Abstract
We investigated the association between disaster experience and the cardiometabolic risk of survivors 2.5 years after disaster onset, adjusting for health information predating the disaster, using natural experiment data stemming from the 2011 Great East Japan Earthquake and Tsunami. We used data from a cohort of adults aged 65 years or older in Iwanuma City, Japan, located 80 km (128 miles) west of the earthquake epicenter. The baseline survey was completed 7 months before the disaster, and the follow-up survey was performed among survivors approximately 2.5 years after the disaster. The survey data were linked to medical records with information on objectively measured cardiometabolic risk factors (n = 1,195). The exposure of interest was traumatic disaster experiences (i.e., housing damage and loss of loved ones). Fixed-effects regression showed that complete housing destruction was significantly associated with a 0.81-unit greater change in body mass index (weight (kg)/height (m)2; 95% confidence interval (CI): 0.24, 1.38), a 4.26-cm greater change in waist circumference (95% CI: 1.12, 7.41), and a 4.77-mg/dL lower change in high-density lipoprotein cholesterol level (95% CI: −7.96, −1.58) as compared with no housing damage. We also observed a significant association between major housing damage and decreased systolic blood pressure. Continued health checkups and supports for victims who lost homes should be considered to maintain their cardiometabolic health.
Keywords: biomarkers, cardiometabolic risk, disasters, fixed-effect analysis, Japan, natural experiment
Beyond the immediate loss of life, natural disasters have been shown to affect the mental and physical health of survivors (1–3). The short-term impact of exposure to a natural disaster (e.g., earthquakes) on the cardiometabolic health of survivors, such as increased incidence of heart attack, has been well established (4–9). Likewise, it is possible that disaster exposure has an adverse impact on cardiometabolic risk factors in the long term due to prolonged psychological distress and changes in health behaviors, including dietary behaviors and physical activity (10–12). However, the empirical evidence is limited, especially studies that include information on the health status of victims predating the disaster.
A handful of studies have examined and found an increase in levels of risk factors several years after a disaster event, suggesting the potential for long-term effects of disaster exposure (13–15). These studies are limited in several ways. First, adjustment for baseline cardiometabolic risks and subjects’ characteristics, such as health-related behaviors and socioeconomic status, is seldom possible. The reason is that researchers studying the health consequences of natural disasters typically collect data retrospectively after the event and do not have predisaster data. Thus, there is a potential for residual confounding bias. For example, persons with preexisting health problems may be more likely to be victimized by a disaster (e.g., because their evacuation tends to be delayed) and to be sick after the disaster. Hence, any association between disaster-related trauma and postdisaster health status is likely to be confounded by predisaster health status. This problem cannot be entirely fixed by retrospectively asking survivors about their predisaster health status because of the potential for recall bias; that is, victims who experienced severe disaster-related trauma are apt to selectively recall their predisaster health problems.
Second, more understanding is needed of the differential effects of disaster by levels of the severity and types of disaster-related experiences. A previous study using data from the 1983 earthquake in Naples, Italy—where the magnitude of the earthquake was small (4.0 on the Richter scale)—found no long-term association between disaster exposure and cardiometabolic risk factors, including blood pressure and serum total cholesterol level (16, 17). On the other hand, studies that examined disasters of larger magnitude, including the Armenian earthquake in 1988 (Richter magnitude 6.8) and the Great East Japan Earthquake in 2011 (Richter magnitude 9.0), did detect long-term associations between disaster exposure and increased cardiovascular mortality and morbidity, as well as risk factors such as blood pressure, waist circumference, and serum triglyceride levels, suggesting the importance of assessing the magnitude-specific impact of natural disasters (13–15). Moreover, there are several types of disaster exposure ranging from property damage to psychological trauma due to loss of someone important to the victim. Investigating type-specific health effects of natural disaster would allow us to better understand the underlying mechanisms and potential interventions. However, because of lack of data, the assessment of natural disaster has been mostly limited to comparisons between evacuees and nonevacuees or to pre-/postdisaster within-individual comparisons of the outcomes (13–17).
Lastly, assessment of cardiometabolic outcomes using a variety of measures is warranted. For instance, adiposity has been measured solely on the basis of body mass index (BMI), a ratio of weight to squared height, and is therefore limited. Older adults are one of the most vulnerable populations during and in the aftermath of natural disaster and thus have been the primary targets of previous studies on health consequences of disaster exposure (7, 18, 19). Since BMI is not the most valid measure of fatness among older adults because of differential loss of muscle mass with aging, other measures of adiposity, such as waist circumference, need to be assessed as well (20). Moreover, few previous studies have assessed biomarkers of cardiometabolic risk such as serum lipid levels, comparing them with the biomarker information that predates disaster.
Our purpose in this study was therefore to examine the severity- and type-specific associations between disaster exposure and a comprehensive profile of cardiometabolic biomarkers. We took advantage of a unique “natural experiment” stemming from the 2011 Great East Japan Earthquake and Tsunami, which killed more than 18,000 people and caused involuntary evacuation and displacement of approximately 345,000 people (21, 22).
The Japan Gerontological Evaluation Study (JAGES), a nationwide cohort study of older adults in Japan, was established 7 months prior to the disaster with a baseline survey that inquired about predictors of healthy aging. Older adults are of particular importance in disaster medicine, since they tend to be disproportionately victimized by natural disasters. In the 2011 Great East Japan Earthquake, older adults constituted 89% of disaster-related deaths (23). Older adults are also vulnerable to health problems in the aftermath of disasters. For instance, we have previously reported that older survivors of the 2011 Great East Japan Earthquake experienced increased risks of functional disability, cognitive decline, and depression (18, 24, 25).
One of the study sites of the JAGES project was Iwanuma City, which was severely damaged by the earthquake and tsunami in 2011. Approximately 2.5 years after the disaster, we conducted a follow-up survey targeting the survivors in Iwanuma and gathered data on their disaster experiences. Thus, we were able to collect rich “predisaster” data on baseline demographic characteristics, as well as a series of anthropometric measures and biomarkers of cardiometabolic risk, in addition to postdisaster data.
METHODS
Data
JAGES is a nationwide Japanese cohort study established in 2010 with the objective of studying the predictors of healthy aging. The baseline survey was conducted in 2010, and we sampled 112,123 older adults residing in 31 municipalities in Japan (response rate = 66.3%).
One of the field sites of the JAGES study is Iwanuma City, located in Miyagi Prefecture, approximately 80 km (128 miles) west of the 2011 earthquake epicenter. Iwanuma had a total population of 44,187 in 2010 (26). In August 2010, we conducted a census of all residents aged 65 years or older in Iwanuma using the official residential register (n = 8,576). A total of 5,058 residents responded to the baseline survey (response rate = 59.0%).
