Highlights
-
•
Acute respiratory distress syndrome due to SARS-CoV-2 and Influenza A co-infection.
-
•
Challenging in co-infection cases identification.
-
•
Improvement of preventive measures and patients’ clinical outcome.
Keywords: Acute respiratory distress syndrome, SARS-CoV-2, Influenza A, COVID-19 epidemic
Abstract
A case of acute respiratory distress syndrome due to SARS-CoV-2 and Influenza A co-infection and a mini-review of the literature is reported. Even in COVID-19 epidemics, the early identification of concurrent respiratory pathogens is important to improve etiological diagnosis, preventive measures and patients’ clinical management and outcome.
Introduction
In December 2019, a novel coronavirus, the severe acute respiratory syndrome coronavirus-2 (SARS- CoV-2) which causes a human disease named coronavirus disease (COVID-19) was identified in the pneumonia outbreaks in Wuhan, China, in December 2019 (Chan et al., 2020). It is currently expanding rapidly to several countries all-around the word, on February 21 the first person-to-person transmission in Italy was reported (Spina et al., 2020). Here, we report a case of SARS-CoV-2 and Influenza A co-infection and a mini-review of the literature.
Case presentation
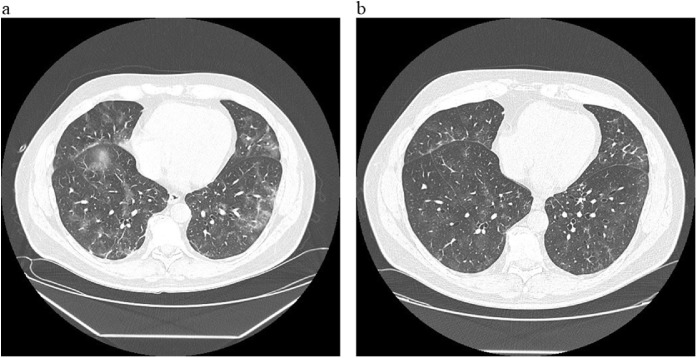
A 56 year-old male general surgeon was admitted to the Lazzaro Spallanzani National Institute for Infectious Diseases in Rome, Italy, on March 6, 2020. He was a smoker, within the overweight range (29-body mass index), with a history of two episodes of acute myocardial infarction treated with coronary angioplasty and stenting, For 4 days, he had been complaining of fever, diarrhea and asthenia after winter ski week in Northern Italy. Two days after the onset of symptoms, a nasopharyngeal swab was positive for SARS-CoV-2 (genes E and S) and Influenza A. On March 6, a chest computed tomography (CT) scan revealed bilateral and multiples peripheral ground glass opacities. Blood tests showed lymphopenia (lymphocyte and monocyte cell count: 0.67 and 0.09 × 109/L respectively), C- reactive protein and serum fibrinogen levels were increased (43.3 g/L and 7980 g/L respectively). An arterial oxygen tension (PaO2)/fractional inspired oxygen (FiO2) P/F Ratio was 320. Oral oseltamivir (75 mg twice per day for 5 days) and lopinavir/ritonavir (400/100 mg twice per days for 14 days) were started together with antibiotic therapy (intravenous ceftriaxone 2 gr and oral azithromycin 500 mg per day) and intravenous methylprednisolone (40 mg twice daily for 5 days with tapered discontinuation). On day eight of hospitalization, he developed respiratory failure (P/F Ratio dropped to 202) and a second chest CT scan showed a worsening of the bilateral ground-glass opacities with fibrotic consolidation in both lower pulmonary lobes (Figure 1 a). At that time, nasopharyngeal swabs were positive for SARS-CoV-2, only. The patient was transferred to the Intensive Care Unit (ICU) and non-invasive ventilation through continuous positive airway pressure (C-PAP) mask was started with positive end-expiratory pressure (PEEP) 7.5 mmHg and FiO2 40%. After three days, he was re-admitted to the High Isolation Unit and discharged in good clinical conditions with persistently negative nasopharyngeal swabs. A SARS-CoV-2 serology by an in house indirect immunofluorescence assay (IgA 1:1280, IgM 1:320 and IgG 1:80) was positive (Colavita et al., 2019). During the following week, low-grade fever re-occurred with nocturnal sweats, a third CT scan showed a reduction of the ground glass areas with residual interstitial damage (Figure 1b) and a further nasopharyngeal swab was negative. Symptoms healed spontaneously few days later.
Figure 1.
Chest computer tomography scan at patient’s worsening and recovery.
(a) Bilateral ground-glass opacities with fibrotic consolidation in both lower pulmonary lobes (b) reduction of the ground glass areas with residual interstitial damage.
