Abstract
Background
Resuscitative endovascular balloon occlusion of the aorta (REBOA) may be a novel intervention to improve cardiopulmonary resuscitation (CPR) quality during cardiac arrest. Zone 1 supraceliac aortic occlusion improves coronary and cerebral blood flow. It is unknown if Zone 3 occlusion distal to the renal arteries offers a similar physiologic benefit while maintaining blood flow to organs above the point of occlusion.
Methods
Fifteen swine were anesthetized, instrumented, and placed into ventricular fibrillation. Mechanical CPR was immediately initiated. After 5 min of CPR, Zone 1 REBOA, Zone 3 REBOA, or no intervention (control) was initiated. Hemodynamic variables were continuously recorded for 30 min.
Results
There were no significant differences between groups before REBOA deployment. Once REBOA was deployed, Zone 1 animals had statistically greater diastolic blood pressure compared to control (median [IQR]: 19.9 mmHg [9.5–20.5] vs 3.9 mmHg [2.4–4.8], p = .006). There were no differences in diastolic blood pressure between Zone 1 and Zone 3 (8.6 mmHg [5.1–13.1], p = .10) or between Zone 3 and control (p = .10). There were no significant differences in systolic blood pressure, cerebral blood flow, or time to return of spontaneous circulation (ROSC) between groups.
Conclusion
In our swine model of cardiac arrest, Zone 1 REBOA improved diastolic blood pressure when compared to control. Zone 3 does not offer a hemodynamic benefit when compared to no occlusion. Unlike prior studies, immediate use of REBOA after arrest did not result in an increase in ROSC rate, suggesting REBOA may be more beneficial in patients with prolonged no-flow time.
Institutional protocol number
FDG20180024A
Keywords: Cardiopulmonary resuscitation, Endovascular, Intra-aortic balloon, Resuscitation, Aortic occlusion
1. Introduction
Each year in the United States there are an estimated 356,000 nontraumatic, out of hospital cardiac arrests [1]. Despite decades of research, improved access to automated external defibrillators, refinement of cardiac life support algorithms, and implementation of therapeutic hypothermia, only 5.5–10.4% of patients who suffer out of hospital cardiac arrest survive to hospital discharge [2,3]. Cardiopulmonary resuscitation (CPR) is inherently inefficient and generates only 20–30% of baseline cardiac output despite proper technique [4]. Resuscitative endovascular balloon occlusion of the aorta (REBOA), an approach currently used in trauma patients to control noncompressible truncal hemorrhage, may be a novel intervention to improve CPR quality [5,6]. A REBOA catheter is placed percutaneously through the femoral artery and advanced retrograde into the aorta. Balloon inflation results in aortic occlusion and redistribution of any remaining cardiac output to the heart and brain. This physiologic shunting of blood flow may be ideal during cardiac arrest, as the limited cardiac output generated with CPR will preferentially flow towards the heart and brain. REBOA improves cerebral and coronary perfusion pressure during cardiac arrest as well as increases the rate of return of spontaneous circulation (ROSC) and overall mortality in animals [5,[7], [8], [9]].
REBOA can be placed into one of three zones of the aorta: Zone 1, the descending thoracic aorta between the left subclavian artery and the coeliac axis; Zone 2, the paravisceral aorta from the coeliac axis to the renal arteries; and Zone 3, the infrarenal aorta [10]. To date, all of the prior studies involving REBOA and cardiac arrest have used Zone 1 aortic occlusion during CPR [5]. This has the greatest effect on reducing circulating volume and theoretically would provide the maximal benefit for coronary and cerebral perfusion [11]. However, Zone 1 occlusion also results in ischemic injury to abdominal organs [12]. Although these organs are poorly perfused during cardiac arrest and CPR under any circumstances, Zone 1 REBOA further increases the risk of clinically significant ischemia of the liver, intestines, and kidneys. In a human case series of the use of REBOA during cardiac arrest, one patient had favorable cardiac and neurologic outcome after ROSC, but required a hemicolectomy due to significant intestinal ischemia [13].
REBOA placement in Zone 3, by contrast, allows perfusion of the abdominal organs which are critical to life if ROSC is achieved, but may not provide beneficial hemodynamic support during CPR. There are no studies of Zone 3 occlusion during CPR in large animal models to determine if a hemodynamic benefit can be achieved without the risk of extensive intra-abdominal ischemia [6]. To fill this knowledge gap, we studied the impact of Zone 1 versus Zone 3 REBOA placement in a ventricular fibrillation porcine model of cardiac arrest. We hypothesized that Zone 1 placement would provide improved hemodynamic support during CPR compared to Zone 3 REBOA placement and control.