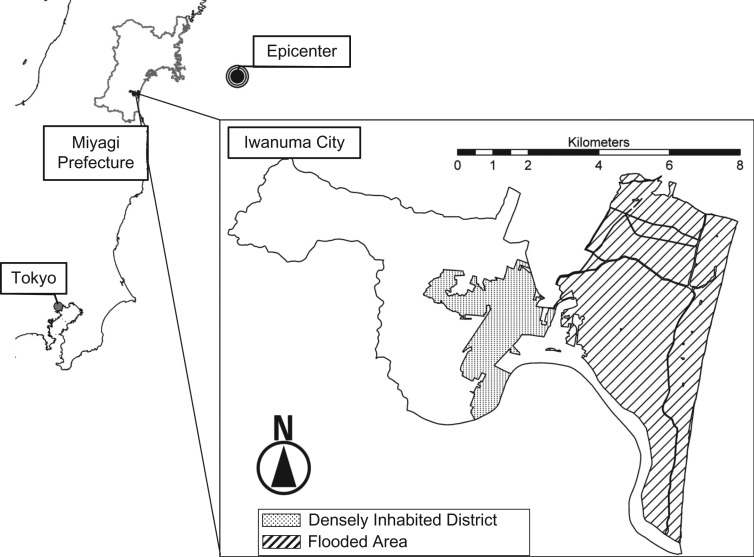
The Great East Japan Earthquake, registering a magnitude of 9.0 on the Richter scale, occurred on March 11, 2011, 7 months after the baseline survey. The subsequent tsunami resulting from the earthquake caused devastating damage to coastal areas of northeastern Japan, including Iwanuma. In Iwanuma, the tsunami killed 180 residents, damaged 5,542 houses, and inundated 48% of the land area (27) (Figure 1).
Figure 1.
Inundated area in Iwanuma City, Japan, after the 2011 Great East Japan Earthquake and Tsunami. The earthquake occurred on March 11, 2011, and registered 9.0 on the Richter scale.
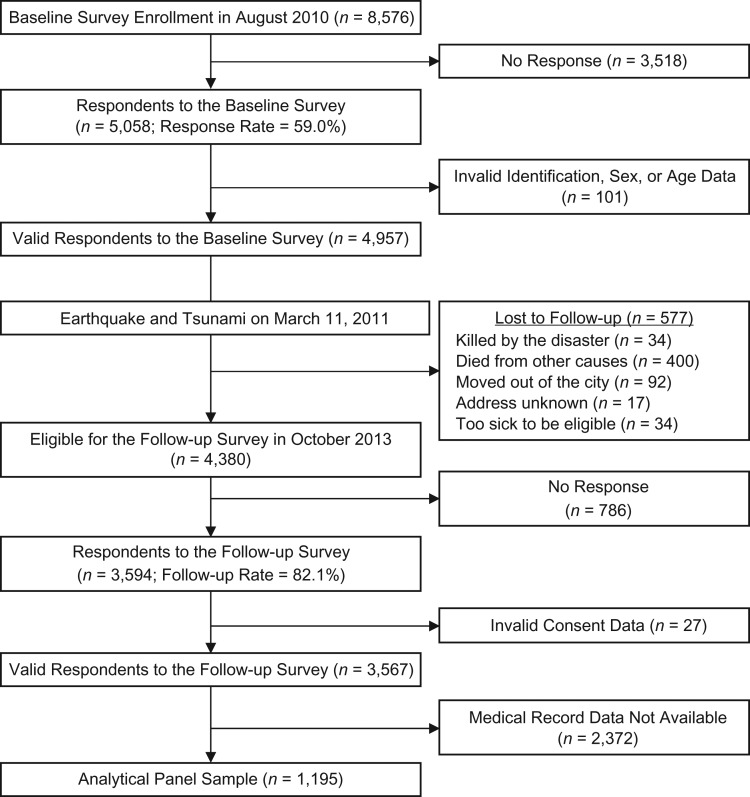
Approximately 2.5 years after the disaster (2013), we recontacted all survivors from the baseline cohort. With the cooperation of the Iwanuma municipal office, we identified the addresses of 99.7% of our original sample. Of the eligible participants from the baseline survey, 3,594 responded to our mailed follow-up survey (follow-up rate = 82.1%). After excluding invalid responses, we obtained panel data for 3,567 subjects. In addition to survey data, we also linked participants to medical record data from regular health checkups conducted by the municipality in 2010 and 2013 (28). During these health checkups, the participant’s height, weight, waist circumference, and blood pressure were measured by public health nurses. Blood samples were also collected from the participants and tested for serum levels of biomarkers (cholesterol, triglycerides, uric acid, and liver function). After exclusion of persons who did not participate in the 2010 and/or 2013 health checkup, a total of 1,195 subjects were linked to the JAGES panel data and used for these analyses. Details on selection of the analytical sample are presented in Figure 2.
Figure 2.
Selection of the analytical sample (n = 1,195) for a study of the association between disaster experience and cardiometabolic risk, Iwanuma City, Japan, 2010–2013. Biomarker information was obtained from medical record data from regular health checkups conducted at municipal public health clinics in 2010 and 2013.
Variables
Exposure variables
In the follow-up survey, we inquired about different types of disaster-related experiences, including housing damage, loss of relatives, loss of friends, and loss of pets. Housing damage was assessed by 2 or more technical officers inspecting residential properties and basing their assessment on objectively established criteria (29). The degree of housing damage was classified into one of 5 levels: “no damage,” “partial damage,” “minor damage,” “major damage,” and “complete destruction” (see Web Table 1, available at https://academic.oup.com/aje). Loss of relatives, loss of friends, and loss of pets were made into binary variables (yes/no).
Outcome variables
Our outcome of interest was a series of biomarkers associated with cardiometabolic risk. Systolic and diastolic blood pressure (mm Hg), BMI, waist circumference (cm), serum triglycerides (mg/dL), and high-density lipoprotein and low-density lipoprotein cholesterol (mg/dL) were measured in both 2010 and 2013. BMI was calculated on the basis of the standard formula: weight (kg)/height (m)2. Waist circumference was measured only among persons under age 75 years.
