Curr Opin Behav Sci 24: 44–49. 10.1016/j.cobeha.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS and Lester LS (1988) A functional behavioristic approach to aversively motivated behavior: predatory imminence as a determinant of the topography of defensive behavior. In Evolution and learning (ed. Bolles RC, Beecher MD), pp. 185–211. Erlbaum, Hillsdale, NJ. [Google Scholar]
- Galatzer-Levy IR, Moscarello J, Blessing EM, Klein J, Cain CK, LeDoux JE. 2014. Heterogeneity in signaled active avoidance learning: substantive and methodological relevance of diversity in instrumental defensive responses to threat cues. Front Syst Neurosci 8: 179 10.3389/fnsys.2014.00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon J, Balsam P. 1989. Spreading associations in time. In Autoshaping and conditioning theory (ed. Locurto CM, Terrace HS, Gibbon J), pp. 219–253. Academic, New York. [Google Scholar]
- Goode TD, Ressler RL, Acca GM, Miles OW, Maren S. 2019. Bed nucleus of the stria terminalis regulates fear to unpredictable threat signals. Elife 8: e46525 10.7554/eLife.46525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Acca GM, Maren S. 2020. Threat imminence dictates the role of the bed nucleus of the stria terminalis in contextual fear. Neurobiol Learn Mem 167: 107116 10.1016/j.nlm.2019.107116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. 2000. The neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system. Oxford University Press, New York. [Google Scholar]
- Helmstetter FJ, Fanselow MS. 1993. Aversively motivated changes in meal patterns of rats in a closed economy: the effects of shock density. Anim Learn Behav 21: 168–175. 10.3758/BF03213397 [DOI] [Google Scholar]
- Hunter WS. 1935. Conditioning and extinction in the rat. Br J Psychol 26: 135–148. 10.1111/j.2044-8295.1935.tb00781.x [DOI] [Google Scholar]
- Kamin LJ. 1956. The effects of termination of the CS and avoidance of the US on avoidance learning. J Comp Physiol Psychol 49: 420–424. 10.1037/h0088011 [DOI] [PubMed] [Google Scholar]
- Kamin CJ, Brimer CJ, Black AH. 1963. Conditioned suppression as a monitor of fear of the CS in the course of avoidance training. J Comp Physiol Psychol 56: 497–501. 10.1037/h0047966 [DOI] [PubMed] [Google Scholar]
- Keehn JD. 1967. Running and bar pressing as avoidance responses. Psychol Rep 20: 591–602. 10.2466/pr0.1967.20.2.591 [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. 2018. Fear paradigms: the times they are a-changing’. Curr Opin Behav Sci 24: 38–43. 10.1016/j.cobeha.2018.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krypotos AM, Effting M, Kindt M, Beckers T. 2015. Avoidance learning: a review of theoretical models and recent developments. Front Behav Neurosci 9: 189 10.3389/fnbeh.2015.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro-Munoz G, LeDoux JE, Cain CK. 2010. Sidman instrumental avoidance initially depends on lateral and basal amygdala and is constrained by central amygdala-mediated Pavlovian processes. Biol Psychiatry 67: 1120–1127. 10.1016/j.biopsych.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Gorman JM. 2001. A call to action: overcoming anxiety through active coping. Am J Psychiatry 158: 1953–1955. 10.1176/appi.ajp.158.12.1953 [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Moscarello J, Sears R, Campese V. 2017. The birth, death and resurrection of avoidance: a reconceptualization of a troubled paradigm. Mol Psychiatry 22: 24–36. 10.1038/mp.2016.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis DJ. 1989. The case for a return to a two-factor theory of avoidance: the failure of non-fear interpretations. In Contemporary learning theories: Pavlovian conditioning and the status of traditional learning theory (ed. Klein SB, Mowrer RR), pp. 227–277. Lawrence Erlbaum Assoc., Hillsdale. [Google Scholar]
- Levis DJ, Stampfl TG. 1972. Effects of serial CS presentation on shuttlebox avoidance responding. Learn Motiv 3: 73–90. 10.1016/0023-9690(72)90049-5 [DOI] [Google Scholar]
- Martinez RC, Gupta N, Lazaro-Munoz G, Sears RM, Kim S, Moscarello JM, LeDoux JE, Cain CK. 2013. Active vs. reactive threat responding is associated with differential c-Fos expression in specific regions of amygdala and prefrontal cortex. Learn Mem 20: 446–452. 10.1101/lm.031047.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NE. 1948. Studies of fear as an acquirable drive: I. Fear as motivation and fear reduction as reinforcement in the learning of new responses. J Exp Psychol 38: 89–101. 10.1037/h0058455 [DOI] [PubMed] [Google Scholar]