2. Methods
2.1. Overview
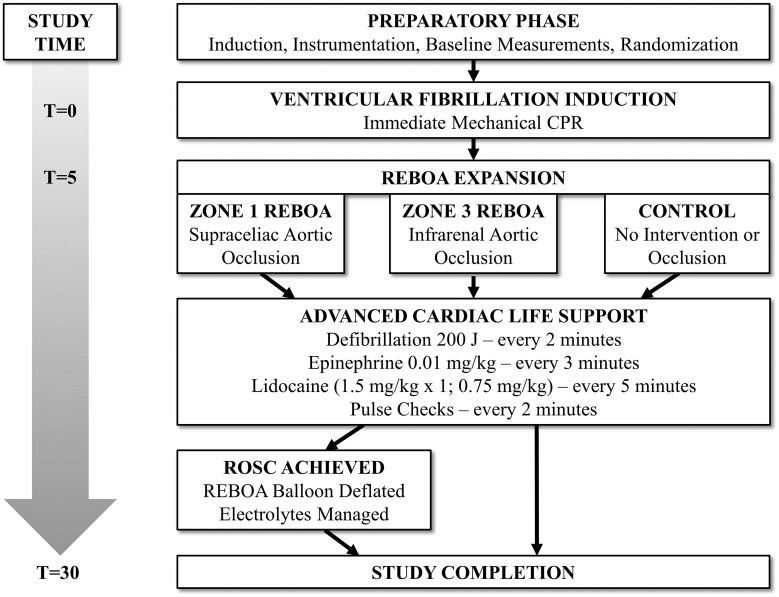
The Institutional Animal Care and Use Committee at David Grant Medical Center, Travis Air Force Base, CA, approved this study. All animal care and use were in strict compliance with the Guide for the Care and Use of Laboratory Animals in a facility accredited by AAALAC International. Healthy adult, castrate male and nonpregnant female Yorkshire-cross swine (Sus scrofa; S&S Farms, Ramona, CA) were acclimated for a minimum of 7 days in well-lit cages with access to food. At the time of experimentation, animals weighed between 50 and 85 kg and were between 4.5 and 6 months of age. Conduct of the protocol, including animal preparation, intervention, and critical care is illustrated in Fig. 1 . Following anesthesia and instrumentation, animals were assigned using a block randomization strategy to one of three intervention arms: Zone 1 REBOA (Zone 1 group, n = 5), Zone 3 REBOA (Zone 3 group, n = 5), or no intervention (control group, n = 5).
Fig. 1.
Experimental design.
2.2. Animal preparation
Animals were premedicated with 6.6 mg/kg intramuscular tiletamine/zolazepam (TELAZOL; Zoetis US, Parsippany, NJ). Following isoflurane induction and endotracheal intubation, general anesthesia was maintained with 2% isoflurane in 100% oxygen. To offset the vasodilatory effects of general anesthesia, an intravenous infusion of norepinephrine (0.01 μg/kg per minute) was titrated to achieve a target mean arterial pressure (MAP) between 65 and 75 mmHg. Animals were mechanically ventilated with tidal volumes of 7 to 10 mL/kg and a respiratory rate of 10 to 15 breaths per minute, sufficient to maintain end-tidal CO2 at 40 ± 5 mmHg.
The animals were placed supine after induction. The right carotid artery was exposed and circumferentially dissected for the placement of a perivascular flow probe (Transonic Systems Inc., Ithaca, NY). Both external jugular veins were cannulated to facilitate medication and fluid administration, as well as for transvenous pacer (TVP) placement. A TVP wire was placed through the right external jugular through a 9-Fr resuscitation catheter (Arrowg+ard Blue® MAC; Teleflex, Wayne, PA). TVP lead placement was confirmed within the right ventricle using both fluoroscopy and electrical monitoring on the TVP lead. The left femoral artery was cannulated with a 7-Fr sheath for distal blood pressure monitoring. The right femoral artery was cannulated with a 7-Fr sheath for REBOA catheter placement (ER-REBOA; Prytime Medical, Boerne, TX). Fluoroscopy was used to confirm the position of the REBOA balloon in either Zone 1 or Zone 3. No REBOA was placed in the control animals. A mechanical CPR device (LUCAS 3 Chest Compression System; Physio-Control, Scheelevägen, Sweden) was placed around the chest with the suction cup placed against the sternum. Padding was placed around the animal's chest to ensure the CPR device remained properly positioned throughout the experiment. The CPR device factory defaults of 102 beats per minute and a compression depth of 2.1 in. were used. Baseline laboratory and arterial blood gas samples were obtained.