Time-varying confounders
As potential time-varying confounders, we adjusted for age group, household income, medical treatment for major diseases, current alcohol drinking, current smoking, and depressive symptoms. Age was categorized into “75 years or older” and “65–74 years” to control for the difference in medical cost-sharing expenses under the national health-care insurance system (30). In Japan, older adults renew their health-care insurance when they turn 75 years of age. Under the Japanese insurance system, copayment associated with health-care services for persons aged ≥75 years is less than it is for persons aged ≤74 years. Annual household income was equivalized in order to adjust for the number of members within households. We created 4 binary variables (yes/no) representing current medical treatment for hypertension, stroke, diabetes, and dyslipidemia. Current alcohol drinking and current smoking were also binarized (yes/no). Depressive symptoms were measured using the validated Japanese short version of the Geriatric Depression Scale (31). We used a cutoff score of 5 or above to indicate clinical depression. We did not adjust for physical activity and dietary behavior, since they were considered potential mediators of the relationship between disaster damage and cardiometabolic risk.
Statistical methods
We used a fixed-effects regression approach to estimate the association of disaster damage with cardiometabolic biomarkers. Because we used 2-wave panel data, the fixed-effects regression model was equivalent to the first-difference model, which is represented by the following equation.
where yit is the value of the dependent variable for the ith individual at time t, Xit is a vector of the exposure variable as well as time-varying confounders, and Δ indicates a difference in the value of each variable between the two time points (i.e., ). Our exposure variable, disaster damage assessed after the disaster (the year 2013), can be interpreted as a “change” variable, because nobody was exposed at baseline. The first-difference model can effectively eliminate confounding bias due to observed and unobserved time-invariant confounders in addition to observed time-varying confounders (32).
Because the biomarker data were available only for persons who voluntarily participated in the health checkups (34% of the respondents to the follow-up survey), there was a possibility of selection bias (33). To address this potential selection bias, we used inverse probability weighting for censoring (34). Inverse probability weighting for censoring can account for selective attrition by applying statistical weights that depend on a person’s probability of being observed and analyzed. For example, respondents who were less likely to participate in the health checkups because of certain characteristics such as poor baseline health would be up-weighted as if the analyses were performed in a pseudopopulation without selective dropout. Specifically, we calculated stabilized censoring weights using baseline health status as well as socioeconomic and demographic characteristics and performed weighted least-squares analysis using robust standard errors. For missing information on covariates used in the study, we performed multiple imputation by chained equations, assuming missingness at random. We used the “mice” package in R statistical software (R Foundation for Statistical Computing, Vienna, Austria) to create 20 imputed data sets and combined results from each imputed data set to obtain the final estimates (35). All analyses were performed using R, version 3.3.2.
Ethical considerations
The survey protocol was approved by the human subjects committee of the Harvard T.H. Chan School of Public Health, as well as the committees of Tohoku University, Nihon Fukushi University, and Chiba University. Informed consent was obtained at the time of data collection.
RESULTS
Demographic characteristics of the baseline JAGES participants, including sex, age, marital status, and working status, were close to those in the actual local census of older residents in Iwanuma (Web Table 2). Moreover, the baseline survey participants were similar to those who participated in the follow-up survey in terms of household income and health status (as measured by the presence of treatment for major diseases, depressive symptoms, and health behaviors) at baseline (Web Table 3). Compared with the whole panel sample, the analytical sample with biomarker data tended to be younger and to report less current medical treatment for major diseases, less current alcohol drinking, and higher income (Web Table 4). These variables were included in the calculation of our inverse probability weights.
Tables 1, 2, and 3 show the baseline characteristics and cardiometabolic biomarker profiles of the analytical sample according to type and severity of disaster damage. Nearly 11% of the population (n = 128) experienced “minor” or “major” housing damage, while 3.8% (n = 44) lost their houses because of complete destruction (Table 1). Moreover, 38.1% of the population lost relatives and/or friends in the disaster, and 3.7% lost their pets (Table 2). Persons in the lowest income tertile group were more likely to experience severe housing damage, as well as loss of loved ones, than those with higher income. Baseline systolic and diastolic blood pressure, BMI, waist circumference, and high-density lipoprotein cholesterol levels were similar across types and severity levels of disaster damage (Table 3). Baseline (predisaster) triglyceride and low-density lipoprotein cholesterol levels tended to be lower among persons with more severe disaster-related damage.
