Highlights
-
•
The global pharmaceutical industry is a highly regulated sector.
-
•
Stark absence of global harmony in these regulatory processes.
-
•
Efforts taken to bring global uniformity, but is far from successful.
-
•
Highly regulated aviation industry enjoys globally harmonized regulatory processes.
-
•
Shout out to international authorities to take a cue from aviation sector.
-
•
This will ensure introduction of best vaccines and timely availability for patients.
Keywords: Aviation, Pharmaceuticals, Regulatory processes, Vaccine
1. Introduction
What does a vaccine have in common with an aircraft? Indeed, a very strange question, and at first glance, the likely answer would be none. One is small, the other one is big. One flies, the other is injected. One is made of metals, plastics, and needs kerosene as fuel, while the other is liquid when injected manually. One helps people to travel as fast, while vaccination saves and protects the lives of millions. The list goes on. So, why this strange question? Well, travelers expect to travel safely, and patients expect to be safely immunized. Both are highly technological and complicated to manufacture. In both cases safety is paramount, and people trust that the appropriate gate keepers (regulators) have ensured that both guarantee their safety. In either case people are in good health prior to travel or vaccination, and they expect to remain that way.
Regulatory oversight is harmonized worldwide for aircraft, but not for vaccines or medicinal products. Incessant efforts in regulatory harmonization of pharmaceuticals were unable to stem significant heterogeneity across countries, resulting in duplication of efforts for initial licensure and post licensure changes. This may contribute to delays in supply, cause shortages and delay patients’ access to the latest vaccines. Aviation regulations, in contrast, are harmonized and an aircraft can benefit from a single worldwide certification based on mutual recognition agreements between certification authorities. In the current COVID-19 pandemic context whereby the entire world is eagerly waiting for a vaccine, this may be the time to really think out the box.
Hence, for the benefit of the population let’s call for United Nations to develop a similar regulatory environment for vaccines, in a timely manner to protect them from serious diseases which know no borders.
2. The challenge
The pharmaceutical industry is highly regulated. All pharmaceutical products need to be authorized by health authorities prior to marketing. Their production must follow Good Manufacturing Practices (GMPs), guidelines and must comply with the relevant pharmacopoeias. National health authorities of every country have their unique regulatory requirements, and worldwide there are ~40 different pharmacopoeias. Furthermore, more than 60 authorities have a local release process for each vaccine batch. The pharmaceutical regulatory landscape remains heterogeneous despite numerous harmonization efforts. Heterogeneity is increasing with emergent countries developing their own regulatory framework.
Many vaccines comprise multiple antigens. Vaccine production requires a huge number of raw materials, and typically takes 18–24 months, with the simplest taking 6 months, and the most complex taking up to 36 months. The number of quality control tests on each vaccine batch varies between 100 and more than 1000, and testing accounts for up to 70% of production time. Moreover, each batch of vaccine released by the manufacturer is retested and released by a National Control Laboratory (NCL) of a reference country as well as by some NCLs upon importation, resulting in multiple tests on the same batch.
Once marketing authorization has been granted, any change to the production process requires a Post-Approval Change (PAC), or variation, that may also need to be approved by regulators. Changes are frequently made to increase production capacity or robustness, and can include changes to equipment, processes, raw materials, suppliers, or quality control testing, as well as any changes needed to comply with new quality or regulatory requirements, or pharmacopoeias. For a global vaccine manufacturer, a single PAC may need submitting to more than 100 individual regulatory agencies for notification or approval. A vaccine company with a large portfolio typically submits 6000–8000 variation dossiers per year. About 4 years is needed to get a single PAC approved worldwide.
Due to manufacturing complexity and duration, only one version of a manufacturing process can be used at a time. Consequently, stocks of vaccine at different production stages using “pre-change” processes must be constituted to cover demand during the period until all regulators have approved the change, which is both challenging and costly. Regulatory complexity can therefore delay efforts to increase manufacturing capacity, delay the application of the latest scientific and technology innovations, and is a factor contributing to worldwide vaccine supply issues. Ultimately this delays patients’ access to the latest and highest quality vaccines. It is estimated that due to this heterogeneity and complexity, each new manufacturing improvement necessitating regulatory approval places about half of the world population at risk of vaccine supply shortage (IFPMA, 2016 [1])
How could we dramatically improve the lifecycle management of vaccines so that populations can reliably benefit from the latest and highest quality standard vaccines in a timely manner?
