Abstract
Objective
The aim of this manuscript is to investigate transversally Ear Nose Throat (ENT) symptoms COVID-19 infection correlated and to study the neurotropism and neuroinvasiveness of the virus in the head-neck district through the investigation of the sense of smell, taste, tearing, salivation and hearing.
Methods
A total of 50 patients with laboratory-confirmed COVID-19 infection were included in our study. For each patient we evaluated the short version of the Questionnaire of Olfactory Disorders-Negative Statements (sQOD-NS), the Summated Xerostomia Inventory-Dutch Version (SXI-DV), The Standardized Patient Evaluation of Eye Dryness (SPEED), Schirmer test I, the Hearing Handicap Inventory For Adults (HHIA) and the Tinnitus Handicap Inventory (THI). All the tests we carried out were performed during the active phase of the symptomatology from COVID-19 (Condition A) and 15 after SARS-COV-2 RT-PCR test negative (Condition B).
Results
A total of 46 patients (92%) had olfactory dysfunction related to the infection. The 70% of patients reported gustatory disorders. Cough, fever, headache and asthenia were the most prevalent symptoms. There was a statistically significant difference (p < 0,001) in sQOD-NS, SXI-DV, SPEED, Schirmer test, HHIA and THI between Condition A and Condition B.
Conclusions
In our population there was an alteration of the sense of taste, of the sense of smell, dry eyes and of the oral cavity and an auditory discomfort, symptoms probably linked to the neurotropism of the virus. Furthermore, anosmia, dysgeusia and xerostomia are early symptoms of COVID-19, which can be exploited for an early quarantine and a limitation of viral contagion.
Keywords: COVID-19, SARS-CoV-2, Head and neck, ENT, Neurotropism, Dry eye, Anosmia, Hearing loss
1. Introduction
A new sequence of human RNA coronavirus was identified in December 2019 in the city of Wuhan, Hubei province in China. The infection started in December 2019 spreading around the world due to its particularly high contagious transmission human-to-human. In March 2020 the World Health Organization (WHO) declared COVID-19 outbreak a pandemic disease, indeed it is responsible for >350,000 deaths worldwide. Coronaviruses (CoVs), family of Coronaviridae, are single-stranded enveloped positive sense RNA viruses, ranging from 60 nm to 140 nm in diameter with spikes like projections on its surface giving it a crown like appearance under the electron microscope. Previous outbreaks of coronaviruses (CoVs) include the severe acute respiratory syndrome (SARS)-CoV and the Middle East respiratory syndrome (MERS)-CoV which have been previously characterized as agents with a great public health impact. In particular, SARS-CoV-2 is the seventh member of the family of coronaviruses, which is the beta-CoV with over 70% similarity in genetic sequence to SARS-nCoV. It seems that SARS-CoV-2 and MERS CoV have a zoonotic reservoir nature, bats and snakes in particular [[1], [2], [3], [4], [5]]. The common general symptoms of the infection are fever, dry, cough, sputum production, myalgia, arthralgia, headache, diarrhea, dyspnea and fatigue, similar to rhinoviruses, influenza viruses, parainfluenza viruses, respiratory syncytial viruses, adenoviruses and enteroviruses. In more severe cases, this virus could cause pneumonia and could lead to severe acute respiratory distress syndrome (ARDS) and even death. Symptoms regarding otorhinolaryngology field are also registered like pharyngodynia, nasal congestion, rhinorrhea, smell and taste disorders in COVID-19 patients. British Rhinology Society underline how an important part of the COVID-19 patients referred anosmia, ageusia and, also, dysgeusia [[6], [7], [8], [9], [10]]. Literature showed higher viral concentration in the nasal cavity as compared to the throat [3]. Anosmia and dysgeusia have already been reported in paucisymptomatic and asymptomatic COVID-19 positive patient, representing in most cases, the first or the only symptomatology manifestation [11,12]. The American Academy of Otolaryngology - Head and Neck Surgery confirmed that anosmia and dysgeusia has been reported by SARS-CoV2 positive patients, and recommended to add these symptoms to the list of screening test for possible COVID-19 infection [13]. Post viral anosmia is one of the most important causes of loss of sense of smell in adults [[14], [15], [16]]. As curiosity, since the outbreaks has spreads in Europe, frequency of searches for smell-related information has increased with the onset of COVID-19 infection in Italy, Spain, UK, USA, Germany, France, Iran and Netherlands [17].
Coronaviruses, and beta-coronaviruses to which the SARSCoV-2 belongs, do not limit their presence to the respiratory tract, in fact they frequently invade the Central Nervous System (CNS). This propensity has been convincingly documented for the SARS-CoV, MERS-CoV, and the coronavirus responsible for porcine hemagglutinating encephalomyelitis (HEV 67N).
Previous findings demonstrate that Angiotensin-Converting Enzyme 2 (ACE2) represents the key, but not the exclusive site of entry of the virus into the cell. The ACE2 is expressed in the brain, being particularly present in the brain stem [18]. The intranasal administration of SARS-CoV-1 10 or MERS-COV 11 resulted in the rapid invasion of viral particles into the brain, possibly through the olfactory bulb via the trans-synaptic route. This pathway when the virus enters peripheral nerves and spreads to the CNS through synaptic contacts has been well documented for several viruses including CoVs.
One method to highlight the neuroinvasiveness of the virus in the ENT district is to evaluate the sense of smell and taste as it is already done in several studies. Furthermore, to analyze the neurotropism of further cranial nerves, we thought of studying tearing, salivation and hearing.