Discussion
Previous cases of viral pneumonia SARS-CoV-2 and influenza coinfection have been reported in literature. During the new coronavirus epidemic, a total number of 37 cases were described. Table 1 summarizes the characteristics of the coinfected patients. Fourteen cases belonged to epidemiological studies and clinical data were not available. All patients had a similar clinical presentation (fever, cough and shortness of breath) and 9 of them (9/37, 24.3%) presented a progressive worsening with ARDS. Furthermore, six patients needed ICU monitoring and were subsequently discharged in good clinical conditions with the exception of three patients who died.
Table 1.
Clinical characteristics of SARS-CoV-2 and influenza coinfection, COVID-19 epidemic.
| References | Sex | Age | Comorbidities | Coinfection | Oseltamivir | Antivirals | Glucorticoids | ARDS | ICU | NIV | IMV | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azekawa et al. (2020) | F | 78 | Dyslipidemia, hypothyroidism | Influenza A | Yes | No | No | No | No | No | No | Survived |
| Blasco et al. (2020) | NA | NA | NA | Influenza A and RSV | NA | NA | NA | NA | NA | NA | NA | NA |
| Cuadrado-Payán et al. (2020) | M | 53 | ERSD | Influenza A | Yes | Yes | NA | Yes | Yes | No | Yes | Survived |
| M | 78 | T2DM | Influenza A | Yes | Yes | NA | Yes | Yes | No | Yes | Survived | |
| M | 56 | T2DM | Influenza A and B | No | No | NA | No | No | No | No | Survived | |
| F | 81 | ERSD | Influenza B | Yes | Yes | NA | Yes | Yes | No | Yes | Survived | |
| de Souza Luna et al. (2020) | NA | 36 | NA | Influenza B | NA | NA | NA | NA | NA | NA | NA | NA |
| Ding et al. (2020) | F | 47 | None | Influenza A | Yes | Yes | Yes | No | No | No | No | Survived |
| M | 50 | Hypertension, cancer | Influenza A | Yes | Yes | Yes | Yes | No | Yes | No | Survived | |
| F | 66 | Hypertension, CVD, HBV | Influenza B | Yes | Yes | No | No | No | No | No | Survived | |
| M | 39 | HBV | Influenza B | Yes | Yes | Yes | No | No | No | No | Survived | |
| F | 49 | None | Influenza A | Yes | Yes | No | No | No | No | No | Survived | |
| Garazzino et al. (2020) | NA | <17 | NA | Influenza A | NA | NA | NA | NA | NA | NA | NA | NA |
| Hashemi et al. (2020) | F | 78 | NA | Influenza A | Yes | Yes | NA | Yes | NA | NA | NA | Dead |
| M | 75 | NA | Influenza A | Yes | Yes | NA | Yes | NA | NA | NA | Dead | |
| Khodamoradi et al. (2020) | F | 74 | Hypertension, CVD | Influenza A | Yes | Yes | No | No | No | No | No | Survived |
| M | 40 | None | Influenza A | Yes | Yes | No | No | No | No | No | Survived | |
| M | 64 | None | Influenza A | Yes | Yes | No | No | No | No | No | Survived | |
| M | 50 | None | Influenza A | Yes | Yes | No | No | No | No | No | Survived | |
| Kim et al. (2020) | NA | NA | NA | Influenza A | NA | NA | NA | NA | NA | NA | NA | NA |
| Konala et al. (2020b) | M | 57 | Hypertension, T2DM, CVD, AICD | Influenza A | Yes | Yes | No | No | No | No | No | Survived |
| F | 35 | Sickle cell trait | Influenza A | Yes | Yes | No | No | No | No | No | Survived | |
| F | 68 | T2DM, hypertension, GERD | Influenza B | Yes | Yes | NA | Yes | Yes | No | Yes | Dead | |
| Konala et al. (2020a) | F | 66 | T2DM, CVD, hypertension, CKD | Influenza A | Yes | Yes | NA | Yes | Yes | No | Yes | Survived |
| Nowak et al. (2020) | NA | NA | NA | Influenza A | NA | NA | NA | NA | NA | NA | NA | NA |
| Pongpirul et al. (2020) | M | 61 | None | Influenza A | Yes | No | No | No | No | No | No | Survived |
| Richardson et al. (2020) | NA | NA | NA | Influenza A | NA | NA | NA | NA | NA | NA | NA | NA |
| Wehl et al. (2020) | NA | 4 months | None | Influenza A | Yes | No | No | No | No | No | No | Survived |
| Wu et al. (2020b) | NA | <17 | NA | MP, Influenza A&B, RSV | NA | NA | NA | NA | NA | NA | NA | NA |
| Wu et al. (2020c) | M | 69 | None | Influenza A | Yes | NA | NA | Yes | Yes | No | Yes | Survived |
| Zhu et al. (2020) | NA | NA | NA | 2 pts Influenza A | NA | NA | NA | NA | NA | NA | NA | NA |
| 5 pts Influenza B |
SARS-CoV-2: severe acute respiratory syndrome coronavirus-2, COVID-19: coronavirus disease-19, CVD: cardiovascular disease, HBV: hepatitis B virus, NA: not available, ARDS; Acute Respiratory Distress Syndrome, ICU: Intensive Care Unit, NIV: non-invasive ventilation, IMV: invasive mechanical ventilation, T2DM: type 2 diabetes mellitus. AICD: automatic implantable cardioverter defibrillator. RSV: Respiratory syncytial virus. MP: Mycoplasma pneumonia. ESRD: end-stage kidney disease. CKD: chronic kidney disease. GERD: gastroesophageal reflux disease.
