Abstract
There is a strong consensus globally that a COVID-19 vaccine is likely the most effective approach to sustainably controlling the COVID-19 pandemic. An unprecedented research effort and global coordination has resulted in a rapid development of vaccine candidates and initiation of trials. Here, we review vaccine types, and progress with 10 vaccine candidates against SARS-CoV-2 – the virus that causes COVID-19 – currently undergoing early phase human trials. We also consider the many challenges of developing and deploying a new vaccine on a global scale, and recommend caution with respect to our expectations of the timeline that may be ahead.
Keywords: Covid 19, Vaccines, Human trials, Timeline
Introduction
COVID-19 is the disease caused by a novel betacoronavirus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2). The disease was first reported in December 2019 Wuhan, China [1] and the full genome was sequenced and published by January 05, 2020 [2]. By March 11, 2020, COVID-19 had spread globally and was declared a pandemic, with, at the time of writing, June 6th 2020, there has been over 6 million people infected and 380,000 deaths [3].
The disease primarily affects the respiratory tract and disease severity can range from very mild rhinorrhoea to severe acute respiratory distress syndrome and death [4], [5], [6]. Non-respiratory symptoms such as anosmia, diarrhoea, rash, thromboembolic disorders, myocarditis and vasculitis have also been associated with COVID-19 [5], [6], [7], [8], [9], [10], [11], [12], [13]. The median incubation period is estimated to be 5 days with a majority developing symptoms by 11.5 days [14]. COVID-19 patients have been shown to excrete viral nucleic acid at highest levels at the onset of symptoms [15]. This, and other epidemiological data [16] suggests transmissibility within an as yet undefined pre-symptomatic period [16]. Clinical deterioration is usually delayed into the second week of illness and associated with laboratory features of an immune-mediated cytokine storm causing widespread inflammation and disseminated intravascular coagulation, usually with low level viraemia [17], [18].
The case fatality rate (death amongst persons with disease) is consistently reported to be age dependent, with a higher percentage in elderly (aged > 70 years) cases dying, although other factors are also associated with intensive care admission and mortality [9], [6], [19] including sex (male > female), hypertension, obesity and diabetes. The reported case fatality rates [CFR] have been between 0.82% and 9.64%, with variability in CFR likely due to the testing frequency and access as well as other health system capacity factors in different locations [20]. The infection fatality rate (IFT; death amongst all people infected – asymptomatic and not tested) is a better estimate of population mortality and is modelled to be between 0.1% and 0.41% [20].
SARS-CoV-2 is one of three coronaviruses that may cause severe respiratory diseases, including fatality, in humans and have been associated with major outbreaks in the last 20 years; the other two viruses being severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV). Although there were some attempts at development of vaccines against SARS and MERS, including a small number of human phase 1 clinical trials, there are no licenced vaccines for any coronavirus as yet.
In addition to these novel epidemic viruses with zoonotic origins, there are four other endemic human coronaviruses in circulation, HCoV2-229E, -HKU1, -NL63 and -OC43 all predominantly causing mild symptoms of the common cold [21].
History of vaccines for Coronaviruses
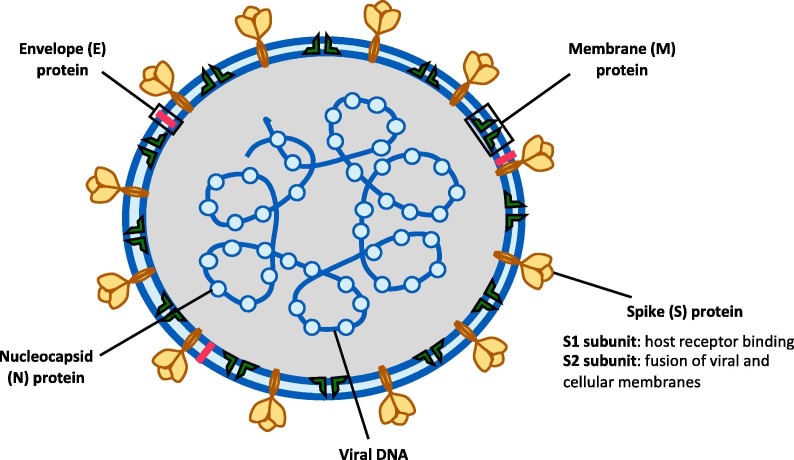
Coronaviruses have a large (30 + kb) single-stranded positive sense RNA genome encased by a helical nucleocapsid (N) and an outer envelope comprised of matrix protein (M), envelope protein (E) and spike proteins (S) [22]. The S protein, which naturally occurs in a trimeric form, contains the receptor-binding domain (RBD) responsible for binding onto the angiotensin converting enzyme 2 (ACE2) and entry into the cell (Fig. 1 ). In SARS-CoV, of all the structural proteins, S protein was found to elicit neutralising antibody and is a major target antigen for vaccine development [23], [24].
Fig. 1.
Schematic of the structure of SARS-CoV2. (Adapted from Lee, C-Y et al, Frontiers in Immunology, 2020)[100].