The lacrimal gland receives sensory nerve supply from the lacrimal nerve, a branch of the ophthalmic nerve, via the communicating branch and the zygomatic nerve, a branch of the maxillary nerve. The greater petrosal nerve, a branch of the facial nerve, conveys the parasympathetic and sympathetic fibers to the lacrimal gland.
The parotid gland receives both sensory and autonomic innervation. Preganglionic parasympathetic fibers leave the brain stem from inferior salivatory nucleus in the glossopharyngeal nerve and then through its tympanic so the lesser petrosal branch pass into the otic ganglion. There, they synapse with postganglionic fibers which reach the gland by hitch-hiking via the auriculotemporal nerve, a branch of the mandibular nerve.
Parasympathetic innervation to the submandibular glands is provided by the superior salivatory nucleus via the chorda tympani, a branch of the facial nerve, that becomes part of the trigeminal nerve's lingual nerve prior to synapsing on the submandibular ganglion.
The vestibulocochlear nerve is the eighth cranial nerve, transmits sound and equilibrium information from the inner ear to the brain because it is splits into two large divisions with different functions: the cochlear nerve and the vestibular nerve. It goes out from cerebellopontine angle. The cochlear nerve travels away from the cochlea where it starts as the spiral ganglia. Processes from the organ of Corti conduct afferent transmission to the spiral ganglia. The inner hair cells of the organ of Corti are responsible for activation of afferent receptors in response to pressure waves reaching the basilar membrane through the transduction of sound. COVID-19 infection could have deleterious effects on cochlear hair cell functions despite being asymptomatic. Typically, virus-induced hearing loss is sensorineural. Occasionally, recovery of hearing after these infections can occur spontaneously.
The aim of this manuscript is to investigate transversally Ear Nose Throat (ENT) symptoms COVID-19 infection correlated and to study the neurotropism and neuroinvasiveness of the virus in the head-neck district through the investigation of the sense of smell, taste, tearing, salivation and hearing.
2. Materials and methods
The study was conducted in the province of Messina, selecting Covid-19 patients who were positive for tests carried out throughout the Messina ASP hospital network. Patients were invited to participate and the informed consent was obtained.
Inclusion criteria: adult > 18 years old; laboratory-confirmed COVID-19 infection (reverse transcription polymerase chain reaction, RT - PCR); native speaker patients and patients clinically able to fulfill the questionnaire.
Exclusion criteria: patients with olfactory, gustatory, tear and salivary dysfunctions before the pandemic; patients with neurodegenerative diseases or with dysfunctions concerning the CNS or the PNS; patients in high flow oxygen therapy with non-invasive ventilation (PEEP, CPAP etc.), as it can alter the physiological hydration of the oral cavity and conjunctiva; patients who were in the intensive-care unit at the time of the data collection.
All the tests we carried out were performed during the active phase of the symptomatology from COVID-19 (Condition A) and 15 after SARS-COV-2 RT-PCR test negative (Condition B).
2.1. Clinical data
Clinical data have been prospectively collected during ENT consultation. The online questionnaire was created with Google Questionnaire based on IFOS questionnaire, composed by the COVID-19 Task Force of Young-Otolaryngologists of the International Federations of Oto-rhino-laryngological Societies (YO-IFOS) [9]. The questionnaire consisted of four general questions (age, sex, ethnicity, and date of diagnosis); three general clinical questions (comorbidities, general, and ENT symptoms associated with COVID-19 infection); seven questions about olfactory function; four questions investigating gustatory function, and one question about the treatment of the COVID-19 infection.
2.2. Olfactory and gustatory data
All patients were asked to complete the short version of the Questionnaire of Olfactory Disorders-Negative Statements (sQOD-NS). The questionnaire has been translated into Italian.
The occurrence of anosmia or hyposmia has been identified in the questionnaire. The impact of olfactory dysfunction on the quality of life (QoL) of patients has been assessed through the validated sQOD-NS. This is a seven-item patient-reported outcome questionnaire including social, eating, annoyance, and anxiety questions. Each item is rated on a scale of 0–3, with higher scores reflecting better olfactory-specific QoL. The total score ranges from 0 (severe impact on QoL) to 21 (no impact on QoL) [19]. The same test was performed also in all recovered patients 15 days after SARS-COV-2 RT-PCR negative. The rest of the olfactory and gustatory questions were based on the smell and taste component of the National Health and Nutrition Examination Survey [10]. This population survey was implemented by the Centers for Disease Control and Prevention to continuously monitor the health of adult citizens in the United States through a nationally representative sample of 5000 persons yearly [20]. The questions have been selected to define the variation, timing, and associated symptoms of both olfactory and gustatory dysfunctions, and, therefore, they suggest a potential etiology.
Note that we assessed the mean recovery time of olfaction through four defined propositions: 1–4 days; 5–8 days; 9–14 days; and >15 days [9].
2.3. Xerostomia symptoms
The Xerostomia Inventory is an 11-item summated rating scale which combines the responses to 11 individual items into a single continuous scale score which represents the severity of chronic xerostomia. We used the Summated Xerostomia Inventory-Dutch Version (SXI-DV), where five of those items (my mouth feels dry when eating a meal; my mouth feels dry; I have difficulty in eating dry foods; I have difficulties swallowing certain foods; and my lips feel dry) are used, with the respondent asked to choose one of three response options (“Never”, scoring 1; “Occasionally”, 2; and “Often”, 3). Higher scores represent more severe symptoms (score from 5 to 15) [21]. The same test was performed also in all recovered patients 15 days after SARS-COV-2 RT-PCR negative.
