Graphical abstract
Keywords: Severe acute respiratory syndrome coronavirus 2, Umifenovir, Ribavirin, Lopinavir/ritonavir and Lianhua Qingwen, Lymphopenia
Abstract
Objectives
The current diagnosis and medicines approach in coronavirus disease 2019 (COVID-19) does not reflect the heterogeneous characteristics of this disease. This study aims to find a new antiviral combination regimen by investigating the frequency of clinically relevant and objectively identified comorbidities, and the clustering of these clinical syndromes and varying results of treatment with antiviral drugs in patients hospitalized with severe COVID-19.
Methods
This study recruited 151 severe COVID-19 infection cases diagnosed in our hospital examination and illustrated the clinical potential during a consecutive 25-day medication period. Potential differences in disease severity and clinical characteristics, hematological profile, and current pharmacologic treatments (single agent, double or triple combinations, and the combined antiviral drugs plus Lianhua Qingwen) among comorbidity clusters were explored.
Results
Although disease severity was comparable among three clusters, it was markedly different in terms of laboratory test status. Coagulable abnormality was mainly present in cluster 1 and cluster 2. Other indicators were normal, except for a significant increase of neutrophils presented in cluster 2. Patients showed the most complicated haematological results in cluster 3, including severe coagulation abnormalities, leukocytosis, neutrophilic granulocytosis, and lymphopenia. Our results for the first time suggest that a quadruple combination therapy (Ribavirin, Lopinavir/ritonavir, Umifenovir, and Lianhua Qingwen) can be considered as a preferred treatment approach to severe COVID-19 patients. After treatment, abnormal coagulation and leukocyte had markedly improved with a better prognosis.
Conclusion
This study expands the understanding of the co-occurrence of combination therapy in patients with COVID-19, which provides the probability of developing novel combined therapy. Furthermore, explore clinical trials of variable antivirus treatments based on subgroup analyses or on using subgroups in the selection criteria would be the next step.
1. Introduction
Since Dec 2019, coronavirus disease 2019 (COVID-19) cases caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have become an epidemic and quickly spread across the world. The novel virus was identified as an enveloped, single-stranded, positive-strand RNA virus that can cause respiratory infections in humans [[1], [2], [3]]. Similar to the emergence of the severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002 and of the Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012, these zoonotic viruses could transmit to humans from various animal species and suggested that additional emergence events are likely to occur [1]. By the time of April 18, 2020, there have been a total of 701,610 confirmed cases with 37,055 deaths in the United States [3]. Globally, 154,215 deaths have been reported among 2,243,710 confirmed cases [4].
The 2020 SARS-CoV-2 outbreak highlights the urgent need for new and more effective therapy, and the emergence of safety to both drug classes among different patients has become a major public health concern. Currently, there are only limited of drugs approved for the treatment of COVID-19, including the use of lopinavir/ritonavir (LPV/r) and Ribavirin (suggested to be used jointly with interferon or LPV/r) [5]. Considering the demand for therapeutic options in response to the 2019-nCoV outbreak, Chinese researchers have been attempting to reuse several existing meds to tackle either the coronavirus itself or the sickness it causes during the continuing occurrence, including an influenza remedy Arbidol (Umifenovir), which is not approved in Western countries [6]. Moreover, the scientific community of China ought to be taken into account in previous experiments with TCM in improving symptoms such as coughing, weakness, and digestive system disorders, also as alleviating anxiety. Traditional Chinese medicine (TCM) decoctions were also expressly recommended in the newest version of the diagnosis and treatment plan issued by the National Health of Commission of China. Several patent herbals drugs, such as Huoxiang Zhengqi capsules, Lianhua Qingwen capsules, and Radix isatidis granula, are being proposed as treatments [7]. Compared with approved antiviral drugs, mechanism of TCM products is less understood. However, numerous clinical investigations have been started to more precisely evaluate their effects.
Furthermore, data relating to treatments of the combined antiviral drugs or combination of antivirals and TCM compared with antiviral drugs as single agents as clinical data were restricted, which could be a viable therapeutic option. Specifically, we hypothesized that antiviral drugs and traditional Chinese medicines would have different therapeutic effects on COVID-19 and would not affect all patients in the same way. Cluster analysis could be a statistical method that enables the researcher to identify groups of individuals due to their differences, which anticipated to clarify this complexity [8]. Although combination drug therapies are strongly recommended as a standard of care for the treatment of COVID-19, effectiveness and safeness of the synergy of variables antivirals on the hospitalized severe or critical illness patients has not been demonstrated to date. Combined with epidemic experience and statistical results, it is a feasible method to establish reference conditions for clinical diagnosis and intervention of COVID-19 patients to analyze the influence of the selection and combination of antivirals on patients.