2.3. Intervention
The animals were placed into ventricular fibrillation by connecting a 9-V alkaline battery to the TVP. Once ventricular fibrillation was confirmed on continuous electrocardiogram monitoring, mechanical CPR was initiated. The animal's isoflurane was discontinued and the ventilator was set at 10 breaths/min with 100% oxygen. After 5 min, the REBOA balloon was inflated, when applicable, and advanced cardiac life support algorithms were initiated. Complete aortic occlusion was confirmed by the loss of distal femoral arterial pressure waveforms during CPR. Cardiac defibrillation (R-series; ZOLL, Chelmsford, MA) was performed at two minute intervals at 200 joules if ventricular fibrillation was observed on electrocardiogram. Intravenous 0.01 mg/kg adrenaline (epinephrine) was administered in three minute intervals. Intravenous 1.5 mg/kg lidocaine was administered after 5 min of ACLS, followed by 0.75 mg/kg lidocaine doses in subsequent five minute intervals. Lidocaine was selected in place of amiodarone in the ACLS algorithm given the hemodynamic side effects of amiodarone in swine [14]. Pulse checks were performed at two-minute intervals where the mechanical CPR device was paused for 5 sec to assess the underlying cardiac rhythm.
If ROSC was achieved ACLS and CPR were discontinued. Additionally, 0.5 mL of fluid was removed from the REBOA balloon every 30 s until completely deflated. If ROSC was not achieved, CPR was continued for a total experimental time of 30 min. Critical care with isotonic crystalloid fluid (Plasma-Lyte A; Baxter Healthcare Corporation, Deerfield, IL) boluses, and noradrenaline titration proceeded in all animals until 30 min. Plasma potassium, glucose, and calcium concentrations were monitored and corrected according to pre-established protocols [12]. If a perfusing rhythm was lost, CPR was restarted and the REBOA catheter was re-inflated. All animals were euthanized at the conclusion of the thirty minute study period.
2.4. Data collection
Physiologic parameters (heart rate, blood pressure proximal and distal to the REBOA balloon), as well as carotid and aortic flow measurements, were collected in real-time using a multichannel data acquisition system (Biopac MP150; Biopac Systems, Inc., Goleta, CA). Arterial blood was collected in five-minute intervals throughout the study for blood gas analysis (RAPIDLab® 1200; Siemens Healthcare Diagnostics, Tarrytown, NY) from the proximal REBOA port.
2.5. Statistical analysis
The primary outcome was the change in diastolic blood pressure after inflation of the REBOA balloon. Secondary outcomes included change in systolic blood pressure, heart rate, carotid flow and rate of ROSC. An a priori power analysis showed that 5 animals in each group would be required to detect a 20% difference in diastolic blood pressure between the Zone 1 and Zone 3 groups with a hypothesized standard deviation of 6 mmHg within each group (power of 80% and an alpha error of 0.05, critical F of 3.89 with an ANOVA test). Data were assessed for normality and are presented as mean ± standard error of the mean or median (interquartile range) for parametric and nonparametric data, respectively. Non-parametric data were analyzed using Kruskal-Wallis analysis of variance, followed by post-hoc testing with Dunn's test. Statistical analysis was performed using commercial software (STATA version 14.0; Stata Corp., College Station, TX). Statistical significance was set as p < 0.05.