2.4. Eye dryness and alteration of tearing
The Standardized Patient Evaluation of Eye Dryness (SPEED) questionnaire was designed by Korb and Blackie in order to quickly track the progression of dry eye symptoms over time. This questionnaire gives a score from 0 to 28 that is the result of 8 items that assess frequency and severity of symptoms. The symptoms assessed include dryness, grittiness, scratchiness, irritation, burning, watering, soreness, and eye fatigue. The questionnaire further assesses whether these symptoms were not problematic, tolerable, uncomfortable, bothersome, or intolerable. The questionnaire also monitored diurnal and symptoms changes over 3 months. The resulting sensitivity and specificity were 0.90 and 0.80 respectively [22].
Clinically, the Schirmer test is most common and Schirmer scores, representing the length of wetting (in mm) on the strip, are routinely used as the key diagnostic criteria for dry eye. The test involves the insertion of a small piece of filter paper into the lower fornix of the eye. We used the Schirmer test I without the application of topical anesthetic prior to strip insertion. In this way, we measured the reflex tear secretion. Scores collected without anesthesia ranging from 5 to <10 mm and <8 mm with anesthesia are generally regarded as abnormal, signifying the presence of dry eye [23]. The same tests were performed also in all recovered patients 15 days after SARS-COV-2 RT-PCR negative.
2.5. Auditory discomfort
We evaluated the patient's auditory discomfort using two subjective tests for hearing loss and for the appearance of tinnitus. The Hearing Handicap Inventory For Adults (HHIA) was developed by Newman et al. [24]. It was used to assess the impact of hearing impairment daily life of the adults. It includes 13 and 12 questions in the emotional and social subsections, respectively. Each of the 25 questions has 3 options: Yes, Sometimes and No with the ratings of 4, 2, and 0, respectively. The maximum overall score is 100. The maximum number of points for social and emotional subsections is 48 and 52, respectively. In our study, given the social isolation imposed by the Italian government for subjects affected by COVID-19, five questions were eliminated from the test. A score of 0 implies no handicap while a score of 80 implies total handicap. A score ranging from 0 to 12 indicates no handicap, a score of 14–34 indicate mild-to-moderate Handicap. Score above 34 indicates significant handicap.
The Tinnitus Handicap Inventory (THI) is a self-administered test used to determine the degree of distress suffered by the patient with tinnitus [25]. It consists of 25 questions divided into three subgroups: functional, emotional, and catastrophic. Eleven items are included on the functional scale, nine on the emotional scale and five on the catastrophic scale. The THI uses a three-point scale: 0 (No), 2 (Sometimes), and 4 (Yes). The total score ranges from 0 to 100, and a higher score indicates a higher frequency of symptoms. The same tests were performed also in all recovered patients 15 days after SARS-COV-2 RT-PCR negative.
2.6. Statistical analysis
Statistical analysis were performed using SPSS 25.0 (IBM SPSS Statistics, New York, USA). The.
data are presented as means with standard deviations or numbers with percentage. Data normality was assessed using the Kolmogorov-Smirnov test of normality. The Wilcoxon Test for nonparametric dependent samples was used to compare measurements of sQOD-NS, SXI-DV, SPEED, HHIA, THI and Schirmer I reflex test in patients during convalescence (Condition A) and after 15 days from RT-PCR negative (Condition B). The Fisher Exact test was used to evaluate the number of patients with olfactory and gustatory dysfunctions, eye dryness, xerostomia, hearing loss and tinnitus in Condition A and in Condition B. A p ≤ 0.05 was been considered significant.
3. Results
A total of 50 patients were included in our study. The mean age of patients was 37.7 ± 17.9 years (range 18–65). There were 30 males and 20 females. The following ethnicities composed the cohort: European (88%) and Asian (12%) The most prevalent comorbidities of patients were gastroesophageal reflux disease (GERD), allergies, asthma and high blood pressure (Table 1 ). At the time of the study, 100% of patients were followed by the acute phase of the disease, until they received a negative SARS-COV-2 RT-PCR test. In recovered patients, the symptoms related to COVID-19 disappeared from an average of 25.32 ± 10.3 days at the end of the study. The mean time of follow-up was 49.7 ± 18.9 days.
Table 1.
Study population.
| Population M ± SD N (%) |
|
|---|---|
| Gender | |
| Male | 30/50 (60%) |
| Female | 20/50 (40%) |
| Age (years) | 37.7 ± 17.9 |
| Ethnicity | |
| European | 44/50 (88%) |
| Asian | 6/50 (12%) |
| Smoker | |
| No | 37/50 (74%) |
| Yes | 13/50 (26%) |
| Duration of COVID-19 symptoms | |
| 1–4 days | 10/50 (20%) |
| 5–8 days | 27/50 (54%) |
| 8–15 days | 10/50 (20%) |
| >15 days | 3/50 (6%) |
| Comorbidities | |
| Diabetes | 3/50 (6%) |
| High blood pressure | 8/50 (16%) |
| CRS | 5/50 (10%) |
| Thyroid disease | 4/50 (8%) |
| Allergies | 10/50 (20%) |
| GERD | 14/50 (28%) |
| Asthma | 7/50 (14%) |
| Heart problems | 2/50 (4%) |
| Autoimmune disease | 4/50 (8%) |
| Follow-up (days) | 49.7 ± 18.9 |
N, number; %, percentage; M, media; SD, standard deviation;
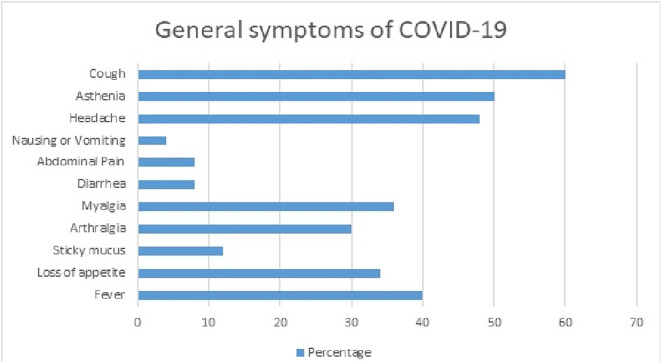
The general symptoms of patients during the infection are described in Fig. 1 . Cough, fever, headache and asthenia were the most prevalent symptoms, accounting for >40% of patients. The majority of the study population had a symptom duration of 5 to 8 days (54%), with an average of 7.14 ± 6.3. The otolaryngological symptoms most related to the infection are reported in Table 2 .