There have been difficulties in the development of coronavirus vaccines historically. Coronavirus vaccines in animal models that mimic human disease have been immunogenic but generally not shown to effectively prevent acquisition of disease [25]. Further, there is a concern that vaccination, as with natural coronaviral infection, may not induce long lived immunity and re-infection may be possible [26]. In some ways more concerning has been vaccine associated disease enhancement. Previous use of coronavirus vaccines (SARS-CoV and MERS-CoV) in some animal models raised safety concerns regarding Th2 mediated immunopathology [27]. Mice vaccinated with two inactivated whole virus vaccines, a recombinant DNA spike protein vaccine or a virus-like particle vaccine developed lung pathology including eosinophilic infiltration 2 days after being challenged with SARS-CoV which were not seen in the lungs of challenged unvaccinated mice [28]. Similar lung immunopathology was observed in several other studies, particularly in aged mice compared to younger mice [29] that were challenged following vaccination [30], [31]. Mice immunised with SARS-CoV N protein vaccine developed severe pneumonia or lung eosinophilic infiltrate upon viral challenge, whereas mice immunised with viral replicon particles expressing glycoprotein did not, suggesting that the N protein antigen may be the antigen linked to this immunopathology. Similar enhanced immunopathology has been seen in mice vaccinated with inactivated MERS-CoV vaccine when challenged with live virus [32]. Vaccine associated disease enhancement may be more of a concern with certain vaccine types. Enhanced disease caused by viral challenge has been notable following administration of inactivated measles and RSV vaccines [33], [34], with the possible mechanisms being a Th2 skewed response resulting from formalin inactivation as well as lack of affinity maturation of the antibodies produced [35].
SARS-CoV-2/COVID-19 vaccines
Context
Developing and scaling-up mass production of a vaccine rapidly in a global pandemic setting is challenging as it requires many activities to be well-coordinated and occurring in parallel, in contrast to the usual decade long, sequential process with pre-clinical testing, phased clinical trials, planned production and distribution. These challenges result in an aggregation of invested resources and elevated financial risk [36]. In outbreaks, delay in vaccine distribution can result in considerable mortality and morbidity as illustrated by the 2013/2014 West African Ebola epidemic which killed more than 11,000 people [37] and resulted in economic and social burden costing over 53 billion dollars [38]. Tragically, a vaccine had been in development and was later shown to be effective in the prevention of Ebola and may have contributed to controlling the outbreak [39], [40]. Unfortunately, the SARS 2003 epidemic ended before vaccine development was complete. Disappointingly, funding agencies then reallocated funds that had been committed to vaccine development, leaving manufacturers with financial loss and setting back other vaccine development programs [36]. In 2017, the Coalition of Epidemic Preparedness Innovation (CEPI) was formed to address these past failures with a mission to develop a coordinated response to emerging infectious disease threats to ensure vaccine development and early deployment in response to epidemics [41].
Diversity of technology platforms
One method for overcoming road blocks has been through the use of new technology platforms to expedite vaccine development [42]. Vaccines licensed in humans have traditionally been live attenuated viruses (e.g. Measles, mumps, rubella), inactivated viruses (e.g. inactivated polio vaccine) protein or polysaccharide conjugated subunit vaccines (protein: acellular pertussis, hepatitis B; polysaccharide conjugated: pneumococcus, meningococcus), and virus-like particles. Over the last decade, a range of new technology platforms have been developed and include vaccines composed of nucleic acid (DNA and RNA) and viral vectors and recombinant proteins.
-
1.
Recombinant vaccines/viral vectors
Viral vector technology involves the delivery of one or more genes that encode a target antigen within an unrelated, engineered virus. The viral vector can be replication competent (live attenuated) or replication deficient. For HIV, Ebola, Zika and Chikungunya, vaccines using viral vectors including adenovirus (Ad), measles virus (MV), vesicular stomatitis virus (VSV), alphaviruses, poxviruses and herpesviruses allowing for insertion of 5 kb or more of the transgene have shown ability to stimulate cellular and humoral immunity [43], [42]. Concerns with this platform are with the likely slower speed of vaccine manufacturing in an outbreak setting given the need for biosafety level 2 (BSL2) laboratories, and possible pre-existing immunity in vaccine recipients to viral vectors such as Ad5 and MV decreasing the effectiveness of the vaccine. Approaches such as the selection of low human prevalence adenoviral serotypes (Ad26 or Ad35) have been used to circumvent such an issue [43], [42]. The recombinant vesicular stomatitis virus-Zaire Ebola virus (rVSV-ZEBOV) Ebola vaccine is currently the only vector-vaccine that has been licensed and available for human use, and only produced and used to a limited extent [44], [45], [46].
A MERS-CoV vaccine (MVA-MERS-S_DF1) using modified vaccinia virus Ankara and expressing the spike (S) protein of MERS-CoV was evaluated in an open label, phase 1 trial on 26 individuals aged 18–55. It showed a favourable safety profile without any severe adverse effects but induced only a relatively limited humoral and T-cell response to the MERS CoV [47]. Reassuringly, the study showed that although vector specific neutralisation antibody was elicited, the vaccine still elicited antibody responses against the transgene following booster immunisation [47]. Results of the phase 1 clinical trial for an alternate vaccine, ChAdOx1 MERS vaccine that uses a replication deficient simian adenoviral vector expressing the spike (S) protein in 24 individuals aged 18–50 years showed that a single dose was able to elicit both humoral and cellular responses against MERS CoV. The majority of solicited and unsolicited adverse events (AEs) reported by participants were mild or moderate and all were self-limiting, and there were no serious AEs related to vaccine administration, which supports progression into phase 1b and 2 trials [48].