Table 1.
Baseline Characteristics of the Analytical Sample According to Housing Damage Incurred During the 2011 Great East Japan Earthquake and Tsunami, Iwanuma, Japan, 2010–2013
| Characteristic | No. of Persons | Housing Damage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Damage | Partial Damage | Minor Damage | Major Damage | Complete Destruction | Missing Data | ||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Total | 1,195 | 489 | 40.9 | 512 | 42.8 | 85 | 7.1 | 43 | 3.6 | 44 | 3.7 | 22 | 1.8 |
| Age category, years | |||||||||||||
| 65–74 | 887 | 367 | 41.4 | 371 | 41.8 | 63 | 7.1 | 35 | 3.9 | 35 | 3.9 | 16 | 1.8 |
| ≥75 | 308 | 122 | 39.6 | 141 | 45.8 | 22 | 7.1 | 8 | 2.6 | 9 | 2.9 | 6 | 1.9 |
| Tertile of equivalized annual household income, yen | |||||||||||||
| 1 (<1,750,000) | 367 | 136 | 37.1 | 153 | 41.7 | 26 | 7.1 | 18 | 4.9 | 27 | 7.4 | 7 | 1.9 |
| 2 (1,750,000–2,475,000) | 343 | 138 | 40.2 | 161 | 46.9 | 22 | 6.4 | 12 | 3.5 | 4 | 1.2 | 6 | 1.7 |
| 3 (>2,475,000) | 347 | 164 | 47.3 | 136 | 39.2 | 27 | 7.8 | 5 | 1.4 | 10 | 2.9 | 5 | 1.4 |
| Missing data | 138 | 51 | 37.0 | 62 | 44.9 | 10 | 7.2 | 8 | 5.8 | 3 | 2.2 | 4 | 2.9 |
| Hypertension treatment | |||||||||||||
| No | 762 | 309 | 40.6 | 341 | 44.8 | 52 | 6.8 | 24 | 3.1 | 24 | 3.1 | 12 | 1.6 |
| Yes | 414 | 174 | 42.0 | 163 | 39.4 | 31 | 7.5 | 18 | 4.3 | 19 | 4.6 | 9 | 2.2 |
| Missing data | 19 | 6 | 31.6 | 8 | 42.1 | 2 | 10.5 | 1 | 5.3 | 1 | 5.3 | 1 | 5.3 |
| Stroke treatment | |||||||||||||
| No | 1,159 | 472 | 40.7 | 500 | 43.1 | 83 | 7.2 | 41 | 3.5 | 42 | 3.6 | 21 | 1.8 |
| Yes | 17 | 11 | 64.7 | 4 | 23.5 | 0 | 0.0 | 1 | 5.9 | 1 | 5.9 | 0 | 0.0 |
| Missing data | 19 | 6 | 31.6 | 8 | 42.1 | 2 | 10.5 | 1 | 5.3 | 1 | 5.3 | 1 | 5.3 |
| Diabetes treatment | |||||||||||||
| No | 1,074 | 444 | 41.3 | 457 | 42.6 | 79 | 7.4 | 37 | 3.4 | 38 | 3.5 | 19 | 1.8 |
| Yes | 102 | 39 | 38.2 | 47 | 46.1 | 4 | 3.9 | 5 | 4.9 | 5 | 4.9 | 2 | 2.0 |
| Missing data | 19 | 6 | 31.6 | 8 | 42.1 | 2 | 10.5 | 1 | 5.3 | 1 | 5.3 | 1 | 5.3 |
| Dyslipidemia treatment | |||||||||||||
| No | 1,039 | 422 | 40.6 | 451 | 43.4 | 70 | 6.7 | 39 | 3.8 | 38 | 3.7 | 19 | 1.8 |
| Yes | 137 | 61 | 44.5 | 53 | 38.7 | 13 | 9.5 | 3 | 2.2 | 5 | 3.6 | 2 | 1.5 |
| Missing data | 19 | 6 | 31.6 | 8 | 42.1 | 2 | 10.5 | 1 | 5.3 | 1 | 5.3 | 1 | 5.3 |
| Depressive symptoms, pointsa | |||||||||||||
| 1–4 | 816 | 357 | 43.8 | 335 | 41.1 | 61 | 7.5 | 24 | 2.9 | 28 | 3.4 | 11 | 1.3 |
| ≥5 | 247 | 85 | 34.4 | 116 | 47.0 | 15 | 6.1 | 14 | 5.7 | 11 | 4.5 | 6 | 2.4 |
| Missing data | 132 | 47 | 35.6 | 61 | 46.2 | 9 | 6.8 | 5 | 3.8 | 5 | 3.8 | 5 | 3.8 |
a Depressive symptoms were measured by Geriatric Depression Scale (31) score.
Table 2.
Baseline Characteristics of the Analytical Sample According to Loss of Loved Ones and Pets During the 2011 Great East Japan Earthquake and Tsunami, Iwanuma, Japan, 2010–2013
| Characteristic | Loss of Relatives/Friends | Loss of Pets | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | Missing Data | No | Yes | Missing Data | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Total | 731 | 61.2 | 450 | 37.7 | 14 | 1.2 | 1,108 | 92.7 | 42 | 3.5 | 45 | 3.8 |
| Age category, years | ||||||||||||
| 65–74 | 526 | 44.0 | 349 | 29.2 | 12 | 1.0 | 818 | 68.5 | 38 | 3.2 | 31 | 2.6 |
| ≥75 | 205 | 17.2 | 101 | 8.5 | 2 | 0.2 | 290 | 24.3 | 4 | 0.3 | 14 | 1.2 |
| Tertile of equivalized annual household income, yen | ||||||||||||
| 1 (<1,750,000) | 210 | 17.6 | 153 | 12.8 | 4 | 0.3 | 333 | 27.9 | 16 | 1.3 | 18 | 1.5 |
| 2 (1,750,000–2,475,000) | 212 | 17.7 | 126 | 10.5 | 5 | 0.4 | 321 | 26.9 | 11 | 0.9 | 11 | 0.9 |
| 3 (>2,475,000) | 223 | 18.7 | 122 | 10.2 | 2 | 0.2 | 325 | 27.2 | 9 | 0.8 | 13 | 1.1 |
| Missing data | 86 | 7.2 | 49 | 4.1 | 3 | 0.3 | 129 | 10.8 | 6 | 0.5 | 3 | 0.3 |
| Hypertension treatment | ||||||||||||
| No | 462 | 38.7 | 295 | 24.7 | 5 | 0.4 | 710 | 59.4 | 24 | 2.0 | 28 | 2.3 |
| Yes | 258 | 21.6 | 147 | 12.3 | 9 | 0.8 | 384 | 32.1 | 16 | 1.3 | 14 | 1.2 |
| Missing data | 11 | 0.9 | 8 | 0.7 | 0 | 0.0 | 14 | 1.2 | 2 | 0.2 | 3 | 0.3 |
| Stroke treatment | ||||||||||||
| No | 707 | 59.2 | 438 | 36.7 | 14 | 1.2 | 1,077 | 90.1 | 40 | 3.3 | 42 | 3.5 |
| Yes | 13 | 1.1 | 4 | 0.3 | 0 | 0.0 | 17 | 1.4 | 0 | 0.0 | 0 | 0.0 |
| Missing data | 11 | 0.9 | 8 | 0.7 | 0 | 0.0 | 14 | 1.2 | 2 | 0.2 | 3 | 0.3 |
| Diabetes treatment | ||||||||||||
| No | 660 | 55.2 | 400 | 33.5 | 14 | 1.2 | 999 | 83.6 | 36 | 3.0 | 39 | 3.3 |
| Yes | 60 | 5.0 | 42 | 3.5 | 0 | 0.0 | 95 | 7.9 | 4 | 0.3 | 3 | 0.3 |
| Missing data | 11 | 0.9 | 8 | 0.7 | 0 | 0.0 | 14 | 1.2 | 2 | 0.2 | 3 | 0.3 |
| Dyslipidemia treatment | ||||||||||||
| No | 635 | 53.1 | 391 | 32.7 | 13 | 1.1 | 965 | 80.8 | 35 | 2.9 | 39 | 3.3 |
| Yes | 85 | 7.1 | 51 | 4.3 | 1 | 0.1 | 129 | 10.8 | 5 | 0.4 | 3 | 0.3 |
| Missing data | 11 | 0.9 | 8 | 0.7 | 0 | 0.0 | 14 | 1.2 | 2 | 0.2 | 3 | 0.3 |
| Depressive symptoms, pointsa | ||||||||||||
| 1–4 | 503 | 42.1 | 305 | 25.5 | 8 | 0.7 | 760 | 63.6 | 26 | 2.2 | 30 | 2.5 |
| ≥5 | 145 | 12.1 | 98 | 8.2 | 4 | 0.3 | 228 | 19.1 | 10 | 0.8 | 9 | 0.8 |
| Missing data | 83 | 6.9 | 47 | 3.9 | 2 | 0.2 | 120 | 10.0 | 6 | 0.5 | 6 | 0.5 |
a Depressive symptoms were measured by Geriatric Depression Scale (31) score.
Table 3.