Even during a pandemic scenario, several respiratory pathogens should be considered in the diagnostic algorithm, for an early etiological identification and appropriate treatment. SARS-CoV-2 and influenza viruses share common route of transmission, same season occurrence and overlapping clinical features (Lai et al., 2020, Chow et al., 2019). Indeed, SARS-CoV-2 exhibits prevalent human-to-human transmission through close contact with an estimated R0 of 3.28 and a median of 2.79 with IQR of 1.6 (Liu et al., 2020).
Respiratory symptoms are always the initial manifestations of both SARS-CoV-2 and influenza infections which could progress towards ARDS. Recently, ground-glass opacities and a higher median PaO2/FIO2 (198.2 vs 107.0) were observed in COVID-19-induced ARDS rather than H1N1 patients (Tang et al., 2020).
Nevertheless, a timely identification of the two co-infections is needed in relation to difference in treatments and prognosis. Antiviral therapy is currently available for influenza infection (i.e. oseltamivir, zanamivir, and peramivir) while experimental off-label drugs (i.e. lopinavir/ritonavir, chloroquine, and hydroxychloroquine) have been commonly used in COVID-19 treatment. In particular, the boosted protease inhibitor lopinavir/ritonavir has been previously associated with significantly fewer adverse clinical outcomes for the treatment of SARS and furthermore, in association with the Interferon Beta-1b, it has been demonstrated to be beneficial in animal studies against the Middle East Respiratory Syndrome (Chu et al., 2004, Chan et al., 2015). Considering the severity of the clinical picture and the prompt availability of lopinavir/ritonavir in our Institute, clinicians opted for this treatment although the antiviral effects remain to be determined (Cao et al., 2020).
Despite a recent report on beneficial effect of steroids treatment in COVID-19 patients who develop ARDS, its routinely use remains controversial with lack of an accurate assessment of the harm/benefit balance (Wu et al., 2020a).
The epidemiological situation in Italy in February 2020 and the recommendations in use at that time allowed a timely identification of our patient with a prompt hospitalization and subsequent diagnostic investigations. Therefore, the early antiviral treatments of both influenza and SARS-CoV-2, together with a brief steroid course and oxygen supplementation, had an impact on the patient’s outcome avoiding the progressive worsening and the evolution towards the severe ARDS phase. In conclusion, even in epidemic setting, the early and prompt identification of concurrent respiratory pathogens is important in order to improve etiological diagnosis, preventive measures and patients’ clinical management and outcome. Further studies are needed to better understand the pathogenic role of viral coinfections in respiratory diseases.
Funding
This paper was funded by Ricerca Corrente Line1on emerging and re-emerging infections funded by the Italian Ministry of Health.
Ethical approval
This study was approved by the Spallanzani Institute Ethical Board and patient’s written informed consent for publication was collected
Conflict of interest
All authors have no conflict of interest to declare.