-
2.
Nucleic acid vaccines
Nucleic acid vaccines utilize antigen-encoding plasmid DNA or RNA, messenger RNA (mRNA) or viral replicons. The nucleic acid, once taken up by a cell will initiate protein synthesis, to which a humoral and cell-mediated immune response is expected to occur, similar to natural infection. Such vaccines have been trialled for veterinary infectious diseases and demonstrated immunogenicity, for example, for foot and mouth disease, deer powassan virus and rabies virus [49], [50], [51]. Phase I trials in humans are underway for nucleic acid vaccines against Ebola, influenza and Zika virus [42]. The benefit of a nucleic acid platform is the ease with which it allows antigen manipulation and the speed of production, as manufacturing can be synthetic and entirely cell free so circumventing the need for BSL2 laboratories. The disadvantages are that nucleic acid, especially mRNA, are fragile and require an uninterrupted cold-chain process for transport and storage [52]. Phase I clinical trials have been conducted on SARS-CoV and MERS-CoV DNA vaccine candidates. A recombinant SARS DNA vaccine candidate coding for the SARS-CoV N protein genome, developed by the National Institute of Allergy and Infectious Diseases (NIAID) was investigated in 10 adults [53]. A MERS-CoV DNA vaccine (GLS-5300), developed by GeneOne Life Science/Inovio and coding for the full length S protein genome, had a higher number of participants (n = 75) [54] Both showed acceptable safety profiles and induced humoral and cellular responses; the MERS-CoV DNA vaccine has advanced into a phase 2 clinical trial [55]. The only other SARS vaccine to have entered a Phase I trial is an inactivated vaccine (ISCV) produced by Sinovac Biotech [56]. There were no reports of human studies in which vaccinated subjects were challenged by the natural virus.
Vaccine candidates
As of 1st June 2020, there are currently 124 candidate vaccines that are under development for prevention of COVID-19 listed by the WHO Health Organization (WHO) landscape summary [57] of which 10 candidate vaccines specifically designed for prevention of COVID-19 (Table 1 ) have entered phase 1, combined phase 1/2 or phase 2 human clinical trials in adults. Most of these trials are enrolling healthy adults (from age 18 years) only, with the upper age limit of inclusion ranging from 50 to 60 years. Two trials are enrolling young participants, one from aged ≥ 3 years and the other ≥ 6 years with no upper age limit in both. One combined phase I/II trial includes older adults (up to age 85 years), while another early phase I trial has been extended in May 2020 to include also older adults (to age 99 years) [58], [59].
Table 1.
Candidate COVID-19 vaccines currently in phase 1 or 2 human clinical trials.
| Platform | Description | Advantages | Disadvantages |
|---|---|---|---|
| Recombinant Viral vector | Unrelated virus engineered to encode the target gene of the pathogen. Viral vectors can be replicating or non-replicating | Induces high cellular and humoral immune responses | Possible pre-existing immunity against vector Risk of reversion to virulence Limitations in scaling-up production |
| Candidate vaccines: |
Ad5-CoV Adenovirus Type 5 vector; antigen: Spike protein Phase I: CanSino Biologics ChiCTR2000030906 [88]/NCT04313127 [89] Phase II: Institute of Biotechnology; Academy of Military Medical Sciences ChiCTR2000031781 [90]/NCT04341389 [91] Participants: 18–60 years; Phase I n = 108Phase II: n = 508 Phase I: vaccine was safe and tolerable. No serious adverse effects. 63 participants (Low dose n = 18 [50%); moderate dose n = 18 [50%]; high dose n = 27 [75%]) developed four fold rise in neutralising antibody titres by Day 28. Pre-existing immunity to Ad5 neutralising antibody titre was observed in half of the participants before vaccination. Out of these, a low proportion seroconverted[76], [1] AZD1222 (previous known as ChAdOx1 nCoV-19) Simian adenoviral vaccine vector; antigen: Spike protein Phase I/II: University of Oxford NCT04324606 [92] Participants: 18–55 years; n = 1090 |
||
| Inactivated | Pathogen virus inactivated by chemicals or radiation | Easy to prepare High safety |
Variable efficacy |
| Candidate vaccines: |
PiCoVacc Phase I/II: Sinovac Biotech Co NCT04352608 [93] Participants: 18–59 years; Phase I n = 144; Phase II n = 600 Phase I/II: Sinovac Biotech Co NCT04383574 [94] Participants: ≥60 years; Phase I n = 72; Phase II n = 350 Inactivated novel coronavirus vaccine Phase I/II: Beijing Institute of Biological Products/Sinopharm ChiCTR2000032459 [95] Recruitment: Age >/= 3 years; Phase I n = 480; Phase II n = 1168 Inactivated novel coronavirus vaccine Phase I/II: Wuhan Institute of Biological Products/Sinopharm ChiCTR2000031809 [95] Participants: Age >/= 6 years; Phase I n = 288; Phase II n = 1168 |
||
| Live attenuated | Live virus whose genome(s) is mutated, inducing immune response but not disease | Induces long-term immunity | Expensive to produce |
| Candidate vaccines: | Nil in clinical trials as yet | ||
| Protein subunit | Components of target antigen protein produced in laboratory; some vaccines may use nanoparticle technology | High safety Scalability |
High cost Lower immunogenicity and may require adjuvant or repeat doses |
| Candidate vaccines: |
NVX-CoV2373 Phase I: Novovax NCT04368988 [96] Participants: 18–59 years; n = 131 |
||