Fig. 1.
General Symptoms of COVID-19.
Table 2.
Otolaryngological complaints associated with COVID-19 Infection.
| Severity | Absence (0) | Tolerable (1) | Medium (2) | Unbearable (3) | Exhausting (4) |
|---|---|---|---|---|---|
| Nasal obstruction | 5 (10) | 5 (10) | 15 (30) | 20 (40) | 5 (10) |
| Rhinorrhoea | 10 (20) | 13 (26) | 18 (36) | 5 (10) | 4 (8) |
| Postnasal drip | 20 (40) | 15 (30) | 10 (20) | 3 (6) | 2 (4) |
| Sore throat | 23 (46) | 12 (24) | 8 (16) | 5 (10) | 2 (4) |
| Face pain | 20 (40) | 10 (20) | 6 (12) | 7 (14) | 7 (14) |
| Ear pain | 35 (70) | 5 (10) | 4 (8) | 3 (6) | 3 (6) |
| Tearing alteration | 12 (24) | 14 (28) | 10 (20) | 6 (12) | 8 (16) |
| Dry mouth | 25 (50) | 16 (32) | 5 (10) | 3 (6) | 1 (2) |
| Dysphagia | 4 (8) | 26 (52) | 10 (20) | 7 (14) | 3 (6) |
| Dyspnoea | 30 (60) | 11 (22) | 4 (8) | 4 (8) | 1 (2) |
| Dysphonia | 43 (86) | 6 (12) | 1 (2) | 0 (0) | 0 (0) |
Percentages are in brackets. Patients had to rate each of the following symptoms in terms of their relationship with your COVID-19 infection.
A total of 46 patients (92%) had olfactory dysfunction related to the infection. Among them, 21 (42%) patients were anosmic and 25 (50%) were hyposmic. Phantosmia was related to 16% of patients during the disease course.
Considering the patients with a clinically resolved infection (absence of general and ENT symptoms, negative SARS-COV-2 RT-PCR test), the olfactory dysfunction persisted after the resolution of other symptoms in 18% of cases (p < 0.001) (Table 4). As regards the onset of olfactory symptoms, for 20 (40%) patients it started before the other symptoms related to COVID-19, for 23 (46%) patients during the other symptoms, for the other 7 (14%) after the other symptoms. For 56% of patients, the olfactory symptoms lasted from 5 to 8 days, with an average duration of 7.3 days.
Table 4.
Comparison between the number of patients with olfactory and gustatory dysfunctions, eye dryness, Xerostomia and tinnitus during convalescence (Condition A) and after 15 days from RT-PCR SARS-COV-2 negativity (Condition B).
| Condition A | Condition B | p value | Odd ratio (95% CI) | |
|---|---|---|---|---|
| Olfactory dysfunction | 46/50 | 9/50 | <0.001 | 52.39 (14.9–182.9) |
| Gustatory dysfunction | 35/50 | 4/50 | <0.001 | 26.81 (8.1–87.9) |
| Eye dryness | 32/50 | 6/50 | <0.001 | 13.03 (4.6–36.5) |
| Xerostomia | 16/50 | 1/50 | 0.002 | 23.05 (2.9–182.2) |
| Hearing loss | 20/50 | 9/50 | 0.017 | 3.03 (1.21–7.59) |
| Tinnitus | 10/50 | 5/50 | 0.16 | 2.25 (0.7–7.1) |
The impact of olfactory dysfunction on the quality of life (QoL) of patients has been assessed through the validated sQOD-NS during the convalescence period with a mean of 9.45 ± 6.3 and after 15 days of negative SARS-COV-2 RT-PCR test with an average of 16.95 ± 7.8. This difference was statistically significant (p < 0.001), emphasizing the high impact of olfactory dysfunction during illness (Table 3 ).
Table 3.
Comparison between sQOD-NS, SXI-DV, SPEED, Schirmer (mm) and THI in the study population during convalescence (Condition A) and after 15 days from RT-PCR SARS-COV-2 negativity (Condition B).
| Condition A | Condition B | p value | |
|---|---|---|---|
| sQOD-NS | 9.45 ± 6.3 | 16.95 ± 7.8 | <0.001 |
| SXI-DV | 8.04 ± 1.9 | 5.08 ± 0.2 | <0.001 |
| SPEED | 8.58 ± 6.39 | 1.29 ± 1.65 | <0.001 |
| Schirmer | 9.22 ± 6.12 | 22.34 ± 16.32 | <0.001 |
| HHIA | 13.2 ± 14.9 | 4.24 ± 5.55 | <0.001 |
| THI | 6.6 ± 12.1 | 1.14 ± 3.2 | <0.001 |
sQOD-NS short Questionnaire of Olfactory Disorders-Negative Statements; SXI-DV Summated Xerostomia Inventory-Dutch Version; SPEED Standardized Patient Evaluation of Eye Dryness; HHIA Hearing Handicap Inventory For Adults; THI Tinnitus Handicap Inventory.