3. Results
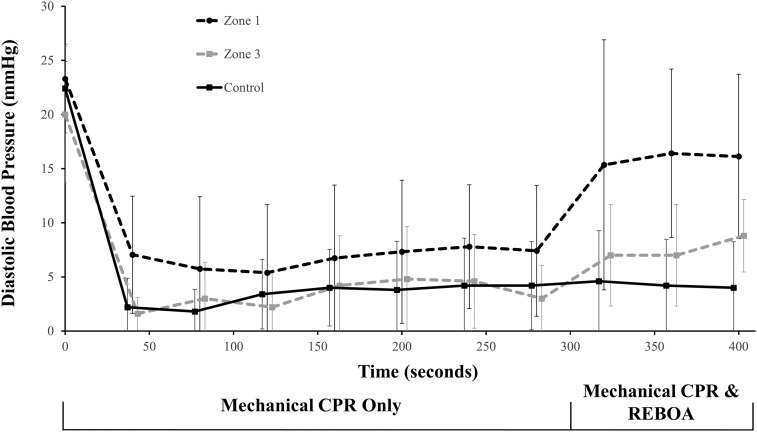
There were no significant differences between the animals' baseline characteristics (Table 1 ). Analysis of the period after REBOA inflation and prior to ROSC showed an overall difference in diastolic blood pressure between groups (p = .04). Pairwise analysis showed that once REBOA was deployed, Zone 1 animals had statistically greater diastolic blood pressure compared to control (median [IQR]: 19.9 mmHg [9.5–20.5] vs 3.9 mmHg [2.4–4.8], p = 0.006) (Table 2 , Fig. 2 ). There were no differences in diastolic blood pressure between Zone 1 and Zone 3 (8.6 mmHg [5.1–13.1], p = 0.10) or between Zone 3 and control (p = 0.10). There were no statistically significant differences between systolic blood pressure or carotid blood flow.
Table 1.
Baseline physiologic, laboratory and hemodynamic characteristics. Data are listed as mean ± standard deviation, where appropriate. Kg = kilogram, C = celsius, L = liter, dL = deciliter, g = grams, mEq = milliequivalent, mmol = millimole, mmHg = millimeters of mercury, min = minute, mL = milliliter
| Zone 1 (n = 5) | Zone 3 (n = 5) | Control (n = 5) | p | |||
|---|---|---|---|---|---|---|
| Physiologic | Weight | kg | 69.92 (11.5) | 70.12 (5.2) | 62.46 (10.5) | 0.4 |
| Sex | male:female | 4:1 | 3:2 | 3:2 | 0.7 | |
| Temperature | °C | 36.4 (0.5) | 36.08 (1.2) | 36.12 (0.7) | 0.8 | |
| pH | – | 7.48 (0.01) | 7.46 (0.03) | 7.5 (0.05) | 0.3 | |
| Laboratory | White Blood Cells | 10^9 cells/L | 13.97 (3.1) | 14.5 (2.8) | 14.61 (3.3) | 0.9 |
| Hemoglobin | g/dL | 9.8 (0.3) | 9.1 (1.2) | 9.0 (0.8) | 0.3 | |
| Potassium | mEq/L | 3.8 (0.1) | 3.7 (0.3) | 3.7 (0.4) | 0.9 | |
| Calcium ion | mg/dL * 0.25 | 1.2 (0.0) | 1.2 (0.1) | 1.2 (0.1) | 0.5 | |
| Lactate | mmol/L | 1.8 (0.6) | 3.03 (0.9) | 2.37 (0.5) | 0.1 | |
| Hemodynamic | Systolic Blood Pressure | mmHg | 90.1 (9.0) | 88.5 (9.9) | 90.7 (6.8) | 0.9 |
| Diastolic Blood Pressure | mmHg | 53 (7.1) | 54.7 (8.0) | 55.4 (5.1) | 0.8 | |
| Heart Rate | beats/min | 77.7 (12.7) | 79.2 (7.2) | 85.6 (10.5) | 0.5 | |
| Carotid Blood Flow | mL/min | 374.4 (139.2) | 357.9 (93.2) | 361.3 (90.3) | 1 | |
Table 2.
Pre- and post-REBOA deployment systolic blood pressure, diastolic blood pressure, and carotid flow. Blood pressures are represented in mmHg. Carotid flow is represented in mL/min. Pre-REBOA period is from initiation of ventricular fibrillation until REBOA deployment (t = 5 min). Post-REBOA deployment period is from REBOA deployment until ROSC was achieved. Post-REBOA diastolic blood pressure was statistically significant by Kruskal-Wallis testing, represented by *. mmHg = mmHg = millimeters of mercury, mL = milliliter, min = minute
| Zone 1 (n = 5) | Zone 3 (n = 5) | Control (n = 5) | p | ||
|---|---|---|---|---|---|
| Pre-REBOA Deployment | SBP (mmHg) | 61.5 (56.7, 79.1) | 65.9 (51.9, 74.2) | 93.9 (68.4, 98.5) | 0.4 |
| DBP (mmHg) | 6.2 (3.9, 12.0) | 4.9 (1.4, 6.3) | 4.3 (1.5, 4.8) | 0.57 | |
| Carotid Flow (mL/min) | 88.0 (82.9, 118.5) | 99.6 (86.6, 100.4) | 107.3 (96.13, 113.15) | 0.76 | |
| Post-REBOA Deployment | SBP (mmHg) | 84.0 (76.1, 94.5) | 84.4 (73.5, 86.6) | 101.8 (89.4, 105.5) | 0.31 |
| DBP (mmHg) | 19.9 (9.5, 20.5)* | 8.6 (5.1, 13.1) | 3.9 (2.4, 4.8) | 0.04* | |
| Carotid Flow (mL/min) | 89.4 (74.6, 102.0) | 99.6 (86.6, 100.5) | 119.0 (110.4, 122.3) | 0.36 | |
Fig. 2.