- Mobbs D, Kim JJ. 2015. Neuroethological studies of fear, anxiety, and risky decision-making in rodents and humans. Curr Opin Behav Sci 5: 8–15. 10.1016/j.cobeha.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD. 2007. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science 317: 1079–1083. 10.1126/science.1144298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, Petrovic P, Dolan RJ, Frith CD. 2009. From threat to fear: the neural organization of defensive fear systems in humans. J Neurosci 29: 12236–12243. 10.1523/JNEUROSCI.2378-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Hagan CC, Dalgleish T, Silston B, Prevost C. 2015. The ecology of human fear: survival optimization and the nervous system. Front Neurosci 9: 55 10.3389/fnins.2015.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscarello JM, Hartley CA. 2017. Agency and the calibration of motivated behavior. Trends Cogn Sci 21: 725–735. 10.1016/j.tics.2017.06.008 [DOI] [PubMed] [Google Scholar]
- Moscarello JM, LeDoux JE. 2013. Active avoidance learning requires prefrontal suppression of amygdala-mediated defensive reactions. J Neurosci 33: 3815–3823. 10.1523/JNEUROSCI.2596-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowrer OH, Lamoreaux RR. 1946. Fear as an intervening variable in avoidance conditioning. J Comp Psychol 39: 29–50. 10.1037/h0060150 [DOI] [PubMed] [Google Scholar]
- Neffinger GG, Gibbon J. 1975. Partial avoidance contingencies. J Exp Anal Behav 23: 437–450. 10.1901/jeab.1975.23-437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare WP. 1969. Age, sex, and strain differences in the aversive threshold to grid shock in the rat. J Comp Physiol Psychol 69: 214–218. 10.1037/h0028196 [DOI] [PubMed] [Google Scholar]
- Rescorla RA. 1968. Probability of shock in the presence and absence of CS in fear conditioning. J Comp Physiol Psychol 66: 1–5. 10.1037/h0025984 [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Solomon RL. 1967. Two process learning theory: relationships between Pavlovian conditioning and instrumental learning. Psychol Rev 74: 151–182. 10.1037/h0024475 [DOI] [PubMed] [Google Scholar]
- Rigoli F, Pavone EF, Pezzulo G. 2012. Aversive Pavlovian responses affect human instrumental motor performance. Front Neurosci 6: 134 10.3389/fnins.2012.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satorra-Marin N, Coll-Andreu M, Portell-Cortes I, Aldavert-Vera L, Morgado-Bernal I. 2001. Impairment of two-way active avoidance after pedunculopontine tegmental nucleus lesions: effects of conditioned stimulus duration. Behav Brain Res 118: 1–9. 10.1016/S0166-4328(00)00306-5 [DOI] [PubMed] [Google Scholar]
- Solomon RL, Brush ES. 1954. Experimentally derived conceptions of anxiety and aversion. In Nebraska symposium on motivation (ed. Jones MR), pp. 201–305. Nebraska Press, Lincoln, NE. [Google Scholar]
- Stein L, Sidman M, Brady JV. 1958. Some effects of two temporal variables on conditioned suppression. J Exp Anal Behav 1: 153–162. 10.1901/jeab.1958.1-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, LeDoux JE. 2004. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not a specific cue-conditioned fear stimulus. Neuroscience 128: 7–14. 10.1016/j.neuroscience.2004.06.015 [DOI] [PubMed] [Google Scholar]
- Terburg D, Scheggia D, Triana Del Rio R, Klumpers F, Ciobanu AC, Morgan B, Montoya ER, Bos PA, Giobellina G, van den Burg EH, et al. 2018. The basolateral amygdala is essential for rapid escape: a human and rodent study. Cell 175: 723–735 e716. 10.1016/j.cell.2018.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kolk BA. 2006. Clinical implications of neuroscience research in PTSD. Ann N Y Acad Sci 1071: 277–293. 10.1196/annals.1364.022 [DOI] [PubMed] [Google Scholar]
- Waddell J, Morris RW, Bouton ME. 2006. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav Neurosci 120: 324–336. 10.1037/0735-7044.120.2.324 [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. 1997. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci 17: 9375–9383. 10.1523/JNEUROSCI.17-23-09375.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Krieckhaus EE, Conte R. 1968. Effects of fear conditioning on subsequent avoidance behavior and movement. J Comp Physiol Psychol 65: 413–421. 10.1037/h0025832 [DOI] [PubMed] [Google Scholar]