In this paper, we report hematological profile results that indicated the effects of single or combined antivirals and TCM groups. Data were collected from patients in Union Hospital of Huazhong University of Science and Technology during their receiving treatment to ensure data quality. After classifying severe COVID-19 by clinical clustering analysis, the adequate grouping of baseline characteristics enables the identification of COVID-19 phenotypes that are preferentially responsive to antiviral treatment.
2. Methods
2.1. Subject enrollment
After careful medical chart review, we compiled the clinical data of laboratory-confirmed hospitalized cases from the Union Hospital of Huazhong University of Science and Technology in Wuhan between January 29th, 2020, and February 22nd, 2020. Patients diagnosed with COVID-19 based on the World Health Organization interim guidance were enrolled. This study was approved by the Ethics Committee of Union Hospital and registered at the Chinese Clinical Trial Registry (ChiCTR2000030803). Ethics Review Committees System approved the ethics review with the approval NO.2020-0096-1. The diagnosis of COVID-19 was according to 'Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia' released by the National Health Commission & State Administration of Traditional Chinese Medicine. It was confirmed by RNA detection of the SARS-CoV-2 in the clinical laboratory of Union Hospital. Confirmed cases denoted the patients whose real-time reverse-transcription polymerase-chain-reaction (RT-PCR) assay findings for nasal and pharyngeal swab specimens were positive.
2.2. Clinical evaluations at Wuhan Union Hospital
Clinical data, including recent exposure history, clinical symptoms and signs, comorbidities, and laboratory results at admission, were reviewed and abstracted by senior medical practitioners and entered into a computerized database for further verification. Because the severity of avian influenza was reported to be poorly predicted by clinical outcomes, according to American Thoracic Society guidelines for community-acquired pneumonia, patients were classified as severe or non-severe COVID-19 because of its global acceptance. Comorbidities were identified based on the patient's self-report at the time of admission. Comorbidities were initially treated as a categorical variable (Yes vs. No) and were subsequently classified according to single and multiple numbers. Moreover, comorbidities were classified according to the organ system, including the respiratory system, cardiovascular system, and endocrine system. The endpoint of our study was a synthetic measure, including the intensive-care unit (ICU), invasive ventilation, or death.
2.3. Design and patients
A total of 151 COVID-19 patients [67 (44.37%) male with a mean age of 59.9 years (S.D. 13.7)] were identified. The following data were collected from medical records by using a structured form: demographic characteristics (gender, age), disease condition, clinical characteristics (fever, cough), therapeutic medication, blood clotting tests (D-dimer) and complete blood count (White blood cell, WBC; Platelet, PLT; Neutrophil, NE; Lymphocyte, LY; Monocytes, MO), prognosis.
In this study, we chose seven variations of therapeutic antiviral drugs: umifenovir (200 mg each time for adults, three times daily, no longer than ten days), ribavirin (500 mg each time for adults, twice or three times of intravenous injection daily, no longer than ten days), lopinavir/ritonavir (200 mg/50 mg per pill for adults, two pills each time, twice daily, no longer than ten days), lianhua qingwen (four capsules each time, three times daily for a week), peramivir and sodium chloride (300 mg each time, one time of intravenous injection daily, for three to five days), oseltamivir (75 mg each time, twice daily for five days), ganciclovir (250 mg each time, twice of intravenous injection daily, for one to two weeks). The recommended doses of the seven prescribed drugs are based on the treatment experience and current Chinese guidelines for COVID-19.
We refer to the five different therapeutic regimens as umifenovir (group 1); umifenovir and lianhua qingwen (group 2); umifenovir, ribavirin and LPV/r (group 3); umifenovir, ribavirin, LPV/r, and lianhua qingwen (group 4); umifenovir, ribavirin, LPV/r, peramivir and sodium chloride, oseltamivir, penciclovir, ganciclovir (group 5).