Comparison of Zone 1, Zone 3, and control animal diastolic blood pressure pre- and post-REBOA balloon inflation. Graph represents the first 400 s of data. REBOA balloon inflated at t = 300 s. The data for Zone 1 animals includes five animals until t = 319 s, when one animal achieved ROSC without further intervention. The remainder of the Zone 1 data (t = 319 until t = 400), represents the remaining four Zone 1 animals. Zone 3 and control data represents all animals. The data are shown offset at each time point for improved visibility. Error bars represent standard error of the mean.
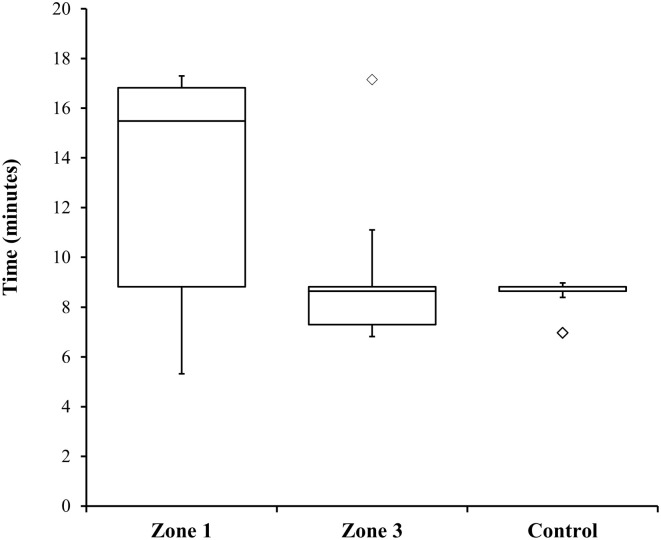
ROSC was obtained on all animals in all groups. There were no statistically significant differences in the time to ROSC between groups (Fig. 3 ). Table 3 shows the final rhythm, time to ROSC, and additional notes for each animal. Zone 1 animals showed divergent patterns to ROSC. One Zone 1 animal achieved ROSC 20 sec after REBOA balloon inflation without any additional intervention. Three Zone 1 animals had a notable delay in achieving ROSC (at 15 min, 16 min and 17 min, respectively). Two animals in the Zone 1 group obtained ROSC but subsequently devolved into ventricular fibrillation prior to the end of the experiment (at 19 min and 24 min, respectively). One animal in the Zone 3 group remained persistently in ventricular tachycardia with a pulse once ROSC was obtained. One Zone 3 animal had a delay in ROSC compared to the rest of its group (16 min). All control animals remained in normal sinus rhythm once ROSC was obtained.
Fig. 3.
Time to achieve return of spontaneous circulation. The ends of the whisker are set at 1.5 times the interquartile range above the third quartile and 1.5 times the interquartile range below the first quartile. Minimum or maximum values outside of this range are shown as outliers.
Table 3.