Distribution (Mean (Standard Deviation)) of Cardiometabolic Risk Factors in the Analytical Sample According to Disaster Damage Incurred During the 2011 Great East Japan Earthquake and Tsunami, Iwanuma, Japan, 2010–2013
| Characteristic | Overall | Housing Damage | Emotional Loss | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Loss of Relatives/Friends | Loss of Pets | |||||||||
| No Damage | Partial Damage | Minor Damage | Major Damage | Complete Destruction | No | Yes | No | Yes | ||
| Systolic blood pressure, mm Hg | 129.5 (16.4) | 129.5 (15.6) | 129.4 (17.7) | 130.8 (13.2) | 128.1 (14.6) | 128.5 (16.7) | 129.6 (16.6) | 129.4 (16.3) | 129.5 (16.5) | 130.4 (17.8) |
| Diastolic blood pressure, mm Hg | 75 (10.3) | 75.5 (10.3) | 74.3 (10.4) | 75.6 (10.0) | 75.6 (9.7) | 75.1 (9.7) | 75 (10.4) | 75.1 (10.2) | 75 (10.4) | 77.4 (9.5) |
| Body mass indexa | 23.5 (3.0) | 23.2 (2.8) | 23.4 (2.9) | 24 (3.3) | 23.3 (3.1) | 24.5 (3.1) | 23.3 (2.9) | 23.6 (3.0) | 23.4 (2.9) | 24.3 (3.3) |
| Waist circumference, cm | 84.4 (8.6) | 83.9 (8.6) | 84.7 (8.3) | 85.6 (9.9) | 82.4 (8.3) | 85 (9.0) | 84 (8.4) | 84.9 (8.9) | 84.3 (8.5) | 86.1 (9.0) |
| Triglycerides, mg/dL | 119.2 (69.4) | 116.7 (58.6) | 122.1 (79.8) | 118.1 (72.4) | 112.4 (55.0) | 111.6 (62.2) | 121.3 (75.5) | 116.1 (58.7) | 119.1 (70.0) | 122.7 (63.9) |
| HDL cholesterol, mg/dL | 59.5 (15.2) | 58.8 (15.2) | 60 (15.2) | 58.6 (15.0) | 63.6 (17.5) | 58.8 (14.1) | 59.5 (15.5) | 59.5 (14.7) | 59.5 (15.4) | 58.8 (12.2) |
| LDL cholesterol, mg/dL | 119.4 (27.5) | 120.3 (27.6) | 120.6 (27.3) | 118 (27.9) | 112.3 (24.6) | 109.2 (25.7) | 120.2 (27.8) | 118.5 (27.0) | 120.2 (27.6) | 110.8 (26.6) |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein.
a Weight (kg)/height (m)2.
The fixed-effects models showed that after adjustment for all time-invariant and observed time-varying confounders, there were strong associations between housing destruction (reference group: no damage) and changes in BMI, waist circumference, and high-density lipoprotein cholesterol level (Table 4). Specifically, persons who experienced destruction of their property reported a 0.81-unit greater change in BMI (95% confidence interval (CI): 0.24, 1.38), a 4.26-cm greater change in waist circumference (95% CI: 1.12, 7.41), and a 4.77-mg/dL lower change in high-density lipoprotein cholesterol level (95% CI: 7.96, 1.58). For triglycerides, the point estimates also suggested increases when comparing postdisaster values with predisaster values (relative to persons who escaped property damage), although the 95% confidence intervals were wide and included the null value. Major housing damage was significantly associated with a 4.36-mm Hg lower change in diastolic blood pressure (95% CI: −8.67, −0.06), as compared with no housing damage. No associations with cardiometabolic biomarkers were observed for other types of disaster experience, including less severe categories of housing damage, loss of relatives, loss of friends, or loss of pets. Changes in depressive symptoms were not associated with the outcomes.
Table 4.
Changes in Cardiometabolic Risk Factors According to Level of Disaster Damage (Fixed-Effects Regression) Among Persons Aged 65 Years or Older (n = 1,195) After the 2011 Great East Japan Earthquake and Tsunami, Iwanuma, Japan, 2010–2013
| Variable | Systolic Blood Pressure, mm Hg | Diastolic Blood Pressure, mm Hg | Body Mass Indexa | Waist Circumference, cmb | Triglycerides, mg/dL | HDL Cholesterol, mg/dL | LDL Cholesterol, mg/dL | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| Housing damage | ||||||||||||||
| Partial damage (vs. no damage) | 1.23 | −1.94, 4.39 | 1.41 | −0.27, 3.08 | −0.002 | −0.20, 0.19 | 0.21 | −0.72, 1.14 | −5.50 | −15.70, 4.71 | 0.69 | −0.77, 2.14 | −2.61 | −6.62, 1.40 |
| Minor damage (vs. no damage) | −3.58 | −8.81, 1.64 | −1.33 | −4.08, 1.43 | 0.18 | −0.17, 0.53 | 0.09 | −1.21, 1.39 | −3.67 | −22.40, 15.10 | −1.51 | −3.81, 0.78 | −1.26 | −8.88, 6.35 |
| Major damage (vs. no damage) | −3.61 | −13.50, 6.24 | −4.36 | −8.67, −0.06 | 0.10 | −0.52, 0.72 | −0.48 | −3.86, 2.90 | 1.59 | −22.60, 25.80 | −2.21 | −6.18, 1.77 | −5.92 | −15.20, 3.31 |
| Complete destruction (vs. no damage) | 1.03 | −6.89, 8.95 | 2.23 | −2.16, 6.61 | 0.81 | 0.24, 1.38 | 4.26 | 1.12, 7.41 | 21.20 | −20.20, 62.60 | −4.77 | −7.96, −1.58 | 5.14 | −4.94, 15.20 |
| Emotional loss | ||||||||||||||
| Loss of relative(s) (Y/N)c | 0.27 | −3.24, 3.78 | 0.20 | −1.41, 1.80 | 0.14 | −0.10, 0.38 | −0.12 | −1.09, 0.85 | 4.97 | −5.96, 15.90 | 0.05 | −1.75, 1.85 | 3.72 | −0.19, 7.64 |
| Loss of friend(s) (Y/N) | −2.85 | −6.45, 0.75 | −1.79 | −3.69, 0.10 | −0.09 | −0.34, 0.16 | −0.58 | −1.80, 0.64 | −0.88 | −12.60, 10.80 | −1.53 | −3.31, 0.24 | 2.25 | −2.57, 7.07 |
| Loss of pet(s) (Y/N) | −1.44 | −10.70, 7.79 | −2.97 | −8.38, 2.43 | −0.09 | −0.73, 0.54 | 1.66 | −1.90, 5.23 | −3.46 | −27.50, 20.50 | 0.68 | −3.65, 5.01 | 0.60 | −15.40, 16.60 |
| Age categoryd | −0.04 | −3.93, 3.86 | −0.26 | −2.15, 1.63 | 0.06 | −0.13, 0.25 | −1.41 | −14.10, 11.30 | −0.21 | −1.65, 1.22 | −1.83 | −6.90, 3.24 | ||
| Equivalized annual household income (per 1,000,000-yen increment) | −1.35 | −2.69, −0.01 | −0.44 | −1.11, 0.23 | 0.04 | −0.04, 0.13 | 0.58 | 0.15, 1.01 | −4.51 | −9.40, 0.39 | 0.17 | −0.48, 0.82 | −0.36 | −2.01, 1.28 |
| Hypertension treatment (Y/N) | −5.10 | −9.12, −1.08 | −3.23 | −5.60, −0.85 | −0.05 | −0.39, 0.28 | 1.23 | −0.27, 2.73 | −9.94 | −29.40, 9.48 | 0.38 | −2.23, 2.99 | −4.32 | −10.30, 1.61 |