A total of 35 patients (70%) reported gustatory disorders, which was characterized by impairment of the following four taste modalities: salty, sweet, bitter, and sour. The gustatory dysfunction consisted of reduced, avoidance or distorted ability to taste flavours in 28%, 42% and 16% of patients, respectively. All patients with taste disorders also have a sense of smell dysfunction among the symptoms. The olfactory and gustatory disorders were constant and unchanged over the days in 54% of patients, whereas they fluctuated in 42% of patients. Among the patients who reported gustatory and olfactory disorders, 4% revealed that these disorders occurred during their rhinorrhoea or nasal obstruction episodes. The gustatory dysfunction persisted after the resolution of other symptoms in 8% of cases (p < 0.001) (Table 4 ).
We performed the Schirmer test for all patients to evaluate reflex tearing. The average result is 9.22 ± 6.12 mm, with a pathological value of <10 mm on 36 patients (72%). The same test was performed 15 days after the RT-PCR negativity with an average result of 22.34 ± 16.32 mm, with a statistically significant difference (p < 0.001). A total of 36 patients reported dry eyes. We performed the SPEED test on all patients during the period of florid symptoms, with an average of 8.58 ± 6.39. The same test was repeated 15 days after the result of the negative RT-PCR with an average of 1.29 ± 1.65, demonstrating a statistically significant result (p < 0.001) (Table 3). Eye dryness persisted after the resolution of COVID-19 symptoms and RT-PCR negative in 6 patients (12%) (p < 0.001) (Table 4).
Xerostomia was reported by 16 patients (32%), beginning with the other symptoms of the disease. During this period, the mean of the SXI-DV was 8.04 ± 1.9. The same test was performed 15 days after the negative result of the RT-PCR test, with an average of 5.08 ± 0.2, showing a statistically significant difference (p < 0.001) (Table 3). At the disappearance of the symptoms related to COVID, xerostomia was present only in 1 patient (2%) (p = 0.002) (Table 4).
In our study, we found that some patients had auditory symptoms. To give greater significance to our work, we took into consideration patients who experienced auditory discomfort for the first time or worsened it. 20 patients (40%) reported the appearance or worsening of hearing loss with a HHIA of 13.2 ± 14.9. 15 days after the negative RT-PCR test, 9 patients still reported the presence of hearing loss (p = 0.017) and the total mean of HHIA was 4.24 ± 5.55 (p < 0.001).
Tinnitus appeared or worsened in 10 patients (20%) with a THI of 6.6 ± 12.1. In condition B, tinnitus still persisted in 5 patients with an average THI of 1.14 ± 3.2 (p < 0.001).
4. Discussion
In the last months, an important number of medical doctors, otorhinolaryngologists in particular, health workers, and common people described anosmia or hyposmia and ageusia or dysgeusia as concomitant symptoms of COVID-19 infection. In this context, our unit conducted this study to investigate the prevalence and the short-term evolution of both olfactory and gustatory disorders, adding a study on tearing, salivation, and hearing. In our research, 92% of patients reported olfactory dysfunction, 42% of them having anosmia. The olfactory dysfunction may appear before (40%) the general symptoms, supporting the importance to add anosmia or hyposmia as symptoms of alert in some COVID-19 possible positive patients. Furthermore, establish potential screening markers for Covid-19 infection positivity considering anosmia or hyposmia and ageusia may carry some prognostic potential on the severity of the disease, due to the possibility to isolating and acting as soon as possible with the COVID-19 therapy. A survey on olfactory and taste disorders conducted in Hospital Sacco (Milan, Italy) noted that a 33,9% of patients have at least one taste or olfactory disorder and 18,6% both alteration [12]. In Germany, was estimated that >2 in 3 cases confirmed reported alteration of smell and taste. In another study of 3191 COVID-19 positive patients self-isolated at home, a 15.3% reported smell or taste loss [15]. In the study of Mao et al., patients indicated the most common complaints were hypogeusia (5.6%) and hyposmia (5.1%) [26]. Yan C. et al. noted that 68% of patients had an olfactory impairment and a 71% had gustatory alteration [27]. COVID-19 Task Force of the YO-IFOS, Jerome R. et al., analyzed 417 mild-to-moderate COVID-19 patients, with 85.6% and 88.0% of patients reporting olfactory and gustatory dysfunctions, respectively [9].