Rhythm and ROSC Details by Animal. Final rhythm, time to ROSC (in minutes) and relevant additional notes are listed by animal. NSR = normal sinus rhythm. ROSC = return of spontaneous circulation. PEA = pulseless electrical activity
| REBOA zone | Animal number | Final rhythm | Time to ROSC (minutes) | Additional notes |
|---|---|---|---|---|
| Control | 1 | Normal sinus rhythm | 7.0 | Remained in NSR once ROSC obtained |
| 2 | Normal sinus rhythm | 8.7 | Remained in NSR once ROSC obtained | |
| 3 | Normal sinus rhythm | 8.8 | PEA 2 min before ROSC then remained in NSR | |
| 4 | Normal sinus rhythm | 9.0 | Remained in NSR once ROSC obtained | |
| 5 | Normal sinus rhythm | 8.7 | Remained in NSR once ROSC obtained | |
| Zone 1 | 1 | Ventricular fibrillation | 17.3 | Devolved into ventricular fibrillation at 24 min |
| 2 | Ventricular fibrillation | 16.8 | PEA at 19 min, ventricular fibrillation at 21 min | |
| 3 | Normal sinus rhythm | 5.3 | Remained in NSR once ROSC obtained | |
| 4 | Normal sinus rhythm | 15.5 | Remained in NSR once ROSC obtained | |
| 5 | Normal sinus rhythm | 8.8 | Remained in NSR once ROSC obtained | |
| Zone 3 | 1 | Normal sinus rhythm | 8.7 | Remained in NSR once ROSC obtained |
| 2 | Ventricular tachycardia with a pulse | 7.3 | Remained in ventricular tachycardia with pulse once ROSC obtained | |
| 3 | Normal sinus rhythm | 6.8 | Remained in NSR once ROSC obtained | |
| 4 | Normal sinus rhythm | 8.8 | PEA 2 min before ROSC then remained in NSR | |
| 5 | Normal sinus rhythm | 17.2 | Remained in NSR once ROSC obtained |
4. Discussion
The use of REBOA during nontraumatic cardiac arrest is currently undergoing initial human trials. It is essential to understand the optimal zone in the aorta where REBOA should be positioned to balance hemodynamic benefits with potential ischemic injury caused by the intervention. In this pilot study we describe the hemodynamic effects of Zone 1 versus Zone 3 REBOA balloon placement in a ventricular fibrillation porcine model of cardiac arrest. We have demonstrated an improvement in diastolic blood pressure with Zone 1 REBOA but not with Zone 3 placement. There were no statistically significant improvements in systolic blood pressure, carotid flow, or change in time to achieve ROSC across all groups.
Zone 3 placement has been suggested to have a better safety profile compared to Zone 1 REBOA due to the decreased ischemic burden of a more distal point of occlusion. In this model of cardiac arrest, Zone 3 REBOA placement was not associated with improvement in blood pressure or carotid flow. We suspect the volume of vasculature within the abdomen is substantial enough that the small changes in blood flow to the lower extremities are not sufficient to provide a hemodynamic benefit. Prior translational studies and clinical studies have demonstrated a similar trend following hemorrhage, with less proximal hemodynamic improvement with Zone 3 placement compared to Zone 1. This was hypothesized to be due to a combination of the large amount of blood flow to the viscera in conjunction with decreased blood volume from hemorrhage [10,12]. We have demonstrated that in the low cardiac output state of CPR a similar trend is observed with no discernable improvement in proximal hemodynamics. Given these substantial risks and no hemodynamic benefits, Zone 3 placement of REBOA should not be considered to augment CPR following nontraumatic cardiac arrest.
In this study, Zone 1 REBOA placement improved diastolic blood pressure relative to control animals. The results are consistent with prior studies which showed that Zone 1 REBOA improves hemodynamic s during cardiac arrest. The improvement in diastolic blood pressure is of clinical significance given the role of diastolic blood pressure in coronary perfusion pressure calculations (coronary perfusion pressure = diastolic blood pressure - left ventricular end-diastolic pressure). Although left ventricular end-diastolic pressure was not measured during this study, we believe that the diastolic blood pressure is a satisfactory surrogate to draw initial conclusions. Previous research has shown that a threshold coronary perfusion pressure of 15 mmHg is required to achieve ROSC [15]. The diastolic blood pressures of both Zone 3 and control animals were below this threshold, which suggests that this is likely inadequate during prolonged resuscitation on a patient with underlying cardiac injury compared to the Zone 1 animals.
Despite these low diastolic blood pressures, all Zone 3 REBOA and control animals achieved ROSC. The swine model used for this study did not have any underlying cardiac injury, had immediate mechanical CPR initiation with limited low-flow time, and rapid initiation of ACLS. These factors likely influenced the time to ROSC of all groups. The entire control cohort achieved ROSC within a two minute period, with four of the five animals regaining pulses after the first dose of adrenaline and defibrillation. It is likely that with a no-flow period prior to initiation of CPR or with delayed ACLS interventions in-line with the emergency medical services response during out-of-hospital cardiac arrest, that the control group would not have obtained ROSC as uniformly as was observed in this study. However, this study design was chosen to help ensure that a greater portion of the animals would actually achieve ROSC so the relationship between ROSC and REBOA placement could be evaluated.