| Stroke treatment (Y/N) | 3.39 | −7.22, 14.00 | −5.25 | −10.90, 0.41 | 0.44 | −0.05, 0.93 | 0.59 | −2.53, 3.72 | −5.74 | −39.50, 28.00 | −0.54 | −3.68, 2.60 | −14.00 | −28.80, 0.81 |
| Diabetes treatment (Y/N) | −0.99 | −6.54, 4.56 | 3.02 | −0.18, 6.22 | −0.09 | −0.51, 0.33 | 0.40 | −1.23, 2.03 | −4.56 | −32.90, 23.80 | 2.47 | −2.33, 7.26 | 3.21 | −5.92, 12.30 |
| Dyslipidemia treatment (Y/N) | −1.73 | −5.67, 2.22 | −0.77 | −2.74, 1.21 | 0.06 | −0.18, 0.30 | 0.42 | −0.71, 1.56 | −12.70 | −39.70, 14.20 | 0.07 | −1.74, 1.87 | −3.02 | −11.60, 5.51 |
| Current alcohol intake (Y/N) | 2.93 | −1.85, 7.72 | 0.69 | −1.88, 3.26 | 0.64 | 0.30, 0.97 | 1.86 | 0.35, 3.38 | 21.40 | 1.27, 41.50 | 0.70 | −0.99, 2.40 | −2.40 | −9.73, 4.94 |
| Current smoking (Y/N) | −2.87 | −10.40, 4.62 | −1.84 | −5.41, 1.73 | −0.63 | −1.18, −0.07 | −2.06 | −4.80, 0.69 | −12.90 | −51.30, 25.50 | 1.31 | −1.94, 4.56 | −1.48 | −12.10, 9.15 |
| Depressive symptoms, pointse | −0.24 | −0.73, 0.26 | −0.09 | −0.38, 0.20 | −0.03 | −0.08, 0.01 | 0.01 | −0.17, 0.19 | −0.60 | −2.65, 1.45 | 0.04 | −0.24, 0.32 | −0.57 | −1.39, 0.26 |
Abbreviations: CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein; N, no; Y, yes.
a Weight (kg)/height (m)2.
b Waist circumference was measured only for persons under age 75 years.
c For “yes/no” variables, yes = 1 and no = 0.
d Age ≥75 years = 1; age 65–74 years = 0.
e Depressive symptoms were measured by a continuous Geriatric Depression Scale (31) score ranging from 0 to 15.
DISCUSSION
To our knowledge, this is the first study to demonstrate a long-term adverse impact of natural disaster on cardiometabolic risk factor levels while incorporating rigorous adjustment for predisaster characteristics, using a unique natural experiment setting stemming from the 2011 Great East Japan Earthquake and Tsunami.
After adjusting for all observed and unobserved time-invariant confounders as well as a series of time-varying confounders, we observed strong associations between property loss (i.e., complete housing destruction) and deterioration in cardiometabolic risk profile. The associations with complete property destruction were particularly marked for cardiometabolic risk factors, including BMI, waist circumference, and serum cholesterol level. The magnitude of association appeared to be clinically significant. For example, an increase in waist circumference of 4.26 cm (95% CI: 1.12, 7.41) among subjects with complete housing destruction (relative to those without housing damage) was of sufficient magnitude to put them at higher risk of diabetes and cardiovascular diseases. Evidence suggests that adding 2.75 cm to the average waist circumference of this population (84.4 cm) would put them at 1.7 times’ higher risk of type 2 diabetes mellitus and 1.39 times’ higher risk of coronary heart disease (36, 37). We also observed a significant association between major housing damage and a decrease in systolic blood pressure. This finding is puzzling, as it appears to be inconsistent with the direction of change in other cardiometabolic parameters. However, Ebner et al. (38) also found that evacuation after the Fukushima Daiichi nuclear power plant accident (which coincided with the 2011 Great East Japan Earthquake) was associated with decreased blood pressure 2 years later, despite all other cardiometabolic parameters (waist circumference, dyslipidemia, hyperuricemia) trending in a worse direction. The underlying pathway through which housing damage was associated with lower blood pressure remains unclear. The pathway may be changes in access to medical treatment, but chance cannot be ruled out.
Our results add to findings from previous studies that exposure to a natural disaster may increase cardiometabolic risk in the long term (13–15). However, the mechanism for this association remains to be clarified. Three possible explanations include: 1) lingering psychosocial stress and trauma resulting in changed health behaviors (e.g., worse sleep quality, decreased physical activity, worse dietary practices); 2) changes in economic circumstances resulting in deteriorated access to healthy nutrition; and/or 3) changes in the residential environment (e.g., local food environment/built environment) stemming from residential relocation.
Regarding the first explanation, we did not find an association between loss of loved ones and changes in cardiometabolic risk. This lack of long-term associations with health for loss of loved ones is consistent with the existing evidence (24). Moreover, there was no association between changes in Geriatric Depression Scale score and cardiometabolic risk. Thus, our results do not seem to support the psychosocial explanation. Regarding the second explanation, the associations with cardiometabolic risk for home loss remained significant after adjustment for changes in household income.
Notably, in our data, only complete housing destruction was associated with deteriorated cardiometabolic risk, while we found no association for less severe degrees of housing damage. It is plausible that severity of housing damage is correlated with the level of psychological trauma, as well as financial difficulty (10, 11). However, we did not find a dose-response relationship between severity of housing damage and cardiometabolic risk markers. This leads us to conclude that there was some factor at work that was unique to the situation of victims who experienced complete housing destruction and forced residential relocation.