3. The dream
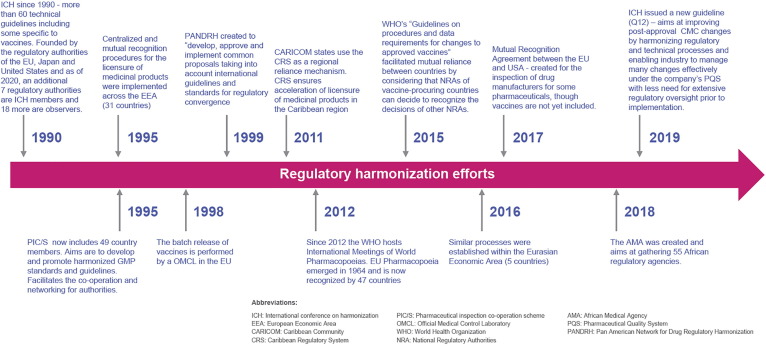
Several efforts have been put into place to bring about regulatory harmonization in the pharmaceutical sector (Fig. 1 ). Despite these harmonization efforts, their success could be described as partial. For example, only 28 health authorities attend ICH conferences out of a total of about 180 countries in the world, and guidelines are not compulsory. Furthermore, some guidelines remain unimplemented or only partially implemented years after completion. Several authors have been increasingly highlighting the need for an improved, more harmonized regulatory environment [2], [3], [4], [5], [6], [7], [8], [9], [10].
Fig. 1.
Timeline depicting some of the various efforts undertaken over the years to bring about harmonization in regulatory processes pertaining to the pharmaceutical sector.
So, is this current environment good enough to address these issues to help reduce the risk of vaccine shortages and ensure timely access of new vaccines? Nonetheless, gradual efforts will continue towards better harmonization and convergence, and developing reliance mechanisms between regulatory agencies. This is likely the most pragmatic way forwards, being based on existing international bodies and multilateral agreements. However, this is extremely slow. For example, even in the EU where European drug licensing procedures were set up 25 years ago, some elements are still managed nationally (e.g. Clinical Trial Applications)!
One should acknowledge existence of scientific specificities or local practices depending on populations. However, with current globalization and movements of populations, does it still make sense that the same individual may be vaccinated according to different practices, may be warned of different adverse events, … depending on the country where he is vaccinated, and while the same manufactured and controlled batch is supplied to all countries?
We need a different model, one that would dramatically reshape the worldwide regulatory landscape for pharmaceuticals. THE DREAM is to conceive an efficient, globally and mutually recognized regulatory mechanism based on a single set of requirements for the review, assessment and worldwide approval of medicines including their post-approval lifecycle management!
4. The aircraft and the vaccine
Is the above dream achievable? The regulatory environment for civil aviation indicates the answer is ‘Yes’.
Regulations for the aviation industry started in 1944 when 52 nations set up the regulatory framework for civil aviation. The International Civil Aviation Organization (ICAO), which has 187 country adherents sets international standards, recommended practices, and policies which guide ICAO member states in setting their national regulations.
Based on the ICAO standards, regional regulatory bodies in Europe, the Americas, and Asia are responsible for the certification of new aircraft models, based on the manufacturer’s dossier and ICAO Certification Specifications. These bodies also review and certify any changes made to the aircraft type. Each aircraft model is approved by a single regulatory body, based on mutual recognition agreement between regulators. For each individual aircraft, a single certificate of airworthiness, recognized worldwide, is granted by one National Aviation Authority (NAA). Finally, the aircraft manufacturer and owner are periodically audited by authorities alongside regular regional assessments. This system enables aircrafts to operate worldwide without additional regulatory requirements, unless otherwise indicated (e.g. Blacklisted companies in the EU). Globally, four billion passengers trusted this system in 2017, and flying remains the safest way to travel with 1 accident per 7.4 million flights.