| Virus like particle | Non-infectious self- assembling viral structural proteins | Induces strong immune response | Limitations in manufacturing production |
| Candidate vaccines: | Nil in clinical trial as yet | ||
| mRNA | mRNA encoding target antigen (may be complexed with lipid- or polymer-based nanoparticles) | Easier to design Induces strong immune response Rapid manufacture |
Requires mRNA to be encapsulated otherwise unstable under physiological conditions |
| Candidate vaccines: |
BNT162 Phase I/II: BioNTech/Pfizer Four candidates, two candidates include a nucleoside modified mRNA, one a uridine containing mRNA and the fourth self- amplifying mRNA. Each combined with a lipid nanoparticle formulation. Germany 2020–001038-36 [97] Participants: 18–55 years; n = 196 USA NCT04368728 [96] Participants: 18–85 years; n = 7600 mRNA-1273 Lipid nano-particle (LNP)-encapsulated mRNA vaccine; Coding antigen: full length S-protein Phase I/II: Moderna/National Institute of Allergy and infectious diseases NCT04283461 [59] Participants: 18–55 years, extended to 99 years n = 155 Interim Phase 1 Data: 15 participants seroconverted by day 15; 8 participants: all developed neutralizing antibodies[77] |
||
| DNA | DNA that encodes the target antigen | Easier to design Rapid manufacture |
May require a special approach to administer the vaccine (e.g. electroporation device) May requires adjuvant Uncertainty of safety issues |
|
INO-4800 Phase I: Inovio Pharmaceuticals NCT04336410 [98] Participants : 18–50 years n = 40 Plasmid DNA oral vaccine (bacTRL-IL-Spike-1) Phase 1: Symvivo NCT04334980 [99] Participants: 19 = 55 years; n = 84 |
|||
Re-purposed vaccines of for COVID-19 or off target effects of other vaccines
Licensed vaccines such as BCG and oral polio vaccine have been shown to have nonspecific, modulatory effects on the immune system and provide protection against other infectious diseases [60], [61], [62], [63], [64]. This has led to the suggestion that these vaccines may have an effect in the prevention of COVID-19 [65]. Three multi-centred randomised controlled trials on BCG vaccine administration are underway in health care workers in Australia [66], Netherlands [67] and South Africa [68]. A measles vaccine trial to prevent COVID-19 in health care workers in Egypt has been registered [58] and oral polio vaccines are being considered in the United States of America [69].
Discussion
Even if sustained immunity is attained after infection by SARS-CoV2, estimates are that 60–70% of a population would need to be immune to achieve herd immunity against SARS-CoV2 [70]. The safest and most controlled way for effective and sustainable prevention of COVID-19 in a population is to have an efficacious and safe vaccine and the majority of the population successfully vaccinated. In addition, the vaccine should also be readily mass-produced inexpensively, and be easily transportable with minimal cold chain requirements to have global utility. Immunity after primary COVID-19 infection seems to protect against re-infection in primate models and is likely to occur in humans [71]; whether this can be mimicked in vaccines and for how long immunity may last is still uncertain. Following SARS-CoV infection, IgG and neutralising antibody was detectable for 1–3 years following infection which suggests that vaccine-induced protection is unlikely to be long-lasting and may require re-immunization [72], [73], [74], [75].
The rapid progression of new vaccine candidates against SARS-CoV-2 into pre-clinical and clinical studies is encouraging. Several phase 1 trial results have recently been released. Ad5-CoV vaccine, conducted in 108 participants showed reasonable safety or tolerability profile and has now progressed to phase II. Humoral and cell mediated responses were seen in participants, albeit less in those with pre-existing Ad5 vector immunity [76]. Interim phase 1 data, released on May 18 on mRNA-1273 demonstrated seroconversion and development of neutralizing antibodies in 8 individuals [77]. It is an open question of whether these candidates will display the necessary efficacy and safety profile in humans to progress further into phase 3 trials and subsequently licensure and use to control COVID-19 transmission. In a review of 11 epidemic infectious diseases, only 1 out of 11 (21 preclinical vaccine candidates) have been shown to go through to end of phase 2a trials, at the estimated cost of USD $319–469 million (range $137 million to $1.1 billion) [78]. In the current climate, some vaccine developers are have shown willingness to start or progress later phase trial preparations even before definitive results of earlier phase trials are available [79].
There have been international collaborative efforts to expedite vaccine development and production. The prior establishment of CEPI has been an integral existing platform that has supported the rapid development of COVID-19 vaccines without having to establish new mechanisms with attendant costs. CEPI is supporting the nine COVID-19 vaccine candidates briefly presented here. Since its formation it has established measures to finance early development of vaccines, up to phase 3 clinical trials. However, it does not have a role in the manufacturing or deployment of vaccines and a consortium of public and philanthropic funders will be required for complete preparedness for vaccine manufacture and delivery.