There is a notable dichotomy in the time to achieve ROSC between the animals in the control group and the animals which received REBOA. Although there were no statistically significant differences, the animals which received REBOA had a trend towards delayed ROSC and a higher likelihood to devolve into a non-perfusing rhythm. This was especially evident within the Zone 1 REBOA group, with three of the five animals having either a delay in ROSC or devolving into ventricular fibrillation prior to study completion. This delay may be due to myocardial injury or higher relative post-ROSC aortic strain due to Zone 1 REBOA placement. Unlike similar studies where direct cardiac injury was induced by coronary artery occlusion [11], the animals in this study had healthy hearts prior to induction of ventricular fibrillation and REBOA placement. Myocardial injury is a known consequence of Zone 1 REBOA placement in traumatically injured swine and it is possible that such injury will lead to a delay in ROSC and limit the hemodynamic s benefits of REBOA placement [10]. While this study was not powered to detect a difference in ROSC rate, these data suggest that the early use of REBOA in cardiac arrest may be detrimental to myocardial function post-ROSC, and use should likely wait until early ACLS fails.
These conclusions must take into context the limitations of this study. First, we were unable to blind the investigators after randomization. While the porcine cardiovascular model is considered close to humans [16], even small differences within chest structure or abdominal vasculature may impact CPR effectiveness, vascular compliance, or the hemodynamic impacts of REBOA. Balloon deflation was performed according to a pre-specified volume removal/unit of time; in some animals in the Zone 1 this may have precipitated a rapid decline in diastolic arterial blood pressure. More controlled balloon deflation may provide better diastolic support and prevent abrupt decreases in myocardial perfusion pressure. Finally, this study was not designed nor powered to evaluate post-ROSC sequelae of REBOA or to assess the functional outcomes related to these interventions. Future investigations will be needed to assess neurologic outcomes, the ideal timing and strategy to deflate the REBOA balloon once ROSC is achieved, the possible cardiac side effects of REBOA during cardiac arrest, and which patient attributes maximize the benefits of REBOA during cardiac arrest.
5. Conclusion
In our swine model of ventricular fibrillation-induced cardiac arrest, Zone 1 REBOA improved diastolic blood pressure when compared to control. Zone 3 placement did not provide any hemodynamic benefits compared to no occlusion. Although it was not statistically significant, animals receiving REBOA had a delay in ROSC. Unlike prior studies which saw an improvement in ROSC rate and time to ROSC with REBOA placement, our study suggests that further research is required to determine the cardiovascular impacts of REBOA placement during cardiac arrest. REBOA placement may provide a benefit to those with prolonged no-flow states or with more significant underlying cardiac injury. However, given our results, Zone 1 REBOA placement should be considered for upcoming human trials of REBOA during nontraumatic cardiac arrest.
Financial support
This work was supported by David Grant USAF Medical Center's Clinical Investigation Facility, Travis Air Force Base, California. The work reported herein was performed under United States Air Force Surgeon General approved Clinical Investigation No. FDG20180024A.
CRediT authorship contribution statement
Craig D. Nowadly: Conceptualization, Investigation, Formal analysis, Data curation, Writing - original draft, Funding acquisition. Guillaume L. Hoareau: Conceptualization, Investigation, Formal analysis, Data curation, Writing - review & editing, Funding acquisition. J. Kevin Grayson: Conceptualization, Investigation, Formal analysis, Data curation, Writing - review & editing, Funding acquisition. M. Austin Johnson: Conceptualization, Investigation, Formal analysis, Data curation, Writing - review & editing, Funding acquisition.
Acknowledgments
Acknowledgements
The authors would like to thank Jolife AB/Physio-Control and Mr. Fredrik Arnwald for their support in providing the LUCAS 3 Chest Compression System© to facilitate the completion of this project.
Animal use statement
The animals involved in this study were procured, maintained, and used in accordance with the Laboratory Animal Welfare Act of 1966, as amended, and NIH 80-23, Guide for the Care and Use of Laboratory Animals, National Research Council.
Disclaimers
The views expressed in this material are those of the authors, and do not reflect the official policy or position of the U.S. Government, the Department of Defense, the Department of the Air Force, or the University of California Davis.
Declaration of competing interest
C.D.N. reports no conflict of interest.
GLH reports no conflict of interest.
JKG reports no conflict of interest.
M.A.J. is a founder and stockholder of Certus Critical Care, Inc. that produces a product relevant to the subject material.