2.4. Statistical analysis
Unless otherwise specified, values are presented as the mean (S.D.) or the percentage as appropriate. Data processing using Statistical Product and Service Solutions 25 software (SPSS 25). In the two-step cluster analysis, gender, disease condition, fever, cough, therapeutic medication, and prognosis were used as the categorical variables, and age, d-dimer, WBC, PLT, NE, LY, and MO data were used as the continuous variables. Chi-square test was used for comparison between the two groups, and one-way ANOVA was used for comparison between multiple groups. The level of significance was = 0.05, and P < 0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
The final study population consisted of moderate to critical illness COVID-19 patients, and these patients had complete clinical information and the laboratory data required for this study. The mean age at disease onset was 59.9 years (range 22–88 years). Fifty-one patients (33.77 %) have been with significant underlying illnesses, such as hypertension, coronary heart disease, diabetes mellitus, chronic kidney disease, or Respiratory disease. On the end of February 22nd, 2020, 25 of the 151 patients (16.56 %) have been discharged,79 (52.32 %)succeed with better clinical outcomes, and 7 (4.64 %) died, the rest 25 (16.56 %) remain hospitalized after the 25-days period.
3.2. Demographics of antiviral therapy response phenotypes in COVID patients by Cluster Analysis
Clustering 151 patients with 13 variables using a two-step approach revealed three distinct clusters with different antiviral therapy response. The demographics of the study population were illustrated in Table 1 . Medication groups used in patients with different comorbidities were shown in Table 2 .
Table 1.
Clinical feature, laboratory results, and prognosis in patients with coronavirus disease 2019.
| Characteristics | Cluster 1 (N = 96) | Cluster 2 (N = 33) | Cluster 3 (N = 22) | P Value |
|---|---|---|---|---|
| Demographic | ||||
| Age | 58.35 (14.39) | 59.67 (11.81) | 67.00 (11.25) | 0.028 |
| Women | 58 (60.4 %) | 20 (60.6 %) | 6 (27.30 %) | 0.015 |
| Symptoms and Conditions | ||||
| Disease Condition | severe cases 93 (96.9 %) | severe cases 33 (100 %) | critical illness 20 (90.9 %) | <0.001 |
| Fever | 81 (84.4 %) | 29 (87.9 %) | 18 (81.80 %) | 0.829 |
| Cough | 71 (74.0 %) | 24 (72.7 %) | 16 (72.70 %) | 0.964 |
| Blood clotting tests and Complete blood count | ||||
| D-dimer | 1.08 (1.60) | 1.10 (1.45) | 4.01 (3.60) | <0.001 |
| White blood cell | 5.74 (2.83) | 5.85 (1.99) | 8.74 (4.08) | 0.002 |
| Platelet | 234.50 (102.36) | 250.27 (84.64) | 187.23 (107.24) | 0.022 |
| Neutrophil | 5.11 (5.00) | 13.32 (11.33) | 9.36 (6.66) | <0.001 |
| Lymphocyte | 1.12 (0.54) | 1.42 (0.56) | 0.62 (0.28) | <0.001 |
| Monocytes | 0.44 (0.25) | 0.41 (0.14) | 0.29 (0.28) | 0.003 |
| Medication | ||||
| Drug Group | group 1 39 (40.6 %) | group 4 33 (100 %) | group 4 7 (31.8 %) | <0.001 |
| Prognosis | ||||
| Improvement Rate | 68 (70.8 %) | 28 (84.9 %) | 8 (36.4 %) | <0.001 |
Data are presented as n (%) or mean (S.D.) unless otherwise stated. P value represents any difference across the clusters. Increased means over the upper limit of the normal range and decreased means below the lower limit of the normal range.
Table 2.
Medication groups and comorbidities in patients.