In addition to CEPI, the WHO and the U.S. National Institutes of Health (NIH) are contributing to global collaborative efforts to accelerate vaccine development. The WHO Solidarity Trial for vaccines is a large, multi-site individually randomised controlled clinical trial to allow evaluation of the benefits and risks of each COVID-19 candidate vaccines within 3–6 months [80]. Further, Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV), an international public–private partnership, has been established to co-ordinate and speed up the response to the COVID-19 pandemic. Government organisations (in the US and one in Europe), international biopharmaceutical companies and a non-profit organisation are all involved in advancing vaccine development [81].
Vaccine efficacy in under-represented and vulnerable populations also remains an issue. A majority of vaccine trials have focused on healthy people between the ages of 18–65 years, excluding the elderly, pregnant women and children. Given the disproportionate mortality rate in people over the age of 60, the elderly need to be considered in vaccine trials to ensure safety, immunogenicity and efficacy data is collected and are prioritised to receive COVID-19 vaccines in outbreak situations. Pregnancy has not been shown to be a risk factor for disease severity and the disease burden is low in children. However, much is still unknown, especially the role of inducing an adaptive auto-inflammatory response such as Paediatric Inflammatory Multisystem Syndrome Temporally associated with SARS-CoV-2 (PIMS-TS). There is an urgent need for a better understanding of the immunopathogenesis of COVID-19 to give guidance to the immunological assessment of vaccine responses.
There has never been a more rapid pace to vaccine development. The pandemic situation has been a challenge and a trigger to reconsidering the usual approaches to regulatory assessment and licensing processes. Vaccine companies are showing willingness to commit to scaled-up production prior to definitive phase 3 trial results [79]. The implementation of high quality, aligned surveillance for COVID-19 across multiple regions concurrently with vaccine deployment is critical both for evaluating the real-world effectiveness of a new vaccine against SARS-CoV-2, but also for monitoring its safety in so-call ‘post-marketing’ surveillance. The association of rotavirus vaccines with intussusception in children was only detected following licensure and deployment of these vaccines [82], [83]. The experience of the Philippines with a novel dengue vaccine should also promote a degree of caution with respect to vaccine safety during their population usage even if they appear safe in phase 1, 2 and 3 studies. [84], [85] There will be considerable uncertainties regarding the safety of vaccines for COVID-19 given the new vaccine platforms used without prior licensed examples, deployment to population subgroups not included in trials of that particular candidate), additional uncertainty arising from novel adjuvants in quite a number of candidates.
As we have alluded to, the development of a vaccine that shows efficacy in clinical trials is only the beginning of a process to manufacture, deploy and monitor the effectiveness of a new vaccine. The challenges ahead are numerous. As an example, in 2016 as part of the Global Poliovirus Eradication Initiative (GPEI), 155 countries were planned to synchronously shift from trivalent oral polio vaccine (tOPV) to bivalent vaccine (bOPV) [86]. This switch was to be coordinated with concurrent inclusion of at least 1 dose of inactivated polio vaccine (IPV) into routine immunization schedules in 126 OPV using countries. Significant difficulties have been encountered with vaccine manufacturing and supply chains resulting in interruptions and delays in multiple countries [87]. This experience will certainly inform and improve a potential global COVID-19 vaccine deployment, but should influence a level of caution with respect to our expectations even were an effective vaccine developed.
Despite efforts in fast tracking vaccine development, completion dates for early clinical trials are estimated to be late 2020 to mid-2021 and it may still take longer before a vaccine is licensed for use globally, although the pandemic has triggered reconsideration of the usual approaches to regulatory assessment and licensing. This emphasises the need for proven public health strategies such as physical distancing, early detection, self-isolation and outbreak control remain as important mitigation tools.
Educational aims
The reader will be able to:
-
•
Understand of the types of vaccine and vaccine platforms being developed for SARS-CoV-2.
-
•
Develop knowledge regarding the concerns around coronavirus vaccine development.
-
•
Appreciated the issues of rapid vaccine development in outbreak settings.
Directions for future research
-
•
Ongoing progress of candidate vaccines through preclinical and clinical studies.
-
•
Ongoing detailed characterisation of the immunopathogenesis of COVID-19.
-
•
Implementation of large scale post marketing surveillance systems to monitor SARS-CoV2 vaccine safety.
Acknowledgements
Dr Philip Britton is funded by an Early Career Fellowship from the National Health and Medical Research Council (GNT1145817).
Contributor Information
Archana Koirala, Email: Archana.Koirala@health.nsw.gov.au.
Ye Jin Joo, Email: YeJin.Joo@health.nsw.gov.au.
Ameneh Khatami, Email: ameneh.khatami@health.nsw.gov.au.
Clayton Chiu, Email: clayton.chiu@health.nsw.gov.au.
Philip N. Britton, Email: philip.britton@health.nsw.gov.au.
References
- 1.Zhu N. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu F. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centre for Systems Science and Engineering. COVID-19 Dashboard. 2020 [cited 2020 June 3, 2020]; Available from: https://coronavirus.jhu.edu/map.html.
- 4.Guan W.-J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatraju P.K. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020 doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan L. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115 doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giacomelli A. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou P. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song Y. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut. 2020;69(6):1143–1144. doi: 10.1136/gutjnl-2020-320891. [DOI] [PubMed] [Google Scholar]
- 13.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 14.Lauer S.A. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020 doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou L. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He X. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020:1–4. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 17.Zhou F. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang N. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan W.-J. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oke J, Heneghan C. Global COVID-19 case fatality rates. 2002 19 May 2020 [cited 2020 24 May 2020]; Available from: https://www.cebm.net/covid-19/global-covid-19-case-fatality-rates/.
