Highlights
-
•
We describe a patient with acute disseminated encephalitis and COVID-19.
-
•
CNS manifestations primarily occurred without evident pulmonary symptoms.
-
•
Brain MRI of the patient indicated diffuse confluent white matter hyperintensities.
-
•
The patient initially responded to steroids but lastly, died of status epilepticus.
-
•
The occurrence of acute disseminated encephalitis may have been immune-mediated.
Keywords: SARS-CoV-2, Acute disseminated encephalomyelitis, Immune-mediated
Dear Editor,
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is spreading around the world, and the outbreak continues to increase in different parts of the world. Patients with SARS-CoV-2 infection syndrome (COVID-19) characteristically present with fever and respiratory symptoms; however, evidence of multi-organ dysfunction is reported [1].
Central nervous system (CNS) involvement, especially in the form of encephalopathy, is reported in some patients [2], and it has been reported that the virus can be detected in the brain or cerebrospinal fluid. Acute Disseminated Encephalomyelitis (ADEM), is usually a para or post-infectious syndrome characteristically multifocal and monophasic immune-mediated central nervous system disorder [3]. It usually occurs in early childhood after infection with the influenza virus [3]. Recently, the first case of acute hemorrhagic leukoencephalitis associated with COVID-19 has been reported [4]. Herein, we describe clinical manifestations and response to treatment of a patient with concurrent infection of SARS-CoV-2, and ADEM with favorable response to treatment. The patient has given consent to publish his data.
A 58-year-old man presented to the emergency department because of a decreased level of consciousness and the inability to walk. Initial symptoms began with slowly progressive gait disturbance around one month before admission; however, consciousness profoundly deteriorated two days before the admission. There were no complaints of pulmonary symptoms such as cough or dyspnea. On admission, his body temperature was 37.10C; he was drowsy but could obey simple tasks, and speaking consisted of short, simple words. He could move all limbs; nevertheless, the left upper limb moved less. Deep tendon reflexes were brisk and plantar reflexes were upgoing. Initial investigations revealed Hb: 15.5 g/dL, WBC: 17,000 [lymphocyte count: 1020/mm3, normal range: 800–5000/mm3], CRP: 82 mg/L (normal <10 mg/L), ESR: 40 mm/h (normal <20 mm/h), and Ferritin 876 ng/mL (normal range: 12 to 300 ng/mL). CSF examination revealed WBCs: 0 /mm3 (normal range: 0–5/mm3), Glucose: 105 mg/dL (normal <80 mg/dL), and protein: 15 mg/dL (normal <45 mg/dL). The CSF was negative for viruses such as herpes simplex, varicella-zoster, cytomegalovirus, and Epstein-Barr virus. Moreover, we did not found oligoclonal bands in CSF. Blood Interferon-Gamma Release Assays (IGRAs) for Tuberculosis, and ELISA for the Brucella antibody and Human Immunodeficiency Virus (HIV) antibody, were negative.
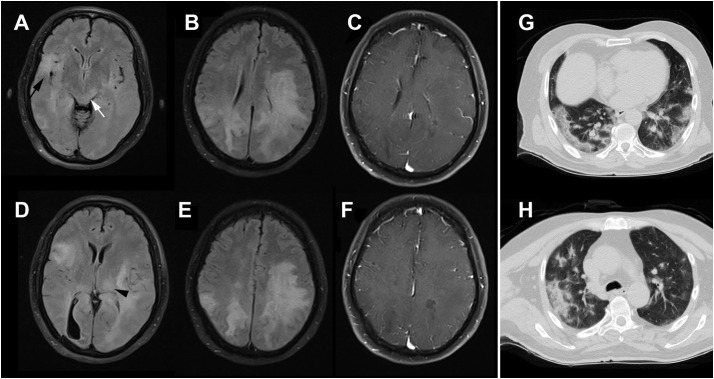
Brain MRI of the patient, indicated diffuse confluent white matter hyperintensity on FLAIR-weighted MRI, particularly at the left-side (Fig. 1 , A-D) without prominent enhancement on T1-weighted brain MRI (Fig. 1, C, F). Moreover, the involvement of cortical as well as deep gray matter, and dorsal midbrain was evident. The chest computed tomography (CT) scan (G, H) indicates bilateral multifocal peripheral consolidations with air-bronchogram consistent with SARS-CoV-2 infection.
Fig. 1.
Brain MRI of the patient indicates diffuse confluent white matter hyperintensity on FLAIR-weighted MRI, particularly at the left-side (A-D) without prominent enhancement on T1-weighted brain MRI (C, F). Moreover, the involvement of cortical (black arrow) as well as deep gray matter (black arrowhead), and dorsal midbrain (white arrow) is evident. The chest computed tomography (CT) scan (G, H) indicates bilateral multifocal peripheral consolidations with air-bronchogram consistent with SARS-CoV-2 infection.
Nasopharyngeal and oropharyngeal swab real-time polymerase chain reaction (rt-PCR) were positive for SARS-CoV-2 virus but negative in the CSF. For the management of ADEM, we started intravenous dexamethasone 8 mg TDS, which resulted in an improvement in mental status after two days. At this time, the patient could communicate verbally; he was oriented to time and person and could walk with aid. Pulmonologists performed pulmonary management in the intensive care unit. However, after 10 days, status epilepticus developed, and unfortunately, the patient died one day later, probably because of status epilepticus.
In this study, we reported a case of adult-ADEM associated with COVID-19 syndrome. Recently, some case reports denoted neurological complications of SARS-CoV-2 infection in central and peripheral nervous systems [4,5]. Poyiadji et al. reported the first case of acute hemorrhagic leukoencephalitis associated with SARS-CoV-2 infection [4]. The patient was a middle-aged woman with a 3-day history of cough, fever, and altered mental status with demyelinating lesions and hemorrhage on brain MRI. SARS-CoV-2 may affect brain parenchyma with two mechanisms; direct invasion of the virus [1] and immune-mediated brain damage [4].
SARS-CoV-2 might act on lymphocytes, principally T lymphocytes, and can induce a storm of cytokine release, producing sequences of immune responses that may play an essential role in the pathogenesis of this disorder [6].
It is significant that in our patient, the neurological syndrome developed before clinical or radiological pulmonary manifestations and never prominent clinical pulmonary symptoms developed.
Considering the temporal association, we hypothesize that SARS-CoV-2 infection might have been responsible for the development of this disorder; nonetheless, definitely establishing a coincidence or a cause-and-effect relationship may not always be possible.
Acknowledgments: None.
Conflicts of interest
All authors declare that they have no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 2.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kheiri B., Abu Sitta E., Salih A., Al Qasmi M., Bachuwa G. Acute disseminated encephalomyelitis following influenza a pneumonia. Clin. Case Rep. 2018;6:436–438. doi: 10.1002/ccr3.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19–associated acute Hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020 doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;0 doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin Y.-Y., Lee K.-Y., Ro L.-S., Lo Y.-S., Huang C.-C., Chang K.-H. Clinical and cytokine profile of adult acute necrotizing encephalopathy. Biom. J. 2019;42:178–186. doi: 10.1016/j.bj.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]