| Medication groups |
|||||
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
| Clusters | |||||
| Cluster 1 (n = 96) | 39 (25.8 %) | 27 (17.9 %) | 17 (11.3 %) | 0 | 13 (8.6 %) |
| Cluster 2 (n = 33) | 0 | 0 | 0 | 33 (21.9 %) | 0 |
| Cluster 3 (n = 22) | 7 (4.6 %) | 1 (0.7 %) | 7 (4.6 %) | 7 (4.6 %) | 0 |
| Comorbidities | |||||
| Hypertension | 17 (11.3 %) | 4 (2.6 %) | 3 (2.0 %) | 5 (3.3 %) | 1 (0.7 %) |
| Coronary heart disease | 4 (2.6 %) | 3 (2.0 %) | 1 (0.7 %) | 3 (2.0 %) | 1 (0.7 %) |
| Diabetes mellitus | 5 (3.3 %) | 1 (0.7 %) | 2 (1.3 %) | 2 (1.3 %) | 1 (0.7 %) |
| Chronic kidney disease | 2 (1.3 %) | 1 (0.7 %) | 0 | 0 | 0 |
| Respiratory disease | 4 (2.6 %) | 1 (0.7 %) | 0 | 1 (0.7 %) | 0 |
| Other diseases | 9 (6.0 %) | 3 (2.0 %) | 1 (0.7 %) | 1 (0.7 %) | 1 (0.7 %) |
Group 1: Umifenovir; Group 2: Umifenovir and Lianhua Qingwen; Group 3: Umifenovir, Ribavirin and Lopinavir/ritonavir; Group 4: Umifenovir, Ribavirin, Lopinavir/ritonavir and Lianhua Qingwen; Group 5: Umifenovir, Ribavirin, Lopinavir/ritonavir, Peramivir and Sodium Chloride, Oseltamivir, Penciclovir, Ganciclovir. (P value represents any difference across the groups).
Data are presented as n (%).
Symptoms of fever and cough were observed in most patients but showed no significant difference between the three clusters.
At baseline, cluster 1 participants (n = 96) were relatively younger and evenly mixed between males and females, most severe cases. Participants in this cluster showed a high d-dimer level, and other hematological indicators were within the normal range. After treatment, 70.8 % of patients showed marked improvement, which was a higher rate than cluster 3 but lower than cluster 2.
The patients in cluster 2 (n = 33) were also young and with a slightly higher proportion of women. All patients in the group were severe cases and used medication group four for therapy. Similar to what was observed in cluster 1, most of the hematological indicators in these participants were within the normal range, except D-dimer and neutrophil count. The average D-dimer value was higher than cluster 1, and patients had the highest level of neutrophil. The improvement ratio was the highest among the three clusters.
Cluster 3 participants (n = 22) were the oldest with critically ill, 72.7 % of whom were men. Patients in this cluster presented the highest D-dimer and leukocyte count levels, and they also had the lowest average value of platelet, lymphocyte, and monocyte counts. They had the second-highest neutrophil count, which exceeded the normal range. Perhaps related to their disease severity, the participants in this cluster did not show significant improvement.
3.3. Validation of the newly identified clusters revealing the effectiveness of different combined medications
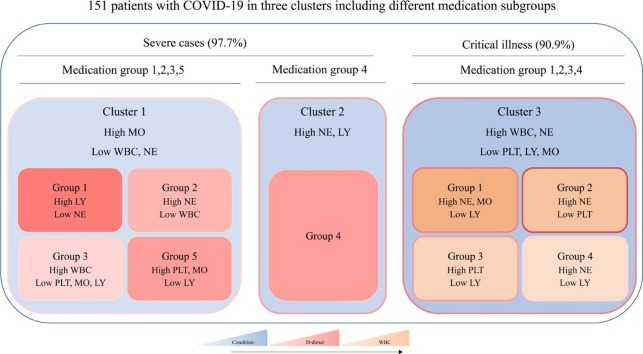
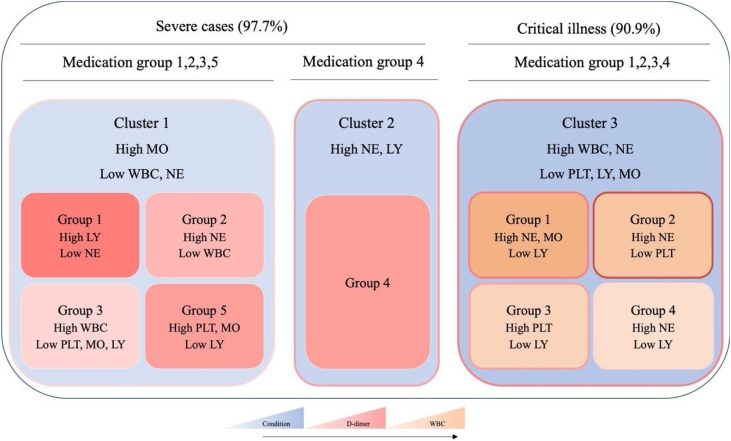
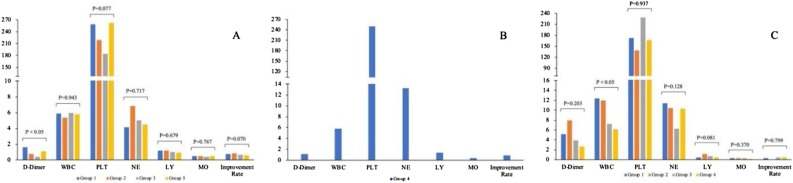
As shown in Fig. 1 , from clusters 1–3, there was a stepwise increase in D-dimer and leukocytes. The results of blood examination were more complicated as the increased severity of disease conditions. To find appropriate treatments, patients were divided into five subgroups based on the use of the drugs in each cluster for the different analysis of haematological profiles. Results of blood clotting tests and complete blood count after antivirus treatment in cluster 1 and cluster 3 were showed in Fig. 2 .