The neuroinvasive properties of SARS-CoV were first suspected when viral RNA was detected in the cerebrospinal fluid of a 32-year-old female patient in Hong Kong in 2004 [28]. The year after, SARS-CoV neuroinvasive properties were indeed demonstrated. The virus was isolated from the brain tissue of a SARS patient who presented neurological symptoms and neuropathology associated with necrosis of neuronal cells and glial cell activation. Moreover, the chemokine CXCL9/Mig (monokine induced by γ-interferon) was expressed by glial cells in association with the infiltration of T cells and macrophages [29]. The same year, another report indicated that SARS-CoV RNA was also detected in the brain of eight different patients who died from SARS, as the presence of the genomic RNA was detected in the cytoplasm of numerous hypothalamic and cortical neurons. Furthermore, edema and scattered red degeneration were observed in the brain of six out of the eight autopsied brains [30]. Therefore, SARS-CoV is neuroinvasive, neurotropic, and could be associated with the development of a neurological disease. Furthermore, the involvement of SARS-CoV in CNS infections was underscored by the findings that made use of transgenic mouse models expressing the human ACE-2, which is the cellular receptor used by the SARS-CoV to infect susceptible cells. Indeed, using these mice, showed that the SARS-CoV could invade the CNS after an intranasal infection primarily through the olfactory bulb [31] or even after an intraperitoneal infection [32], with concomitant neuronal loss. The pathophysiological mechanisms leading to the olfactory and gustatory dysfunctions in the COVID-19 infection, considering the particular tropism of the virus SARS-Cov-2 that enters to the olfactory cells through signal interaction between its spike protein and the ACE2 protein on nasal epithelium target cells. This connection could be responsible for anosmia and related disturbance in smell in COVID-19 patients [11]. Researchers discuss about two mechanisms of infection: first, infection of neuronal olfactory receptor in the olfactory mucosa, after which virus is transported anterogradely to the olfactory bulb; second, diffusion through the channels formed by olfactory ensheathing cells, which form an open connection to the Central Nervous System [33]. Interestingly, studies demonstrated that the virus antigen was first detected 60–66 h post-infection and was most abundant in the olfactory bulb. Regions of the cerebral cortex, basal ganglia and midbrain were also strongly interested after the virus had spread from the cribriform plate [34]. Galougahi KM et al. affirm that olfactory bulb magnetic resonance imaging in CoV-2 patients is considered useful for evaluation of patients with anosmia/hyposmia, is detected a reduction of the volume of olfactory bulb [35,36]. One of the principal points of question from the ORL perspective is to establish the time of recovery of olfactory and gustatory functions. Our results indicate that at least, 56% of patients recovered both olfactory and gustatory functions in 5–8 days from the first symptoms. Clearly, it is a short-term observation, further studies in follow-up is required to correct the analysis. The otolaryngological symptoms in our European cohort were particularly prevalent compared with the Asian cohorts. Guan et al. reported a prevalence of nasal obstruction in 5% of patients in a cohort of 1099 patients [5] and Chen N. et al. reported only a 4% of rhinorrhea in infected patients [37].
A study conducted during the 2003 SARS outbreak detected SARS-CoV in tear samples in SARS patients in Singapore [38]. Lack of eye protection was a primary risk factor of SARS-CoV transmission from SARS patients to healthcare workers in Toronto, prompting a concern that respiratory illness could be transmitted through ocular secretions [39]. Similar concerns have been raised with SARS-CoV-2, especially among eye care providers and those on the front lines triaging what could be initial symptoms of COVID-19. Patients infected with SARS-CoV-2 can present with symptoms of conjunctivitis, including eye redness, ocular irritation, foreign body sensation, tearing, and chemosis. These symptoms have more commonly affected patients with severe systemic symptoms of COVID-19, though they can rarely present as an initial manifestation of the disease. Examination findings include those consistent with mild follicular conjunctivitis, including unilateral or bilateral bulbar conjunctiva injection, follicular reaction of the palpebral conjunctiva, watery discharge, and mild eyelid edema. Bilateral chemosis alone may represent third-spacing in a critically ill patient rather than a true ocular manifestation of the virus. There have been no reports of COVID-19 patients experiencing blurred vision, subconjunctival hemorrhage, eyelid ecchymoses, conjunctival scarring, keratitis, or pseudomembrane formation [40]. SARS-CoV-2 can be detected in RT-PCR by sweeping the lower eyelid fornices to collect tears and conjunctival secretions with a virus sampling swab [41]. In our study, 72% of the patients showed symptoms related to dry eyes, with an altered Schirmer test. In all patients with ocular symptoms, dry eyes appeared during the period of manifestation of the classic symptoms related to COVID-19, but never appeared as an initial symptom, according to the data present in the literature.
SARS-CoV-2 has been consistently detected in whole saliva at an early stage of the disease [42] and in saliva collected from the duct opening of the salivary glands at a late stage. It has been shown that ACE2-positive salivary gland epithelial cells are early targets of SARS-CoV in nonhuman primates and that salivary gland functions may be affected at an early stage of the disease [43]. These findings suggest that oral symptoms may occur due to the impediment of salivary flow in these patients. A cross-sectional survey of 108 patients with confirmed COVID-19 in Wuhan indeed found that 46% of the patients reported dry mouth as one of their symptoms [44]. However, the temporal sequence of oral dryness and COVID-19 diagnosis is not clear and warrants further exploration. In summary, empirical, biological, and clinical evidence supports that oral mucosa is an initial site of entry for SARS-CoV-2 and that oral symptoms, including loss of taste/smell and dry mouth, might be early symptoms of COVID-19 before fever, dry cough, fatigue, shortness breath, and other typical symptoms occur. In our study, 32% of patients complained of dry mouth disorders, the onset of symptoms was earlier than the symptoms related to COVID-19, confirming the data in the literature.
In the manuscript of Chong Cui et al. 3/20 patients presented with symptoms of tinnitus, treated with betahistina with benefit [45].
In the study of Mustafa et al. twenty cases who were confirmed positive for COVID-19 formed the test group for 2 full weeks. Their age ranged between 20 and 50 years to avoid any age-related hearing affection. Patients who had a history of hearing loss or a history of any known cause of hearing loss were excluded from the examined sample. Twenty subjects who had normal hearing with no history of a known cause of hearing loss were used as a control group. No significant difference (p > 0.05) was found at all octave and mid-octave frequencies (250, 500, 750, 1000, 1500, 2000, 3000), 4000. Significant difference was found (p < 0.05) at 4000, 6000, and 8000 Hz between both groups. The high frequency pure-tone thresholds as well as the TEOAE amplitudes were significantly worse in the test group. The results of the current study showed that COVID-19 infection had deleterious effects on the hair cells in the cochlea. Moreover, the absence of the major symptoms does not guarantee a safe healthy cochlear function [46]. We evaluated the auditory discomfort in 50 patients, using two subjective tests for hearing loss and for the appearance of tinnitus, HHIA and THI, where the ear damage was in 40% of patients. The results of the current study showed that COVID-19 infection had deleterious effects on cochlear cell functions despite patients presented mild symptoms. Mechanisms involved in the induction of hearing loss by different viruses vary greatly, ranging from direct damage to inner ear structures, including inner ear hair cells and organ of Corti to induction of host immune-mediated damage.