5. Conclusion
The aircraft industry is around 80 years old, which is very new compared to the use of medicinal products. For both medicinal products and aircraft, the current regulatory framework stems from the middle of the twentieth century. So, why did these two regulatory environments evolve in different directions? The aircraft industry shows us that it is possible to have a unique set of global standards, mutual recognition mechanisms which require internationally recognized, requirements, specifications and regulatory bodies.
This dream is achievable, albeit the hurdles. The current situation is not sustainable, and change is necessary. Continuing to contemplate a worldwide situation where local regulations and requirements continue to emerge despite harmonization efforts and mutual reliance will neither help securing the supply of vaccines nor give access to the latest quality and innovative improvements in a timely manner. It is time to change gears and to think disruptively. The COVID-19 pandemic illustrates that outbreaks know no borders. So, why should vaccines created to fight those diseases be regulated with borders? What if ALL nations could overcome their local regulations and political and economic barriers to collectively modernize the way they work across borders for pharmaceuticals, just like they do for aircrafts? Let us CALL FOR ACTION at the level of the United Nations to join efforts to transform the dream into reality for the benefit of the 7 billion people who need equal and timely access to vaccines and other medicines.
“A dream you dream alone is only a dream. A dream you dream together is reality.”: John Lennon
Declaration
Information on civil aviation regulations has been gathered from OSAC “Organisme pour la Sécurité de l’Aviation Civile” who received delegation from the French authorities for aircraft certification and airworthiness oversight.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: ‘The author is an employee of Sanofi Pasteur. The author declares that there are no other known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The opinions expressed in this article are the author’s, and do not necessarily reflect those of the employer’.
Acknowledgement
T. Gastineau thanks the contribution of P. Juvin, G. Galy, G. Marsh (Sanofi Pasteur) and M. McGoldrick (MSD).
References
- 1.The complex journey of a vaccine – Part II; Immunization supply chain, delivery innovation, and regulatory requirements; IFPMA; 2016. https://www.ifpma.org/wp-content/uploads/2016/01/IFPMA-ComplexJourney-Part_Two_2016.pdf.
- 2.Wechsler J. Efforts accelerate to streamline postapproval change process; Manufacturers and regulatory authorities seek coordinated lifecycle management policies. Biopharm Int 2016;29(9):8,10,50.
- 3.Hoath C., Chang L., Iyer K.R., Kutza J., Murray S., Smith H., Payne R. Postapproval changes for biopharmaceutical drug-substance and drug-product manufacture; Regulatory complexity and impact. Bioprocess Int. 2016;14(11):38–43. [Google Scholar]
- 4.Zerhouni E., Hamburg M. The need for global regulatory harmonization: a public health imperative. Sci Transl Med. 2016;8(338):pp338ed6. doi: 10.1126/scitranslmed.aaf1396. [DOI] [PubMed] [Google Scholar]
- 5.Medicine and vaccine shortages: what is the role of global regulatory complexity for post approval changes? A report in The Economist; 2018. http://graphics.eiu.com/upload/topic-pages/medicine-shortages/Medicine-and-vaccine-shortages-EIU.pdf.
- 6.Lindstrome-Gommers L., Mullin T. International conference on harmonization: recent reforms as a driver of global regulatory harmonization and innovation in medicinal products. Clin Pharmacol Therapeut. 2019;105(4):926–931. doi: 10.1002/cpt.1289. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien J., Lumsden R.S., Diehl D.H., Macdonald J.C. Building a better approach for the benefit of patients: 10 pillars to strengthen regulatory review systems globally. Therapeut Innov Regulat Sci. 2019;XX(X):1–10. doi: 10.1007/s43441-019-00055-9. [DOI] [PubMed] [Google Scholar]
- 8.Dellepiane N., Pagliusi S. Opportunities for improving access to vaccines in emerging countries through efficient and aligned registration procedures: an industry perspective. Vaccine. 2019;37(23):2975–3140. doi: 10.1016/j.vaccine.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Regulatory reliance principles; concept note and recommendations, from PAHO; 2018. http://iris.paho.org/xmlui/handle/123456789/51549.
- 10.Cooke E. WHO’s approaches to promoting reliance, from presentation in July 2019. http://www.nationalacademies.org/hmd/~/media/Files/Presentations/MRA/EmerCooke%20Presentation.pdf.