Fig. 1.
Schematic diagram of three clusters including the subgroup of different medication groups. The density of pink color indicates the mean value of D-dimer in each cluster, blue color indicates the severity of the condition, and yellow color indicates the mean value of WBC (WBC: white blood cell, PLT: platelet, NE: neutrophil, LY: lymphocyte, MO: Monocytes). The mean value or severity increases as the color deepens.
Group 1: Umifenovir; Group 2: Umifenovir and Lianhua Qingwen; Group 3: Umifenovir, Ribavirin and Lopinavir/ritonavir; Group 4: Umifenovir, Ribavirin, Lopinavir/ritonavir and Lianhua Qingwen; Group 5: Umifenovir, Ribavirin, Lopinavir/ritonavir, Peramivir and Sodium Chloride, Oseltamivir, Penciclovir, Ganciclovir. (P value represents any difference across the groups).
Fig. 2.
Laboratory results and prognosis in different medication subgroups in each cluster (A: cluster 1, B: cluster 2, C: cluster 3). (WBC: white blood cell, PLT: platelet, NE: neutrophil, LY: lymphocyte, MO: Monocytes).
Group 1: Umifenovir; Group 2: Umifenovir and Lianhua Qingwen; Group 3: Umifenovir, Ribavirin and Lopinavir/ritonavir; Group 4: Umifenovir, Ribavirin, Lopinavir/ritonavir and Lianhua Qingwen; Group 5: Umifenovir, Ribavirin, Lopinavir/ritonavir, Peramivir and Sodium Chloride, Oseltamivir, Penciclovir, Ganciclovir. (P value represents any difference across the groups).
Medication group one was used mostly in cluster 1, but with the highest D-dimer level. Although medication group two showed the highest improvement rate, medication group three seems to be a better choice, which presented the most significant improvements in D-dimer, and other indicators were within normal limits, except for a slight decrease of lymphocyte. In cluster 2, patients had the highest count of neutrophil, but the improvement rate was also the highest with the treatment of medication group four. In cluster 3, the number of patients in each group was equal, except for group two. Perhaps because they had received more treatment, the leukocyte and monocyte count of patients gradually decreased among groups one to four. With the same improvement rate of patients in group three and group four, both of the therapy reduced the level of D-dimer. The results of complete blood count in group three patients tend to be normal with modestly lower lymphocyte count. Patients in medication group 4 had the lowest level of D-dimer. However, this treatment could not help with neutrophilic granulocytosis and lymphopenia.
Considering the improvement of the disease condition and hematological indicators, group four might be a more effective antivirus therapy for COVID-19 patients.
4. Discussion
Since December 2019, the global accumulated diagnosed COVID-19 patients have more than 2,000,000 people; the epidemic situation has spread to 159 countries and regions. Until now, no specific treatment has been recommended for coronavirus infection except for meticulous supportive care. This study conducted a cluster analysis of COVID-19 patients, aiming to retrospectively summarized the differences in the responses of each cluster to different antiviral interventions, and provides reference opinions for treatment of COVID-19.
The results showed that for the COVID-19 patients with severe disease, the D-dimer level was markedly elevated, and the increase of neutrophil was revealed. Moreover, abnormal coagulation results (high D-dimer level) and partial abnormal of complete blood count (including leukocyte increase, thrombocytopenia, lymphocytopenia, and monocytopenia) appeared in patients who develop a critical illness. In this study, prominent laboratory abnormalities were in agreement with that reported clinical features in COVID-19 cases [[9], [10], [11], [12], [13]].