References
- Azekawa S., Namkoong H., Mitamura K., Kawaoka Y., Saito F. Co-infection with SARS-CoV-2 and influenza A virus. IDCases. 2020;20(April) doi: 10.1016/j.idcr.2020.e00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco M.L., Buesa J., Colomina J., Forner M.J., Galindo M.J., Navarro J. Co-detection of respiratory pathogens in patients hospitalized with coronavirus viral disease-2019 pneumonia. J Med Virol. 2020;(April) doi: 10.1002/jmv.25922. [DOI] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(May (19)):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Yao Y., Yeung M.L., Deng W., Bao L., Jia L. Treatment with lopinavir/ritonavir or interferon-beta1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212(12):1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Kok K.H., Zhu Z., Chu H., To K.K., Yuan S. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(January (1)):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow E.J., Doyle J.D., Uyeki T.M. Influenza virus-related critical illness: prevention, diagnosis, treatment. Crit Care. 2019;23(June (1)):214. doi: 10.1186/s13054-019-2491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colavita F., Biava M., Castilletti C., Lanini S., Miccio R., Portella G. Inflammatory and humoral immune response during Ebola virus infection in survivor and fatal cases occurred in Sierra Leone during the 2014–2016 outbreak in West Africa. Viruses. 2019;11:373. doi: 10.3390/v11040373. Available at: https://www.mdpi.com/1999-4915/11/4/373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado-Payán E., Montagud-Marrahi E., Torres-Elorza M., Bodro M., Blasco M., Poch E. SARS-CoV-2 and influenza virus co-infection. Lancet. 2020;395(May (10236)):e84. doi: 10.1016/S0140-6736(20)31052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza Luna L.K., Perosa D.A.H., Conte D.D., Carvalho J.M.A., Alves V.R.G., Cruz J.S. Different patterns of influenza A and B detected during early stages of COVID-19 in a University Hospital in São Paulo, Brazil. J Infect. 2020;(May) doi: 10.1016/j.jinf.2020.05.036. S0163-4453(20)30313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q., Lu P., Fan Y., Xia Y., Liu M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol. 2020;(March) doi: 10.1002/jmv.25781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garazzino S., Montagnani C., Donà D., Meini A., Felici E., Vergine G. Multicentre Italian study of SARS-CoV-2 infection in children and adolescents, preliminary data as at 10 April 2020. Euro Surveill. 2020;25(May (18)) doi: 10.2807/1560-7917.ES.2020.25.18.2000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi S.A., Safamanesh S., Ghafouri M., Taghavi M.R., Mohajer Zadeh Heydari M.S., Namdar Ahmadabad H. Co-infection with COVID-19 and influenza A virus in two died patients with acute respiratory syndrome, Bojnurd, Iran. J Med Virol. 2020;(May) doi: 10.1002/jmv.26014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodamoradi Z., Moghadami M., Lotfi M. Co-infection of coronavirus disease 2019 and influenza A: a report from Iran. Arch Iran Med. 2020;23(April (4)):239–243. doi: 10.34172/aim.2020.04. [DOI] [PubMed] [Google Scholar]
- Kim D., Quinn J., Pinsky B., Shah N.H., Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323(April (20)):2085–2086. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konala V.M., Adapa S., Gayam V., Naramala S., Daggubati S.R., Kammari C.B. Co-infection with influenza A and COVID-19. Eur J Case Rep Intern Med. 2020;7(April (5)) doi: 10.12890/2020_001656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konala V.M., Adapa S., Naramala S., Chenna A., Lamichhane S., Garlapati P.R. A case series of patients coinfected with influenza and COVID-19. J Investig Med High Impact Case Rep. 2020;8(January–December) doi: 10.1177/2324709620934674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.C., Liu Y.H., Wang C.Y., Wang Y.H., Hsueh S.C., Yen M.Y. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. 2020;(March) doi: 10.1016/j.jmii.2020.02.012. pii: S1684-1182(20)30040-30042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M.D., Sordillo E.M., Gitman M.R., Paniz Mondolfi A.E. Co-infection in SARS-CoV-2 infected patients: where are influenza virus and rhinovirus/enterovirus? J Med Virol. 2020;(April) doi: 10.1002/jmv.25953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongpirul W.A., Mott J.A., Woodring J.V., Uyeki T.M., MacArthur J.R., Vachiraphan A. Clinical characteristics of patients hospitalized with coronavirus disease, Thailand. Emerg Infect Dis. 2020;26(April (7)) doi: 10.3201/eid2607.200598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(April (20)):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina S., Marrazzo F., Migliari M., Stucchi R., Sforza A., Fumagalli R. The response of Milan’s Emergency Medical System to the COVID-19 outbreak in Italy. Lancet. 2020;395(March (10227)):e49–e50. doi: 10.1016/S0140-6736(20)30493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Du R., Wang R., Cao T., Guan L., Yang C. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N. Chest. 2020;(March) doi: 10.1016/j.chest.2020.03.032. pii: S0012-3692(20)30558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehl G., Laible M., Rauchenzauner M. Co-infection of SARS CoV-2 and influenza A in a pediatric patient in Germany. Klin Padiatr. 2020;(May) doi: 10.1055/a-1163-7385. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;(March) doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Xing Y., Shi L., Li W., Gao Y., Pan S. Coinfection and other clinical characteristics of COVID-19 in children. Pediatrics. 2020;(May) doi: 10.1542/peds.2020-0961. [DOI] [PubMed] [Google Scholar]
- Wu X., Cai Y., Huang X., Yu X., Zhao L., Wang F. Co-infection with SARS-CoV-2 and influenza A virus in patient with pneumonia, China. Emerg Infect Dis. 2020;26(June (6)):1324–1326. doi: 10.3201/eid2606.200299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Ge Y., Wu T., Zhao K., Chen Y., Wu B. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020;285(May) doi: 10.1016/j.virusres.2020.198005. [DOI] [PMC free article] [PubMed] [Google Scholar]