In Iwanuma, people who experienced total property destruction lived closer to the coastline (see Figure 1). After the disaster, the majority of survivors were relocated by the municipal authorities to temporary shelters that were built closer to the city center (39). The new residential location also happened to be more convenient to the city center, offering a variety of options for dining out. Hence, a change in the local food environment may have contributed to the change in cardiometabolic risk profiles among persons whose homes were destroyed (12). Moreover, relocation after a natural disaster has been reported to be associated with sleep deprivation (40, 41). Because sleep plays an important role in regulating metabolism as well as appetite, long-term sleep deprivation following relocation may have contributed to the increased cardiometabolic risks (42–44).
Strengths of our study include the availability of predisaster data on demographic characteristics and health status, assessment of the different types/severity of disaster damage, and pre-/postdisaster linkage with objective data on anthropometric measurements and biomarkers. Nonetheless, several limitations should be noted. First, despite the adjustment for all observed and unobserved time-invariant confounders using fixed-effects regression, there may still have been residual confounding by time-varying confounders that we could not account for. For example, fish consumption, which has been associated with better cardiometabolic health, may have been greater among persons who lived in the coastal area before the earthquake. When it is considered as a confounder rather than a mediator, however, this would result in bias towards the null, and thus, the present study underestimates the true causal effect of home loss. Secondly, selection bias may remain. Although we performed inverse probability weighting for censoring due to lack of biomarker data, selection bias is still possible when variables used in the calculation of stabilized weights are insufficient. Third, the assumption of missingness at random may not hold for our multiple imputation procedure for handling missing data. However, we performed complete-case analysis using subjects without any missing data (Web Table 5) and replicated the same association between home loss and deteriorated cardiometabolic risk factors, suggesting that our results were robust.
In conclusion, our natural experiment study demonstrated that loss of homes was associated with a deteriorated cardiometabolic risk profile 2.5 years after the 2011 Great East Japan Earthquake among older adult survivors. Our finding suggests a need for continued health checkups and support to maintain the cardiometabolic health of the victims. The long-term adverse impact of natural disaster exposure on survivors’ cardiometabolic health may be due to postdisaster relocation and resulting changes in living environments. Although future studies assessing underlying mechanisms through which natural disaster exposure affects the cardiometabolic health of survivors are warranted, changes in people’s living environments may need to be considered in the planning of postdisaster evacuations.
Supplementary Material
ACKNOWLEDGEMENTS
Author affiliations: Department of Social and Behavioral Sciences, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Koichiro Shiba, Ichiro Kawachi); Division of Community Medicine and Public Health Practice, School of Public Health, University of Hong Kong, Hong Kong, Republic of China (Hiroyuki Hikichi); Department of International and Community Oral Health, Graduate School of Dentistry, Tohoku University, Miyagi, Japan (Jun Aida); Center for Preventive Medical Sciences, Chiba University, Chiba, Japan (Katsunori Kondo); and Center for Gerontology and Social Science, National Center for Geriatrics and Gerontology, Aichi, Japan (Katsunori Kondo).
This study was supported by the US National Institutes of Health (grant R01 AG042463), the Japan Society for the Promotion of Science (KAKENHI grant JP15H01972), the Japanese Ministry of Health, Labour, and Welfare (Health and Labour Sciences Research Grant H28-Choju-Ippan-002), the Japan Agency for Medical Research and Development (grant 16dk0110017h0002), the Japanese National Center for Geriatrics and Gerontology (Research Funding for Longevity Sciences grant 29-42), and the World Health Organization Centre for Health Development (WHO Kobe Centre) (grant WHO APW 2017/713981).
Conflict of interest: none declared.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- JAGES
Japan Gerontological Evaluation Study
REFERENCES
- 1. Center for Research on the Epidemiology of Disasters EM-DAT: The International Disaster Database. 2017. http://www.emdat.be/. Accessed December 1, 2017.
- 2. Bland SH, O’leary ES, Farinaro E, et al. Long-term psychological effects of natural disasters. Psychosom Med. 1996;58(1):18–24. [DOI] [PubMed] [Google Scholar]
- 3. Noji EK. The Public Health Consequences of Disasters. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 4. Tsubokura M, Takita M, Matsumura T, et al. Changes in metabolic profiles after the Great East Japan Earthquake: a retrospective observational study. BMC Public Health. 2013;13:Article 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aoki T, Fukumoto Y, Yasuda S, et al. The Great East Japan Earthquake disaster and cardiovascular diseases. Eur Heart J. 2012;33(22):2796–2803. [DOI] [PubMed] [Google Scholar]
- 6. Trevisan M, Celentano E, Meucci C, et al. Short-term effect of natural disasters on coronary heart disease risk factors. Arteriosclerosis. 1986;6(5):491–494. [DOI] [PubMed] [Google Scholar]
- 7. Ohira T, Hosoya M, Yasumura S, et al. Evacuation and risk of hypertension after the Great East Japan Earthquake: the Fukushima Health Management Survey. Hypertension. 2016;68(3):558–564. [DOI] [PubMed] [Google Scholar]
- 8. Ogawa K, Tsuji I, Shiono K, et al. Increased acute myocardial infarction mortality following the 1995 Great Hanshin-Awaji earthquake in Japan. Int J Epidemiol. 2000;29(3):449–455. [PubMed] [Google Scholar]
- 9. Omama S, Yoshida Y, Ogasawara K, et al. Influence of the Great East Japan Earthquake and Tsunami 2011 on occurrence of cerebrovascular diseases in Iwate, Japan. Stroke. 2013;44(6):1518–1524. [DOI] [PubMed] [Google Scholar]
- 10. Winning A, Glymour MM, McCormick MC, et al. Psychological distress across the life course and cardiometabolic risk: findings from the 1958 British Birth Cohort Study. J Am Coll Cardiol. 2015;66(14):1577–1586. [DOI] [PubMed] [Google Scholar]
- 11. Holman EA. Psychological distress and susceptibility to cardiovascular disease across the lifespan: implications for future research and clinical practice. J Am Coll Cardiol. 2015;66(14):1587–1589. [DOI] [PubMed] [Google Scholar]
- 12. Mozaffarian D, Hao T, Rimm EB, et al. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hashimoto S, Nagai M, Fukuma S, et al. Influence of post-disaster evacuation on incidence of metabolic syndrome. J Atheroscler Thromb. 2017;24(3):327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ohira T, Nakano H, Nagai M, et al. Changes in cardiovascular risk factors after the Great East Japan Earthquake. Asia Pac J Public Health. 2017;29(2 suppl):47S–55S. [DOI] [PubMed] [Google Scholar]
- 15. Armenian HK, Melkonian AK, Hovanesian AP. Long term mortality and morbidity related to degree of damage following the 1998 earthquake in Armenia. Am J Epidemiol. 1998;148(11):1077–1084. [DOI] [PubMed] [Google Scholar]
- 16. Bland SH, Farinaro E, Krogh V, et al. Long term relations between earthquake experiences and coronary heart disease risk factors. Am J Epidemiol. 2000;151(11):1086–1090. [DOI] [PubMed] [Google Scholar]
- 17. Trevisan M, Jossa F, Farinaro E, et al. Earthquake and coronary heart disease risk factors: a longitudinal study. Am J Epidemiol. 1992;135(6):632–637. [DOI] [PubMed] [Google Scholar]
- 18. Hikichi H, Aida J, Kondo K, et al. Increased risk of dementia in the aftermath of the 2011 Great East Japan Earthquake and Tsunami. Proc Natl Acad Sci. 2016;113(45):E6911–E6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murakami A, Sugawara Y, Tomata Y, et al. Association between housing type and γ-GTP increase after the Great East Japan Earthquake. Soc Sci Med. 2017;189:76–85. [DOI] [PubMed] [Google Scholar]
- 20. Hu FB. Obesity Epidemiology. Oxford, NY: Oxford University Press; 2008:67. [Google Scholar]
- 21. Japan Fire and Disaster Management Agency Summary Report of the 2011 Great East Japan Earthquake [in Japanese]. Tokyo, Japan: Fire and Disaster Management Agency; 2018. http://www.fdma.go.jp/bn/higaihou/pdf/jishin/158.pdf. Accessed February 28, 2019.