In the past studies, many antiviral drugs have been developed for clinical use to treat millions of human beings worldwide, and some of them have been approved efficiency of severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS), such as Ribavirin and LPV/r [[14], [15], [16], [17], [18]]. Ribavirin is a unique guanosine analogue with broad-spectrum activity against many RNA and DNA viruses [19].There are several proposed mechanisms of action, including indirect mechanisms (Enhancement of host T-cell-mediated immunity against viral infection and inhibition of the host enzyme Inosine-5′-monophosphate dehydrogenase) and other hypotheses (Direct inhibition of viruses and as an RNA mutagen) [19,20]. LPV/r is a fixed-dose combination medication for the treatment and prevention of human immunodeficiency virus (HIV) / acquired immunodeficiency syndrome (AIDS). Lopinavir is a potent inhibitor of HIV-1 protease, which showed the effect on inhibition of the protease produces immature, non-infectious virions [21]. The single-use of lopinavir has insufficient bioavailability, but its blood levels could significantly increase by low doses of ritonavir [22].
Additionally, interest in another two drugs has been renewed as a result of the COVID-19 outbreak. Umifenovir is an antiviral treatment for influenza infection used in Russia and China [23], which was claimed to inhibit early membrane fusion from preventing contact between the virus and the target host cells [24]. It was shown to have effects on stimulating the immune response on its capacity to induce interferon production and activate the phagocytic function of macrophages [23]. As a traditional Chinese medicine prescription, Lianhuaqingwen (LH) has shown an aboard spectrum antiviral effects and have been widely used for respiratory tract diseases, such as influenza, acute bronchitis, asthma, and chronic obstructive pulmonary disease (COPD) [25]. Previous studies have confirmed that the mechanisms of LH action may be reduced systemic and airway inflammation by regulation of the inherent and acquired immune systems through inhibition of the release of the corresponding inflammatory factors [26]. Moreover, LH could inhibit viral replication, especially at the early stage of virus infection, and regulates the immune response of virus infection [27].
Some case reports have indicated that, in patients with SARS-CoV infection, MERS-CoV infection, or severe influenza, combination therapy may accelerate clinical recovery compared to monotherapy [[28], [29], [30], [31]]. The combined use of two or more antivirus drugs with different mechanisms of action, acting on different stages of the virus replication cycle, may result in producing drug synergism or additivity to improve the efficacy. At the same time, regulating body immune function could raise the ability to resist viruses while inhibiting virus replication, which helps to shorten the treatment time and reduce the occurrence of the drug resistance. Although studies have shown that combined antiviral therapy has certain advantages over single-drug treatment, many problems exist, including how to select the combination of drugs, the dose and proportion of various medications, and whether the interaction of multiple medications will produce toxicity.
In our study, the use of two-step clustering and subgroup analysis enabled an in-depth analysis of the effects of single and combination drug therapies on the virus population. Following the antiviral therapy, there was indeed an improvement of severe patients' condition, and the effect of a combination of three drugs plus Lianhua Qingwen was superior to single or dual agents. This combination of drugs with different mechanisms of action may result in maximal suppression of virus replication and infection. Meanwhile, regulating uncontrolled inflammatory innate responses and impaired adaptive immune responses caused by SARS-CoV-2, which involves several pathways and targets that combine to become an integrated action in the body. For instance, the virus-host fusion inhibitor umifenovir blocks viral fusion with target membranes, prohibiting viral entry into cells; the lopinavir/ritonavir prevent cellular infection by inhibiting the formation of infectious virions; the RdRp inhibitor ribavirin can incorporate into nascent viral RNA chains and results in premature termination while lianhua qingwen can inhibit virus replication and reducing the cytokine release from host cells to reduce the disease course of clinical trial patients. Besides, ribavirin in combination with LPV/r was shown to the improvement of patients with SARS-CoV infection in 2003. While the data may not reflect all the patients’ conditions, they provide partial evidence by comparing the effects of different drug regimens under similar conditions.