- 21.Zimmermann P., Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39(5):355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boopathi S., Poma A.B., Kolandaivel P. Novel coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J Biomol Struct Dyn. 2019;2020:1–10. doi: 10.1080/07391102.2020.1758788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchholz U.J. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc Natl Acad Sci U S A. 2004;101(26):9804–9809. doi: 10.1073/pnas.0403492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walls A.C. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020 doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roper R.L., Rehm K.E. SARS vaccines: where are we? Expert Rev Vaccines. 2009;8(7):887–898. doi: 10.1586/erv.09.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edridge A. et al., Human coronavirus reinfection dynamics: lessons for SARS-CoV-2. 2020.
- 27.Graham R.L., Donaldson E.F., Baric R.S. A decade after SARS: strategies for controlling emerging coronaviruses. Nat Rev Microbiol. 2013;11(12):836–848. doi: 10.1038/nrmicro3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tseng C.-T. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0035421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deming D. Vaccine efficacy in senescent mice challenged with recombinant SARS-CoV bearing epidemic and zoonotic spike variants. PLoS Med. 2006;3(12) doi: 10.1371/journal.pmed.0030525. p. e525-e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolles M. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol. 2011;85(23):12201–12215. doi: 10.1128/JVI.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yasui F. Prior immunization with severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS-CoV. J Immunol. 2008;181(9):6337–6348. doi: 10.4049/jimmunol.181.9.6337. [DOI] [PubMed] [Google Scholar]
- 32.Agrawal A.S. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Human Vaccines Immunother. 2016;12(9):2351–2356. doi: 10.1080/21645515.2016.1177688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chin J. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89(4):449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 34.Kim H.W. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 35.Delgado M.F. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15(1):34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lurie N. Developing covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382(21):1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 37.WHO Ebola Response Team After Ebola in West Africa — unpredictable risks, preventable epidemics. N Engl J Med. 2016;375(6):587–596. doi: 10.1056/NEJMsr1513109. [DOI] [PubMed] [Google Scholar]
- 38.Huber C., Finelli L., Stevens W. The economic and social burden of the 2014 Ebola outbreak in West Africa. J Infect Dis. 2018;218(Supplement_5) doi: 10.1093/infdis/jiy213. p. S698-S704. [DOI] [PubMed] [Google Scholar]
- 39.Jones S.M. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med. 2005;11(7):786–790. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- 40.Henao-Restrepo A.M. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!) Lancet. 2017;389(10068):505–518. doi: 10.1016/S0140-6736(16)32621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brende B. CEPI—a new global R&D organisation for epidemic preparedness and response. Lancet. 2017;389(10066):233–235. doi: 10.1016/S0140-6736(17)30131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rauch S. New vaccine technologies to combat outbreak situations. Front Immunol. 2018;9:1963. doi: 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robert-Guroff M. Replicating and non-replicating viral vectors for vaccine development. Curr Opin Biotechnol. 2007;18(6):546–556. doi: 10.1016/j.copbio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Regules J.A. A recombinant vesicular stomatitis virus Ebola vaccine. N Engl J Med. 2017;376(4):330–341. doi: 10.1056/NEJMoa1414216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kennedy S.B. Phase 2 placebo-controlled trial of two vaccines to prevent Ebola in Liberia. N Engl J Med. 2017;377(15):1438–1447. doi: 10.1056/NEJMoa1614067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization . The Organization; Geneva: 2019. Preliminary results on the efficacy of rVSV-ZEBOV-GP Ebola vaccine using the ring vaccination strategy in the control of an Ebola outbreak in the Democratic Republic of the Congo: an example of integration of research into epidemic response. [Google Scholar]
- 47.Koch T. Safety and immunogenicity of a modified vaccinia virus Ankara vector vaccine candidate for Middle East respiratory syndrome: an open-label, phase 1 trial. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Folegatti P.M. Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pulido M.R. RNA immunization can protect mice against foot-and-mouth disease virus. Antiviral Res. 2010;85(3):556–558. doi: 10.1016/j.antiviral.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 50.VanBlargan L.A. An mRNA vaccine protects mice against multiple tick-transmitted flavivirus infections. Cell Reports. 2018;25(12) doi: 10.1016/j.celrep.2018.11.082. p. 3382-3392. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saxena S. Induction of immune responses and protection in mice against rabies using a self-replicating RNA vaccine encoding rabies virus glycoprotein. Vet Microbiol. 2009;136(1–2):36–44. doi: 10.1016/j.vetmic.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 52.Zhang C. Advances in mRNA vaccines for infectious diseases. Front Immunol. 2019;10(594) doi: 10.3389/fimmu.2019.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin J.E. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine. 2008;26(50):6338–6343. doi: 10.1016/j.vaccine.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Modjarrad K. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect Dis. 2019;19(9):1013–1022. doi: 10.1016/S1473-3099(19)30266-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.ClinicalTrials.gov. Identifier: NCT03721718. Evaluate the Safety, Tolerability and Immunogenicity Study of GLS-5300 in Healthy Volunteers 2020 [cited 2020 25 May 2020]; Available from: https://clinicaltrials.gov/ct2/show/NCT03721718.