- 22. Japan Reconstruction Agency The Number of Evacuees in the Aftermath of the Tohoku Earthquake and Tsunami [in Japanese]. Tokyo, Japan: Reconstruction Agency; 2015. http://www.reconstruction.go.jp/topics/main-cat2/sub-cat2-1/20150630_hinansha_suii.pdf. Accessed December 1, 2017.
- 23. HelpAge International Displacement and Older People: the Case of the Great East Japan Earthquake and Tsunami of 2011 Chiang Mai, Thailand: HelpAge International; 2013. http://www.helpage.org/silo/files/displacement-and-older-people-the-case-of-the-great-east-japan-earthquake-and-tsunami-of-2011.pdf. Accessed July 20, 2018.
- 24. Tsuboya T, Aida J, Hikichi H, et al. Predictors of decline in IADL functioning among older survivors following the Great East Japan Earthquake: a prospective study. Soc Sci Med. 2017;176:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sasaki Y, Aida J, Tsuji T, et al. Does type of residential housing matter for depressive symptoms in the aftermath of a disaster? Insights from the Great East Japan Earthquake and Tsunami. Am J Epidemiol. 2018;187(3):455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. City of Iwanuma Trajectory of Results From Census From 1950 to 2010 [in Japanese]. Iwanuma, Japan: City of Iwanuma; https://www.city.iwanuma.miyagi.jp/shisei/tokei/joho/documents/kokuchousuii.pdf. Accessed December 1, 2017.
- 27. Miyagi Prefectural Government Current Situations of Damage and Evacuation [in Japanese]. Sendai City, Japan: Miyagi Prefectural Government; 2016. http://www.pref.miyagi.jp/uploaded/attachment/347652.pdf. Accessed December 1, 2017.
- 28. Japan Ministry of Health, Labour and Welfare Specific Health Checkups and Specific Health Guidance Tokyo, Japan: Ministry of Health, Labour and Welfare; 2009. http://www.mhlw.go.jp/english/wp/wp-hw3/dl/2-007.pdf. Accessed December 1, 2017.
- 29. Cabinet Office of Japan Cetification of housing damage from disaster [in Japanese]. 2017. http://www.bousai.go.jp/taisaku/unyou.html. Accessed December 1, 2017.
- 30. Japan Ministry of Health, Labour and Welfare Detailed Information 2. Operation of long life medical care system (medical care system for elderly in the latter stage of life) (FY 2009). Tokyo, Japan: Ministry of Health, Labour and Welfare; 2017. http://www.mhlw.go.jp/english/wp/wp-hw3/dl/2-003.pdf. Accessed December 1, 2017.
- 31. Burke WJ, Roccaforte WH, Wengel SP. The short form of the Geriatric Depression Scale: a comparison with the 30-item form. J Geriatr Psychiatry Neurol. 1991;4(3):173–178. [DOI] [PubMed] [Google Scholar]
- 32. Allison PD. Fixed Effects Regression Models. Thousand Oaks, LA: Sage Publications; 2009. [Google Scholar]
- 33. Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–625. [DOI] [PubMed] [Google Scholar]
- 34. Hernán M, Robins J. Causal Inference. Boca Raton, FL: Chapman & Hall/CRC; Press. In press. [Google Scholar]
- 35. van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):10.18637/jss.v045.i03. [Google Scholar]
- 36. Wang Y, Rimm EB, Stampfer MJ, et al. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81(3):555–563. [DOI] [PubMed] [Google Scholar]
- 37. Flint AJ, Rexrode KM, Hu FB, et al. Body mass index, waist circumference, and risk of coronary heart disease: a prospective study among men and women. Obes Res Clin Pract. 2010;4(3):e171–e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ebner DK, Ohsawa M, Igari K, et al. Lifestyle-related diseases following the evacuation after the Fukushima Daiichi nuclear power plant accident: a retrospective study of Kawauchi Village with long-term follow-up. BMJ Open. 2016;6(7):e011641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Santiago-Fandino V. The 2011 Japan Earthquake and Tsunami: Reconstruction and Restoration. New York, NY: Springer Publishing Company; 2017. [Google Scholar]
- 40. Najarian LM, Goenjian AK, Pelcovitz D, et al. The effect of relocation after a natural disaster. J Trauma Stress. 2001;14(3):511–526. [DOI] [PubMed] [Google Scholar]
- 41. Ursano RJ, McCaughey BG, Fullerton CS. Individual and Community Responses to Trauma and Disaster: the Structure of Human Chaos. New York, NY: Cambridge University Press; 1994. [Google Scholar]
- 42. Spiegel K, Tasali E, Penev P, et al. Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–850. [DOI] [PubMed] [Google Scholar]
- 43. Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Redline S, Berger NA. Impact of Sleep and Sleep Disturbances on Obesity and Cancer. New York, NY: Springer Publishing Company; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.