Although the data are not sufficient for antiviral agent treatments in COVID-19, quadruple combined antiviral therapy has been recommended for critically ill patients considering the decreased improvement rate and the safety data. However, the outcomes of this study offer only a snapshot of antiviral agents, effectiveness, and drawing any conclusions at this point is premature.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was financially supported by the Faculty Research Grant of Macau University of Science and Technology (FRG-20-004-SKL) and Special Fund Project for Science and Technology Innovation Strategy of Guangdong Province (Grant No. 2019B121205004). The content is solely the responsibility of the authors. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005;69(4):635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren S.Y., Gao R.D., Chen Y.L. Fear can be more harmful than the severe acute respiratory syndrome coronavirus 2 in controlling the corona virus disease 2019 epidemic. World J. Clin. Cases. 2020;8(4):652–657. doi: 10.12998/wjcc.v8.i4.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johns Hopkins Coronavirus Resource Center; 2020. Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE)https://coronavirus.jhu.edu/map.html [Google Scholar]
- 4.Coronaviridae Study Group of the International Committee on Taxonomy of, V The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Health Commission, State Administration of Traditional Chinese Medicine . 2020. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 4) p. 3. [Google Scholar]
- 6.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Trends. 2020;14(1):69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 7.Redeploying plant defences. Nat. Plants. 2020;6:177. doi: 10.1038/s41477-020-0628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leonard S.T., Droege M. The uses and benefits of cluster analysis in pharmacy research. Res. Social Adm. Pharm. 2008;4(1):1–11. doi: 10.1016/j.sapharm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. 2020. Clinical Characteristics of Coronavirus Disease 2019 in China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Clercq E., Li G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016;29(3):695–747. doi: 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses - drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15(5):327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Clercq E. New nucleoside analogues for the treatment of hemorrhagic fever virus infections. Chem. Asian J. 2019;14(22):3962–3968. doi: 10.1002/asia.201900841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng V.C., Tang B.S., Wu A.K., Chu C.M., Yuen K.Y. Medical treatment of viral pneumonia including SARS in immunocompetent adult. J. Infect. 2004;49(4):262–273. doi: 10.1016/j.jinf.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morse J.S., Lalonde T., Xu S., Liu W.R. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem. 2020;21(5):730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nyström K., Waldenström J., Tang K.W. Ribavirin: pharmacology, multiple modes of action and possible future perspectives. Future Virol. 2019;14(3):153–160. [Google Scholar]
- 20.Thomas E., Ghany M.G., Liang T.J. The application and mechanism of action of ribavirin in therapy of hepatitis C. Antivir. Chem. Chemother. 2012;23:1–12. doi: 10.3851/IMP2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandwani A., Shuter J. Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther. Clin. Risk Manag. 2008;4(5):1023. doi: 10.2147/tcrm.s3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sham H.L., Kempf D.J., Molla A., Marsh K.C., Kumar G.N., Chen C.M. ABT-378, a highly potent inhibitor of the human immunodeficiency virus protease. Antimicrob. Agents Chemother. 1998;42(12):3218–3224. doi: 10.1128/aac.42.12.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leneva I.A., Russell R.J., Boriskin Y.S., Hay A.J. Characteristics of arbidol-resistant mutants of influenza virus: implications for the mechanism of anti-influenza action of arbidol. Antiviral Res. 2009;81(2):132–140. doi: 10.1016/j.antiviral.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Glushkov R.G., Gus’ kova T.A., Krylova L., Nikolaeva I.S. Mechanisms of arbidole’s immunomodulating action. Vestnik Rossiiskoi akademii meditsinskikh nauk. 1999;3:36–40. [PubMed] [Google Scholar]
- 25.Runfeng L., Yunlong H., Jicheng H., Weiqi P., Qinhai M., Yongxia S. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong L., Xia J.W., Gong Y., Chen Z., Yang H.H., Zhang J. Effect of lianhuaqingwen capsules on airway inflammation in patients with acute exacerbation of chronic obstructive pulmonary disease. Evid. Based Complement. Altern. Med. 2014;2014 doi: 10.1155/2014/637969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding Y., Zeng L., Li R., Chen Q., Zhou B., Chen Q. The Chinese prescription lianhuaqingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function. BMC Complement. Altern. Med. 2017;17(1):130. doi: 10.1186/s12906-017-1585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin Y., Cai L., Cheng Z. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Military Med. Res. 2020;7(4) doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020;92(5):479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng Y.M., Xu X.L., He X.Q., Tang S.Q., Li Y. Comparative effectiveness and safety of ribavirin plus interferon-alpha, lopinavir/ritonavir plus interferon-alpha and ribavirin plus lopinavir/ritonavir plus interferon-alphain in patients with mild to moderate novel coronavirus pneumonia. Chin. Med. J. (Engl.) 2020;133(9):1132–1134. doi: 10.1097/CM9.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y., Fan G., Salam A., Horby P., Hayden F.G., Chen C. Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically ill patients with influenza virus infection. J. Infect. Dis. 2019;221(10):1688–1698. doi: 10.1093/infdis/jiz656. [DOI] [PubMed] [Google Scholar]