References
- 1.Meaney P.A., Bobrow B.J., Mancini M.E., Christenson J., de Caen A.R., Bhanji F. Cardiopulmonary resuscitation quality: improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation. 2013;128:417–435. doi: 10.1161/CIR.0b013e31829d8654. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139 doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 3.Daya M.R., Schmicker R.H., Zive D.M., Rea T.D., Nichol G., Buick J.E. Out-of-hospital cardiac arrest survival improving over time: results from the resuscitation outcomes consortium (ROC) Resuscitation. 2015;91:108–115. doi: 10.1016/j.resuscitation.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang L., Zhang J. Mechanical cardiopulmonary resuscitation for patients with cardiac arrest. World J Emerg Med. 2011;2:165–168. doi: 10.5847/wjem.j.1920-8642.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daley J., Morrison J.J., Sather J., Hile L. The role of resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct to ACLS in non-traumatic cardiac arrest. Am J Emerg Med. 2017;35:731–736. doi: 10.1016/j.ajem.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Daley J., Morrison J. REBOA in nontraumatic cardiac arrest. In: Hörer T., DuBose J.J., Rasmussen T.E., White J.M., editors. Endovascular resuscitation and trauma management : Bleeding and haemodynamic control. Springer International Publishing; Cham: 2020. pp. 135–148. [DOI] [Google Scholar]
- 7.Sesma J., Sara M.J., Espila J.L., Arteche A., Saez M.J., Labandeira J. Effect of intra-aortic occlusion balloon in external thoracic compressions during CPR in pigs. Am J Emerg Med. 2002;20:453–462. doi: 10.1053/ajem.2002.32627. [DOI] [PubMed] [Google Scholar]
- 8.Gedeborg R., Rubertsson S., Wiklund L. Improved haemodynamics and restoration of spontaneous circulation with constant aortic occlusion during experimental cardiopulmonary resuscitation. Resuscitation. 1999;40:171–180. doi: 10.1016/S0300-9572(99)00021-0. [DOI] [PubMed] [Google Scholar]
- 9.Nozari A., Rubertsson S., Wiklund L. Improved cerebral blood supply and oxygenation by aortic balloon occlusion combined with intra-aortic vasopressin administration during experimental cardiopulmonary resuscitation. Acta Anaesthesiol Scand. 2000;44:1209–1219. doi: 10.1034/j.1399-6576.2000.441005.x. [DOI] [PubMed] [Google Scholar]
- 10.Beyer C.A., Hoareau G.L., Tibbits E.M., Davidson A.J., DeSoucy E.D., Simon M.A. Resuscitative endovascular balloon occlusion of the aorta induced myocardial injury is mitigated by endovascular variable aortic control. J Trauma Acute Care Surg. 2019;87:590. doi: 10.1097/TA.0000000000002363. [DOI] [PubMed] [Google Scholar]
- 11.Dogan E.M., Beskow L., Calais F., Hörer T.M., Axelsson B., Nilsson K.F. Resuscitative endovascular balloon occlusion of the aorta in experimental cardiopulmonary resuscitation: aortic occlusion level matters. Shock. 2019;52:67. doi: 10.1097/SHK.0000000000001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tibbits E.M., Hoareau G.L., Simon M.A., Davidson A.J., DeSoucy E.S., Faulconer E.R. Location is everything: the hemodynamic effects of REBOA in zone 1 versus zone 3 of the aorta. J Trauma Acute Care Surg. 2018;85:101–107. doi: 10.1097/TA.0000000000001858. [DOI] [PubMed] [Google Scholar]
- 13.Rødseth Brede Jostein, Thomas Lafrenz, Pål Klepstad, Aardal Skjærseth Eivinn, Trond Nordseth, Edmund Søvik. Feasibility of pre-hospital resuscitative endovascular balloon occlusion of the aorta in non-traumatic out-of-hospital cardiac arrest. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karlis G., Iacovidou N., Lelovas P., Niforopoulou P., Zacharioudaki A., Papalois A. Effects of early amiodarone administration during and immediately after cardiopulmonary resuscitation in a swine model. Acta Anaesthesiol Scand. 2014;58:114–122. doi: 10.1111/aas.12226. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds J.C., Salcido D.D., Menegazzi J.J. Coronary perfusion pressure and return of spontaneous circulation after prolonged cardiac arrest. Prehosp Emerg Care. 2010;14:78–84. doi: 10.3109/10903120903349796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crick S.J., Sheppard M.N., Ho S., Gebstein L., Anderson R.H. Anatomy of the pig heart: comparisons with normal human cardiac structure. J Anat. 1998;193:105–119. doi: 10.1046/j.1469-7580.1998.19310105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]