- 56.Lin J. Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antiviral Therapy. 2007;12(7):1107–1113. [PubMed] [Google Scholar]
- 57.World Health Organization, DRAFT landscape of COVID-19 candidate vaccines. World, 2020.
- 58.ClinicalTrials.gov. Identifier: NCT04357028. Measles Vaccine in HCW (MV-COVID19) 2020; Available from: https://clinicaltrials.gov/ct2/show/NCT04357028.
- 59.ClinicalTrials.gov. Identifier: NCT04283461. Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19) 2020 May 4, 2020 [cited 2020 May 25, 2020]; Available from: https://clinicaltrials.gov/ct2/show/NCT04283461.
- 60.Goodridge H.S. Harnessing the beneficial heterologous effects of vaccination. Nat Rev Immunol. 2016;16(6):392–400. doi: 10.1038/nri.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pollard A.J., Finn A., Curtis N. Non-specific effects of vaccines: plausible and potentially important, but implications uncertain. Arch Dis Child. 2017;102(11):1077–1081. doi: 10.1136/archdischild-2015-310282. [DOI] [PubMed] [Google Scholar]
- 62.Biering-Sørensen S. Early BCG-Denmark and neonatal mortality among infants weighing< 2500 g: a randomized controlled trial. Clin Infect Dis. 2017;65(7):1183–1190. doi: 10.1093/cid/cix525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Upfill-Brown A. Nonspecific effects of oral polio vaccine on diarrheal burden and etiology among Bangladeshi infants. Clin Infect Dis. 2017;65(3):414–419. doi: 10.1093/cid/cix354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aaby P. Non-specific beneficial effect of measles immunisation: analysis of mortality studies from developing countries. BMJ. 1995;311(7003):481–485. doi: 10.1136/bmj.311.7003.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Curtis N. Considering BCG vaccination to reduce the impact of COVID-19. Lancet. 2020 doi: 10.1016/S0140-6736(20)31025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.ClinicalTrials.gov. Identifier: NCT04327206. BCG Vaccination to Protect Healthcare Workers Against COVID-19 (BRACE) 2020; Available from: https://clinicaltrials.gov/ct2/show/NCT04327206.
- 67.ClinicalTrials.gov. Identifier: NCT04328441. Reducing Health Care Workers Absenteeism in Covid-19 Pandemic Through BCG Vaccine (BCG-CORONA); Available from: https://clinicaltrials.gov/ct2/show/NCT04328441.
- 68.ClinicalTrials.gov. Identifier: NCT04379336. BCG Vaccination for Healthcare Workers in COVID-19 Pandemic 2020; Available from: https://clinicaltrials.gov/ct2/show/NCT04379336.
- 69.Global Polio Eradication Initiative. The use of oral polio vaccine (OPV) to prevent SARS-CoV2. 2020 [cited 2020 24 May 2020]; Available from: http://polioeradication.org/wp-content/uploads/2020/03/Use-of-OPV-and-COVID-20200421.pdf.
- 70.Randolph H.E., Barreiro L.B. Herd immunity: understanding COVID-19. Immunity. 2020;52(5):737–741. doi: 10.1016/j.immuni.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bao L. Reinfection could not occur in SARS-CoV-2 infected rhesus macaques. BioRxiv. 2020 [Google Scholar]
- 72.Wu L.-P. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis. 2007;13(10):1562–1564. doi: 10.3201/eid1310.070576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu W. Two-year prospective study of the humoral immune response of patients with severe acute respiratory syndrome. J Infect Dis. 2006;193(6):792–795. doi: 10.1086/500469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cao W.-C. Disappearance of antibodies to SARS-associated coronavirus after recovery. N Engl J Med. 2007;357(11):1162–1163. doi: 10.1056/NEJMc070348. [DOI] [PubMed] [Google Scholar]
- 75.Liu L. Longitudinal profiles of immunoglobulin G antibodies against severe acute respiratory syndrome coronavirus components and neutralizing activities in recovered patients. Scand J Infect Dis. 2011;43(6–7):515–521. doi: 10.3109/00365548.2011.560184. [DOI] [PubMed] [Google Scholar]
- 76.Zhu F.-C. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moderna, Moderna Announces Positive Interim Phase 1 Data for its mRNA Vaccine (mRNA-1273) Against Novel Coronavirus. 2020, Moderna, Inc.
- 78.Gouglas D. Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study. Lancet Global Health. 2018;6(12):e1386–e1396. doi: 10.1016/S2214-109X(18)30346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mullard A. COVID-19 vaccine development pipeline gears up. Lancet. 2020;395(10239):1751–1752. doi: 10.1016/S0140-6736(20)31252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.World Health Organisation. Update on WHO Solidarity Trial – Accelerating a safe and effective COVID-19 vaccine. 2020 [cited 2020 May 24, 2020]; Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-trial-accelerating-a-safe-and-effective-covid-19-vaccine.
- 81.National Institutes of Health. NIH to launch public-private partnership to speed COVID-19 vaccine and treatment options. 2020 April 17, 2020 [cited 2020 May 24, 2020]; Available from: https://www.nih.gov/news-events/news-releases/nih-launch-public-private-partnership-speed-covid-19-vaccine-treatment-options.