Although several viral infections may lead to hearing loss, it's still unknown whether COVID-19 has effects on the auditory system or not. Therefore, this research was designed to address the impact of this novel viral infection on the auditory system, but further research are useful.
The present study has several limitations. We did not use specific objective examinations for olfactory, gustatory and salivary functions, including psychophysical tests or electrophysiological methods [47]. For the audiological study, we carried out only subjective tests. Unfortunately, we were unable to perform an audio-impedance test on patients. The patients in the study had little comorbidities, without respiratory problems. They may be not representative of the infected population. However, we excluded patients in high flow oxygen therapy with non-invasive ventilation, as it can alter the physiological hydration of the oral cavity and conjunctiva and patients who were in the intensive-care unit at the time of the data collection. The lack of consistent follow-up of our patients limits us from inquiring into the recovery time of olfactory and gustatory functions, tearing, salivation and hearing. All of these weaknesses should be considered in future studies to investigate and characterize the neurotropism and neuroinvasiveness of SARS-CoV-2. Given the limitations and weaknesses present in this manuscript, we can consider our work a preliminary study. It would be very interesting to enrich the literature with more detailed studies, with a greater number of patients, checked with objective diagnostic tests, considering our manuscript as a first step.
Coronaviruses are neurotropic, and SARS-CoV-2 most likely is not an exception; coronaviruses may enter the CNS through several routes, most notably through intranasal inoculation and though peripheral nerves using trans-synaptic pathways. Coronaviruses can infect both neurones and neuroglia; neural cells express the entry protein ACE2. With our study, we have tried to confirm the theories on the neuroinvasiveness of the virus, from the clinical point of view. In our studied population, we have shown an alteration of the sense of taste, of the sense of smell, dry eyes and of the oral cavity and an auditory discomfort, symptoms probably linked to the neurotropism of the virus. Furthermore, anosmia, dysgeusia and xerostomia are early symptoms of COVID-19, which can be exploited for an early quarantine and a limitation of viral contagion.
Compliance with ethical standards
Declaration of competing interest
All authors declare no conflict of interest.
Funding
No funding or financial support sources were used for this study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Contributor Information
Francesco Freni, Email: franco.freni@tiscali.it.
Alessandro Meduri, Email: ameduri@unime.it.
Francesco Gazia, Email: ssgazia@gmail.com.
Viviana Nicastro, Email: vivianica@hotmail.it.
Cosimo Galletti, Email: cosimogalletti92@gmail.com.
Pasquale Aragona, Email: paragona@unime.it.
Cosimo Galletti, Email: cosimogalletti88a@gmail.com.
Bruno Galletti, Email: bgalletti@unime.it.
Francesco Galletti, Email: Francesco.Galletti@unime.it.
References
- 1.Lovato A., de Filippis C. Clinical presentation of COVID-19: a systematic review focusing on upper airway symptoms. Ear Nose Throat J. 2020 Apr 13 doi: 10.1177/0145561320920762. published online ahead of print, 2020 Apr 13. [DOI] [PubMed] [Google Scholar]
- 2.Russell B., Moss C., Rigg A., Hopkins C., Papa S., Van Hemelrijck M. Anosmia and ageusia are emerging as symptoms in patients with COVID-19: what does the current evidence say? Ecancermedicalscience. 2020 Apr 3;14:ed98. doi: 10.3332/ecancer.2020.ed98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatr. 2020 Apr;87(4):281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovato A., de Filippis C., Marioni G. Upper airway symptoms in coronavirus disease 2019 (COVID-19) Am J Otolaryngol. 2020 Apr 4:102474. doi: 10.1016/j.amjoto.2020.102474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020 May;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y.C., Chen C.S., Chan Y.J. The outbreak of COVID-19: an overview. J Chin Med Assoc. 2020 Mar;83(3):217–220. doi: 10.1097/JCMA.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020 Apr 13;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 9.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020 Apr 6:1–11. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kowalski L.P., Sanabria A., Ridge J.A., Ng W.T., de Bree R., Rinaldo A. COVID-19 pandemic: effects and evidence-based recommendations for otolaryngology and head and neck surgery practice. Head Neck. 2020 Apr 9;42:1259–1267. doi: 10.1002/hed.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brann D.H., Tsukahara T., Weinreb C. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. bioRxiv. 2020;03(25):009084. doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. 2020 Mar 26:ciaa330. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaira L.A., Salzano G., Deiana G., De Riu G. Anosmia and Ageusia: common findings in COVID-19 patients. Laryngoscope. 2020 Apr 1 doi: 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki M., Saito K., Min W.P., Vladau C., Toida K., Itoh H. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007 Feb;117(2):272–277. doi: 10.1097/01.mlg.0000249922.37381.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopkins C., Surda P., Kumar N. Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology. 2020 Apr 11;130(7):1787. doi: 10.4193/Rhin20.116. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki M., Saito K., Min W.P., Vladau C., Toida K., Itoh H. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007 Feb;117(2):272–277. doi: 10.1097/01.mlg.0000249922.37381.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker A., Hopkins C., Surda P. The use of google trends to investigate the loss of smell related searches during COVID-19 outbreak. Int Forum Allergy Rhinol. 2020 doi: 10.1002/alr.22580. PMID: 32279437; PMCID: PMC7262261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steardo L., Steardo L., Jr., Zorec R., Verkhratsky A. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol (Oxf) 2020 Mar 29:e13473. doi: 10.1111/apha.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattos J.L., Edwards C., Schlosser R.J., Hyer M., Mace J.C., Smith T.L. A brief version of the questionnaire of olfactory disorders in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2019;9(10):1144–1150. doi: 10.1002/alr.22392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhattacharyya N., Kepnes L.J. Contemporary assessment of the prevalence of smell and taste problems in adults. Laryngoscope. 2015;125(5):1102–1106. doi: 10.1002/lary.24999. [DOI] [PubMed] [Google Scholar]
- 21.Thomson W.M., van der Putten G.J., de Baat C., Ikebe K., Matsuda K., Enoki K. Shortening the xerostomia inventory. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011 Sep;112(3):322–327. doi: 10.1016/j.tripleo.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asiedu K. Rasch analysis of the standard patient evaluation of eye dryness questionnaire. Eye Contact Lens. 2017 Nov;43(6):394–398. doi: 10.1097/ICL.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 23.Senchyna M., Wax M.B. Quantitative assessment of tear production: a review of methods and utility in dry eye drug discovery. J Ocul Biol Dis Infor. 2008 Mar;1(1):1–6. doi: 10.1007/s12177-008-9006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman C.W., Weinstein B.E., Jacobson G.P., Hug G.A. The hearing handicap inventory for adults: psychometric adequacy and audiometric correlates. Ear Hear. 1990 Dec;11(6):430–433. doi: 10.1097/00003446-199012000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Newman C.W., Jacobson G.P., Spitzer J.B. Development of the tinnitus handicap inventory. Arch Otolaryngol Head Neck Surg. 1996 Feb;122(2):143–148. doi: 10.1001/archotol.1996.01890140029007. [DOI] [PubMed] [Google Scholar]
- 26.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 Apr 10:e201127. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan C.H., Faraji F., Prajapati D.P., Boone C.E., DeConde A.S. Association of chemosensory dysfunction and COVID‐19 in patients presenting with influenza‐like symptoms. Int Forum Allergy Rhinol. 2020:1–8. doi: 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau K.K., Yu W.C., Chu C.M., Lau S.T., Sheng B., Yuen K.Y. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10(2):342–344. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J., Zhong S., Liu J., Li L., Li Y., Wu X. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis. 2005 Oct 15;41(8):1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005 Aug 1;202(3):415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tseng C.T., Huang C., Newman P., Wang N., Narayanan K., Watts D.M. Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human angiotensin-converting enzyme 2 virus receptor. J Virol. 2007 Feb;81(3):1162–1173. doi: 10.1128/JVI.01702-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Riel D., Verdijk R., Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol. 2015 Jan;235(2):277–287. doi: 10.1002/path.4461. [DOI] [PubMed] [Google Scholar]
- 34.Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008 Aug;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galougahi M.K., Ghorbani J., Bakhshayeshkaram M., Naeini A.S., Haseli S. Olfactory bulb magnetic resonance imaging in SARS-CoV-2-induced anosmia: the first report. Acad Radiol. 2020 Apr 11;27(6):892–893. doi: 10.1016/j.acra.2020.04.002. [S1076-6332(20)30194-X] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gane S.B., Kelly C., Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology. 2020 Apr 2;58(3):299–301. doi: 10.4193/Rhin20.114. [DOI] [PubMed] [Google Scholar]
- 37.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loon S.C., Teoh S.C., Oon L.L., Se-Thoe S.Y., Ling A.E., Leo Y.S. The severe acute respiratory syndrome coronavirus in tears. Br J Ophthalmol. 2004 Jul;88(7):861–863. doi: 10.1136/bjo.2003.035931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu C.W., Liu X.F., Jia Z.F. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020 Feb 22;395(10224):e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu P., Duan F., Luo C., Liu Q., Qu X., Liang L. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020 Mar 31;138(5):575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia J., Tong J., Liu M., Shen Y., Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020 Feb 26 doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.To K.K., Tsang O.T., Chik-Yan Yip C., Chan K.H., Wu T.C., Chan J.M.C. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020 Feb 12:ciaa149. doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu L., Wei Q., Alvarez X., Wang H., Du Y., Zhu H. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J Virol. 2011 Apr;85(8):4025–4030. doi: 10.1128/JVI.02292-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen L., Zhao J., Peng J., Li X., Deng X., Geng Z. Detection of 2019-nCoV in saliva and characterization of oral symptoms in COVID-19 patients. Mar 2020. https://ssrn.com/abstract=3556665 Available at SSRN. [DOI] [PMC free article] [PubMed]
- 45.Cui C., Yao Q., Zhang D., Zhao Y., Zhang K., Nisenbaum E. Approaching otolaryngology patients during the COVID-19 pandemic. Otolaryngol Head Neck Surg. 2020 May 12 doi: 10.1177/0194599820926144. Epub ahead of print. PMID: 32396445; PMCID: PMC7218357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mustafa M.W.M. Audiological profile of asymptomatic Covid-19 PCR-positive cases. Am J Otolaryngol. 2020 Apr 10:102483. doi: 10.1016/j.amjoto.2020.102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lovato A., Galletti C., Galletti B., de Filippis C. Clinical characteristics associated with persistent olfactory and taste alterations in COVID-19: a preliminary report on 121 patients. Am J Otolaryngol. 2020 May 26;41(5):102548. doi: 10.1016/j.amjoto.2020.102548. [DOI] [PMC free article] [PubMed] [Google Scholar]