- 82.Parashar U.D. Value of post-licensure data on benefits and risks of vaccination to inform vaccine policy: the example of rotavirus vaccines. Vaccine. 2015;33(Suppl 4):D55–D59. doi: 10.1016/j.vaccine.2015.05.094. [DOI] [PubMed] [Google Scholar]
- 83.Yen C. Rotavirus vaccination and intussusception - Science, surveillance, and safety: A review of evidence and recommendations for future research priorities in low and middle income countries. Hum Vaccin Immunother. 2016;12(10):2580–2589. doi: 10.1080/21645515.2016.1197452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Larson H.J., Hartigan-Go K., de Figueiredo A. Vaccine confidence plummets in the Philippines following dengue vaccine scare: why it matters to pandemic preparedness. Hum Vaccin Immunother. 2019;15(3):625–627. doi: 10.1080/21645515.2018.1522468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Bruin Y.B. Initial impacts of global risk mitigation measures taken during the combatting of the COVID-19 pandemic. Saf Sci. 2020 doi: 10.1016/j.ssci.2020.104773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.World Health Organization and Global Polio Eradication Initiative . World Health Organization; 2019. Polio endgame strategy 2019–2023: eradication, integration, certification and containment. [Google Scholar]
- 87.Lewis I. A supply and demand management perspective on the accelerated global introductions of inactivated poliovirus vaccine in a constrained supply market. J Infect Dis. 2017;216(suppl_1):S33–S39. doi: 10.1093/infdis/jiw550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chinese Clinical Trial Registry. ChiCTR2000030906 A phase I clinical trial for recombinant novel coronavirus (2019-COV) vaccine (adenoviral vector) 2020 [cited 2020 25 May 2020]; Available from: http://www.chictr.org.cn/showprojen.aspx?proj=51154.
- 89.ClinicalTrials.gov. Identifier: NCT04313127. Phase I Clinical Trial of a COVID-19 Vaccine in 18-60 Healthy Adults (CTCOVID-19) 2020 [cited 2020 25 May 2020]; Available from: https://clinicaltrials.gov/ct2/show/NCT04313127.
- 90.Chinese Clinical Trial Registry. ChiCTR2000031781 A randomized, double-blinded, placebo-controlled phase II clinical trial for Recombinant Novel Coronavirus (2019-nCOV) Vaccine (Adenovirus Vector) 2020 [cited 2020 25 May 2020]; Available from: http://www.chictr.org.cn/showprojen.aspx?proj=52006.
- 91.ClinicalTrials.gov. Identifier: NCT04341389. A Phase II Clinical Trial to Evaluate the Recombinant Vaccine for COVID-19 (Adenovirus Vector) (CTII-nCoV) 2020 [cited 2020 May 25 2020]; Available from: https://clinicaltrials.gov/ct2/show/NCT04341389.
- 92.ClinicalTrials.gov. Identifier: NCT04324606. A Study of a Candidate COVID-19 Vaccine (COV001) 2020 [cited 2020 25 May 2020]; Available from: https://clinicaltrials.gov/ct2/show/NCT04324606.
- 93.ClinicalTrials.gov. Identifier: NCT04352608. Safety and Immunogenicity Study of Inactivated Vaccine for Prophylaxis of SARS CoV-2 Infection (COVID-19) 2020 April 28, 2020 [cited 2020 May 25, 2020].
- 94.ClinicalTrials.gov. Identifier: NCT04383574. Safety and Immunogenicity Study of Inactivated Vaccine for Prevention of SARS-CoV-2 Infection (COVID-19) 2020 12 May 2020 [cited 2020 25 May 2020]; Available from: https://clinicaltrials.gov/ct2/show/NCT04383574.
- 95.Chinese Clinical Trial Registry. ChiCTR2000031809 A randomized, double-blind, placebo parallel-controlled phase I/II clinical trial for inactivated Novel Coronavirus Pneumonia vaccine (Vero cells) 2020 April 13, 2020 [cited 2020 May 25, 2020]; Available from: http://www.chictr.org.cn/showprojen.aspx?proj=52227.
- 96.ClinicalTrials.gov. Identifier: NCT04368988. Evaluation of the Safety and Immunogenicity of a SARS-CoV-2 rS (COVID-19) Nanoparticle Vaccine With/Without Matrix-M Adjuvant 2020 May 15, 2020 [cited 2020 May 25, 2020]; Available from: https://clinicaltrials.gov/ct2/show/NCT04368988.
- 97.EU Clinical Trials Register. EudraCT Number: 2020-001038-36. A Multi-site Phase I/II, 2-Part, Dose-Escalation Trial Investigating the Safety and Immunogenicity of four Prophylactic SARS-CoV-2 RNA Vaccines Against COVID-2019 Using Different Dosing Regimens 2020 April 20, 2020 [cited 2020 May 25, 2020]; Available from: https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number%3A2020-001038-36.
- 98.ClinicalTrials.gov. Identifier: NCT04336410. 2020 April 24, 2020 [cited 2020 May 2, 2020]; Available from: https://clinicaltrials.gov/ct2/show/NCT04336410.
- 99.ClinicalTrials.gov. Identifier: NCT04334980. 2020 April 22, 2020 [cited 2020 May 25, 2020]; Available from: https://clinicaltrials.gov/ct2/show/NCT04334980.
- 100.Lee C.Y.-P. Serological approaches for COVID- 19: epidemiologic perspective on surveillance and control. Front Immunol. 2020:11(879). doi: 10.3389/fimmu.2020.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]